Nano-layered manganese oxides as low-cost, easily synthesized, environmentally friendly and efficient catalysts for epoxidation of olefins†‡
Mojtaba
Amini
ab,
Mohammad Mahdi
Najafpour
*cd,
Sara
Nayeri
c,
Babak
Pashaei
c and
Mojtaba
Bagherzadeh
a
aChemistry Department, Sharif University of Technology, P.O. Box: 11155-3516, Tehran, Iran
bDepartment of Chemistry, Faculty of Science, University of Maragheh, Golshahr, P.O. Box: 55181-83111731, Maragheh, Iran
cDepartment of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, P.O.: Box: 45137-66731, Iran
dCenter of Climate Change and Global Warming, Institute for Advanced Studies in Basic Sciences (IASBS), Iran. E-mail: mmnajafpour@iasbs.ac.ir; Tel: (+98) 241 415 3201
First published on 22nd February 2012
Abstract
Incorporation of calcium(II), zinc(II) and aluminium(III) to manganese oxides greatly improved the activity of manganese oxide towards the epoxidation of olefins in the presence of anhydrous tert-butyl hydroperoxide as an oxidant.
Manganese is not only earth abundant and low cost but also environmentally friendly. Manganese oxides in particular are known to be useful, versatile, and environmentally friendly catalysts for important reactions, and they are attractive for use in many applications.1–5 Manganese oxides have been used extensively for the oxidation of a variety of molecules, especially water oxidation,2 the decomposition of nitrogen oxides,3 the oxidation of methane4 and ethylene,5 ethylbenzene,6 carbon monoxide7 and also the oxidation of a few organic compounds.8 Mn2O3/TiO2 heterogeneous mixed oxides have shown better photocatalytic activity than pure TiO2 for oxidation of different organic compounds.9
Properties of the manganese oxides, similar to other metal oxides, are influenced by the structure and morphologies, including crystallite sizes, orientations, stacking manners and aspect ratios, which are sensitive to the preparation methodology used in their synthesis.10 Recently, the synthesis of manganese oxide nanomaterials has attracted considerable attention.11
The epoxidation of olefins is an important catalytic reaction in the industry because of the epoxides' versatility in preparing many chemical intermediates.12,13 Many manganese complexes were reported for epoxidation of olefins using peracids and hydroperoxides as oxidants.13 However, few manganese oxides, as low-cost, easily synthesized and environmentally friendly compounds, have been used as a catalysts for epoxidation of olefins.14,15 Recently, we showed that the incorporation of calcium in the structure of manganese oxides greatly improves water oxidation activity of manganese oxides as layered materials.16 In this paper, we reported layered manganese oxides contain calcium(II), zinc(II) or aluminium(III) as a catalytic system for the epoxidation of various olefins by anhydrous tert-butyl hydroperoxide (TBHP) as an oxidant. These oxides can be easily synthesized from cheap, environmentally friendly and abundant starting materials without using any organic compound, and they are therefore suitable for potentially large-scale applications (ESI†).
In the IR spectrum of these oxides, a broad band at ∼3200–3500 cm−1 relating to antisymmetric and symmetric O–H stretching and at ∼1630 cm−1 relating to H–O–H bending are observed (Fig. S2, ESI†). These oxides contain small amounts of OH groups, indicated by IR spectroscopy. A sharp absorption bands characteristic for a MnO6 core in the 400–500 cm−1 region assigned to the stretching vibrations of Mn–O bonds in manganese oxide was also observed in the FTIR spectra of the brown solid. To characterize the morphology of the prepared oxides, oxides were studied by scanning electron microscopy (SEM) and Transmission Electron Microscopy (TEM). SEM and TEM images are shown in Fig. 1 and Fig. 2. These images indicate that the powders are composed of plate like crystals with a lateral dimension of ∼70 nm.
 | ||
| Fig. 1 SEM micrographs of layered zinc (top), aluminium (middle) and calcium (below) manganese oxides (scale bar 300 nm). | ||
