Au–thymine, thymidine and thymidine 5′-monophosphate nanoparticles: chemical characterisation and cellular uptake studies into U87 cancer cells†
Svetlana
Avvakumova
a,
Giorgio
Scari
b and
Francesca
Porta
*a
aThe University of Milan, Department of Inorganic, Metallorganic and Analytical Chemistry “L. Malatesta”, via Venezian, 21, 20133, Milan, Italy. E-mail: francesca.porta@unimi.it
bThe University of Milan, Department of Biology, via Celoria, 26, 20133, Milan, Italy
First published on 7th March 2012
Abstract
Gold nanoparticles capped by thymine, thymidine and thymidine 5′-monophosphate were prepared and characterised by spectroscopy and microscopy techniques. AuNPs incubated with U87 cancer cells were taken up and showed a particular ability to overcome intracellular boundaries, being present in various cell compartments.
Recently, gold nanoconjugates have attracted great attention for their properties and promising applications in biology and medicine.1 Gold nanoparticles (AuNPs), when functionalized with the appropriate surface moieties, can readily enter living cells.2 Recently, the ability of some peptides to negotiate intra- and intercellular membranes independently of receptors and temperature was reported.2,3 Thus, taking advantage of this property and binding a therapeutic agent able to penetrate the nucleus to nanoconjugates could deliver directly into the nucleus.1 The nanoparticles able of penetrating the nucleus must be small enough (<30 nm) to enter cells, bypass or escape endosomal/lysosomal pathways; penetrate nuclear membranes or access importins to pass through the nuclear pore complex, and, finally, they must possess a low cytotoxicity.3 Nevertheless, no information has been reported on the nuclear penetration of AuNPs functionalised with simple small molecules, like nucleotides or nucleosides. In the present work, we focused our attention on some nucleic acid components: thymine (1), thymidine (2) and thymidine 5′-monophospate (3), analogs widely used in cancer treatments. Azidothymidine, for example, is known to be efficient for the treatment of HIV/AIDS, functioning as a chain terminator of DNA synthesis.4 The tritiated thymidine is used for cell proliferation assay: once incorporated into dividing cells, it induces cell cycle arrest and apoptosis.5 Interestingly, the incorporation of thymidine in DNA was demonstrated in the fifties by Reichard et al.6 But only some years later was it discovered that the thymidine kinase enzyme, which may be activated only in cells expressing a tumor marker, was responsible for this process.7,8 Hence, we were stimulated by these interesting arguments towards the study of the characteristics of AuNPs capped by (1)–(3), to understand their ability to penetrate the cell nucleus and investigate the fate of (1)–(3) capped gold nanoparticles inside the cell.
In the present study, water soluble AuNPs were prepared via sodium borohydride reduction in the presence of thymine (1), thymidine (2) and thymidine 5-monophosphate (3) (see ESI,† III for molecular structure) (also referred as T, dT and 5′-TMP further in the text) as stabilising agents. Opaque reddish sols were obtained. The experimental details are available in part II of the ESI.†
UV-visible spectroscopy allows us to monitor the reactions and determine the running time of the growth process of AuNPs (2 h) The spherical morphology and mean particle sizes (10–13 nm) were determined by TEM microscopy. Fig. 1 reports the micrographs of Au–T and Au–dT NPs and their relative histograms. Dynamic light scattering (DLS) results (Table 2, part VII of the ESI† ) are in accordance with the mean diameter values obtained by TEM, and are indicative of good particle distribution.
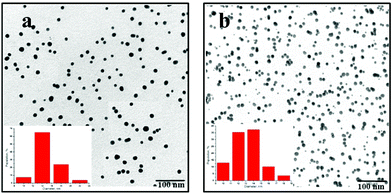 | ||
| Fig. 1 TEM micrographs and size distribution histograms of a) Au–thymidine (d = 13 nm) and b) Au–thymine (d = 11 nm). | ||
Concerning Au-5′-TMP NPs, we observed two different populations with 12 and 83 nm mean diameters, indicative of the presence of small aggregates which, however, do not propagate the aggregation.
Even though molecules (1)–(3) do not possess a thiol moiety, they are able to bond covalently to gold,9 AuNP sols remained stable for a long time after preparation (from some days to some weeks) and could be steadily dispersed under biophysical salted conditions. In order to estimate the colloidal stability of the Au–(1–3) nanoconjugates, ζ-potential measurements were carried out. It is well known that the particle charge is of great importance in the cellular uptake studies.10 It was discovered that the electrical charge of the NPs plays a key role in determining cytotoxicity: cationic AuNPs display a moderate toxicity, while their anionic counterparts exhibit no toxic (or very low) effects.11 The ζ-potential values were found to exceed −40 mV, confirming an excellent colloidal stability in those conditions (Table 2, part VII, ESI†). The ζ-potential values of the AuNPs followed the order Au–T < Au–dT < Au-5′-TMP, increasing with the AuNPs hydrodynamic diameter increase. The rise in the ζ-potential value can be associated with the formation of small nanoparticle arrangements, causing a light scattering increase and a mobility bias by the largest particles in the poly-dispersed sample.12 At the same time, the more negative values of the ζ-potential for Au-5′-TMP NPs are due to the presence of negatively charged phosphate moieties on AuNP surface.13 Nevertheless, it is well known that the interpretation of the ζ-potential values of “uncapped” particles, with sizes in the 5–100 nm range, represents a delicate challenge. Therefore, the ligand-coated NPs provide further complexity, because the hydrodynamic size and surface-charge shielding depend on the ligand conformation.14
To better characterise the ligand shell of AuNPs, 1H, 31P NMR and ATR-FTIR techniques were applied. The ATR-FTIR spectra of free ligands (1–3) show sharp characteristic bands of NH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and phosphate groups, which, after interaction with Au, change both in intensity and shifting, as already observed in amine and peptide capped gold nanoparticles (part VIII, IX, ESI†).15 In Au–thymine, Au–(1), major changes due to increased wavenumbers of both the N–H stretching and bending vibrations, and minor variations of the C
O and phosphate groups, which, after interaction with Au, change both in intensity and shifting, as already observed in amine and peptide capped gold nanoparticles (part VIII, IX, ESI†).15 In Au–thymine, Au–(1), major changes due to increased wavenumbers of both the N–H stretching and bending vibrations, and minor variations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching modes were observed (Table 3, Fig. 13, ESI†). In Au–thymidine, Au–(2), the N-H stretching band undergoes a large downshift, involving C
O stretching modes were observed (Table 3, Fig. 13, ESI†). In Au–thymidine, Au–(2), the N-H stretching band undergoes a large downshift, involving C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O more than in Au-(1) (the band of CO stretching mode shortens in intensity and shifts of 20 cm−1). A sharp band appears at 778 cm−1, assigned to out of plane C–H deformation vibrations, characteristic of a ribose moiety and thymine ring (Table 4, Fig. 14, ESI†). In Au-thymidine 5′-monophosphate, Au–(3), a remarkable difference was observed in the position and intensity of the signal due to the NH bending vibration (that varies from 1447 (free) to 1390 cm−1 (bound to Au). The phosphate ion bands, which appear in the characteristic zone (1150–900 cm−1)16 for free ligands, completely disappear after interaction with gold, suggesting P–O vibrations in a plane parallel to the gold surface (Table 5, Fig. 15, ESI†). Moreover, it was proved by Kryachko, that thymine can create nonconventional N–H⋯Au hydrogen bonds, similar to weak conventional H–bonds. As a result, the N–H stretching mode undergoes a red shift and an intensity increase.17 Our data are perfectly in agreement with Kryachko's findings on Au clusters and thus, we suggest the presence of H–bonds between gold and the molecules (1–3) in our nanoconjugates. AuNPs are nonconventional proton acceptors and thymine (or thymine derivatives) act as proton donors.
O more than in Au-(1) (the band of CO stretching mode shortens in intensity and shifts of 20 cm−1). A sharp band appears at 778 cm−1, assigned to out of plane C–H deformation vibrations, characteristic of a ribose moiety and thymine ring (Table 4, Fig. 14, ESI†). In Au-thymidine 5′-monophosphate, Au–(3), a remarkable difference was observed in the position and intensity of the signal due to the NH bending vibration (that varies from 1447 (free) to 1390 cm−1 (bound to Au). The phosphate ion bands, which appear in the characteristic zone (1150–900 cm−1)16 for free ligands, completely disappear after interaction with gold, suggesting P–O vibrations in a plane parallel to the gold surface (Table 5, Fig. 15, ESI†). Moreover, it was proved by Kryachko, that thymine can create nonconventional N–H⋯Au hydrogen bonds, similar to weak conventional H–bonds. As a result, the N–H stretching mode undergoes a red shift and an intensity increase.17 Our data are perfectly in agreement with Kryachko's findings on Au clusters and thus, we suggest the presence of H–bonds between gold and the molecules (1–3) in our nanoconjugates. AuNPs are nonconventional proton acceptors and thymine (or thymine derivatives) act as proton donors.
Additional details were collected by 1H and 31P NMR spectroscopy, using lyophilised AuNPs sols re-dispersed in D2O. The NMR data of the compounds (1)–(3) and Au–(1–3) NPs are reported in the ESI† (IV, V). The C–H and CH3 chemical shifts of thymine and thymidine change slightly after interaction with gold (Fig. 2–4, ESI†). Only the H5' proton signals of the Au–(3) NPs changed significantly (from 3.89 to 4.14 ppm) because of their proximity to the phosphate group, which presumably interacts strongly with the gold core (Fig. 2, A, a and b). In fact, 31P NMR spectra of Au-5′-TMP NPs (Fig. 2, B, a and b) showed a large chemical shift of the P signal from 3.79 to 0.27 ppm, as reported by Loweth et al. in gold colloids.18
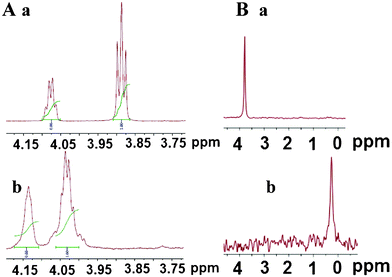 | ||
| Fig. 2 A) 1H NMR spectra of 4.25–3.75 ppm region of 5′-TMP (a) and Au-5′-TMP (b). B) 31P NMR spectra of 5′-TMP (a) and Au-5′-TMP (b). | ||
![Graphical behaviour of the mortality of U87 cells incubated with Au-(1–3) NPs, varying Au concentration [■ = AuT; ● = Au–dT; ▲ = Au-5′-TMP; ★= Au–Citrate]; an image of U87 cells treated with Au–T NPs (19 μM).](/image/article/2012/RA/c2ra20386c/c2ra20386c-f3.gif) | ||
| Fig. 3 Graphical behaviour of the mortality of U87 cells incubated with Au-(1–3) NPs, varying Au concentration [■ = AuT; ● = Au–dT; ▲ = Au-5′-TMP; ★= Au–Citrate]; an image of U87 cells treated with Au–T NPs (19 μM). | ||
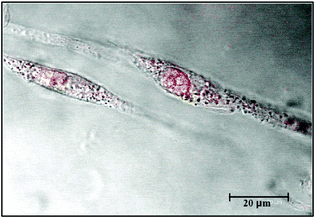 | ||
| Fig. 4 U87 cells uptake of Au-thymine NPs after 1h: image obtained by confocal microscopy. The image was red coloured for clarity. | ||
The cytotoxicity Au–(1–3) NPs was investigated for further cell uptake studies using human glioblastoma U87 cell line. Glioblastoma is the most common and aggressive malignant brain tumor in humans. The median survival for patients diagnosed with this disease is approximately 15 months, even after aggressive treatment.19 The in vitro cytotoxicity of all Au nanoconjugates was studied and compared to Au–citrate NPs in the 9.8–73 μM Au concentration range using trypan blue exclusion assay. The graph reported in Fig. 3 shows the increasing cytotoxicity of nanoconjugates. We found that all Au–(1–3) NPs are slightly toxic up to 9.8 μM. Then, the values rise up to 83% (in the case of Au–T NPs) when the concentration increases to 73 μM. The cytotoxicity values for Au–dT and Au-5′-TMP NPs present a maximum at 62% and 71%, respectively, and their subsequent decrease is likely due to the detachment of cells during the washing step. In Fig. 3, an image of U87 cells treated with Au–T NPs (19 μM) is reported, where dead cells stain blue, while live cells exclude trypan blue and are left unstained. This method cannot distinguish necrotic vs. apoptotic cells, but can evaluate the damage. The images of the cells treated with Au–T, Au–dT and Au-5′-TMP NPs are reported in Fig. 19, part XII, ESI.†
For cellular uptake tests, U87 cells were seeded to Petri dishes one day before the incubation experiments and 2 mM gold sols were added to the cell culture media and incubated for 1 h. The results were evaluated by confocal microscopy, observing both the cellular morphology and the uptake of Au–(1–3) nanoconjugates, after 1 h. (Fig. 16, 17, 18 in the ESI†).
Fig. 4 shows the presence of a good deal of Au-thymine NPs inside the cell and, in particular, highlights the particles inside the nucleus and in the nucleolus.
Also in the case of the Au–thymidine nanoconjugates, plenty of Au–dT NPs are visible inside the cell body, but the morphology of the cells appear damaged due to the presence of many membrane blebs (Fig. 17, ESI†). Finally, the Au-5′-TMP treated cells show morphology conservation and a large amount of nanoparticles distributed ubiquitously in the cellular compartments (e.g. cytoplasm and nucleus) (Fig. 18, ESI†).
In order to confirm the location of AuNPs inside the cell and demonstrate the presence of particles in the nucleus, we performed TEM microscopy analyses. Here we report in detail on the intracellular fate of AuNPs modified with 5`-TMP, as we observed a large amount of Au-(3) NPs in the cells. Fig. 5 and 6 give an idea of the AuNPs’ fate once in contact with U87 cells. We observe gold nanoparticles approaching and crossing cellular membranes, as well as being encapsulated into an endosome. However, the particles are not only entrapped in vesicles, we find them also free in the cytosol, crossing the nuclear pore complex and even in the nucleus (Fig. 6). This is due to their ability to overcome intracellular boundaries and can be related to the mechanism of endosomial escape.2
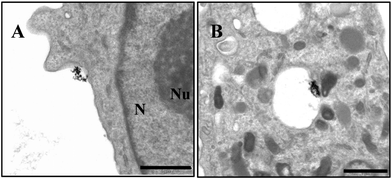 | ||
| Fig. 5 TEM micrographs of U87 cell uptake of Au-5′-TMP NPs after 1 h: A) AuNPs approaching cellular membrane; B) AuNPs enclosed in an endosome (Scale bar 1 μm); N = nucleus; Nu = nucleolus. | ||
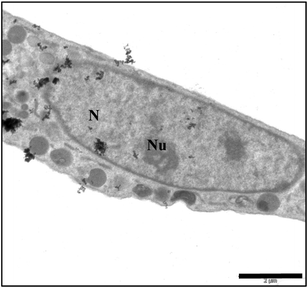 | ||
| Fig. 6 TEM micrographs of U87 cell uptake of Au-5′-TMP NPs after 1 h: AuNPs in various cell compartments (Scale bar 2 μM); N = nucleus; Nu = nucleolus. | ||
In conclusion, Au–T, Au–dT and Au-5′-TMP sols, prepared by using a simple reductive method, are stable and long living enough to be taken up by U87 cancer cells without degradation. The interesting aspect of these sols is represented by their highly negative value of ζ- potential, which confirms the kinetic stability. The thymine derivatives are simpler capping agents for gold NPs than the peptides or proteins used in many cell-targeting and cell-penetration experiments, but in spite of their simplicity show interesting abilities in the crossing the intracellular barriers. It is worth a mention that Au-5′-TMP NPs are able to penetrate even into the nucleus. These studies are in progress to investigate the fate of Au–T and Au–dT NPs inside the cells.
Acknowledgements
We gratefully acknowledge FONDAZIONE CARIPLO, Ricerca Scientifica e Tecnologica sui Materiali Avanzati , 2009, for financial support. Paolo Verderio, PhD student of The University of Milano “Bicocca”, for the help in z-potential measurements.References
- D. A. Giljohann, D. S. Seferos, W. L. Daniel, M. D. Massich, P. C. Patel and C. A. Mirkin, Angew. Chem. Int. Ed., 2010, 49, 3280–3294 CrossRef CAS.
- (a) Z. Krpetic, S. Saleemi, I. A. Prior, V. See, R. Qureshi and M. Brust, ACS Nano, 5, 5195–5201 CrossRef CAS; (b) R. L. Juliano, R. Alam, V. Dixit and H. M. Kang, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2009, 1, 324–335 CrossRef CAS; (c) M. Brust, Z. Krpetic, P. Nativo and F. Porta, Bioconjugate Chem., 2009, 20, 619–624 CrossRef.
- A. G. Tkachenko, H. Xie, Y. Liu, D. Coleman, J. Ryan, W. R. Glomm, M. K. Shipton, S. Franzen and D. L. Feldheim, Bioconjugate Chem., 2004, 15, 482–490 CrossRef CAS.
- D. T. Chiu and P. H. Duesberg, Genetica, 1995, 95, 103–109 CrossRef CAS.
- H. R. Maurer, Cell Proliferation, 1981, 14, 111–120 CrossRef CAS.
- B. P. Reichard, J. Biol. Chem., 1951, 188, 839–846 Search PubMed.
- F. J. Bollum and R. Van Potter, J. Biol. Chem., 1958, 233, 478–482 CAS.
- S. M. Weissman, R. M. S. Smellie and J. Paul, Biochim. Biophys. Acta, 1960, 45, 101–110 CrossRef CAS.
- P. D. Jadzinsky, G. Calero, C. J. Ackerson, D. A. Bushnell and R.D. Kornberg, Science, 2007, 318, 430–433 CrossRef CAS.
- Y. Zhang, M. Yang, J.-H. Park, J. Singelyn, H. Ma, M. J. Sailor, E. Ruoslahti, M. Ozkan and C. Ozkan, Small, 2009, 5, 1990–1996 CrossRef CAS.
- C. M. Goodman, C. D. McCuster, T. Yilmaz and V. M. Rotello, Bioconjugate Chem., 2004, 15, 897–900 CrossRef CAS.
- T. L. Doane, C. H. Chuang, R. J. Hill and C. Burda, Acc. Chem. Res. DOI:10.1021/ar200113c.
- W. Zhao, W. Chiuman, J. C. F. Lam, M. A. Brook and Y. Li, Chem. Commun., 2007, 3729–3731 RSC.
- A. V. Delgado, F. González-Caballero, R. J. Hunter and L. K. Koopal, J. Colloid Interface Sci., 2007, 309, 194–224 CrossRef CAS.
- (a) F. Porta, Z. Krpetic, L. Prati, A. Gaiassi and G. Scari, Langmuir, 2008, 24, 7061–7064 CrossRef CAS; (b) F. Porta, G. Speranza, Z. Krpetic, V. Dal Santo, P. Francescato and G. Scari, Mater. Sci. Eng., B, 2007, 140, 187–194 CrossRef CAS.
- (a) C. L. Angell, J. Chem. Soc., 1961, 504–515 RSC; (b) W. Haiss, B. Roelfs, S. N. Port, E. Bunge, H. Baumgartel and R. J. Nichols, J. Electroanal. Chem., 1998, 454, 107–113 CrossRef CAS.
- E. S. Kryachko and F. Remacle, Nano Lett., 2005, 5, 735–739 CrossRef CAS.
- (a) V. Mark, C. H. Dungan, M. M. Crutchfield and J. R. Van Walzer, Topics in Phosphorous Chemistry, Interscience Publishing, Monsanto Co., St Louis, 1967, vol. 5, ch. 4, pp. 319–323 Search PubMed; (b) C. J. Loweth, W. B. Caldwell, X. G. Peng, A. P. Alivisatos and P. G. Schultz, Angew. Chem., Int. Ed., 1999, 38, 1808–1812 CrossRef CAS.
- http://en.wikipedia.org/wiki/Glioblastoma_multiforme .
Footnote |
| † Electronic Supplementary Information (ESI) available: Detailed procedures and compound characterization. See DOI: 10.1039/c2ra20386c/ |
| This journal is © The Royal Society of Chemistry 2012 |
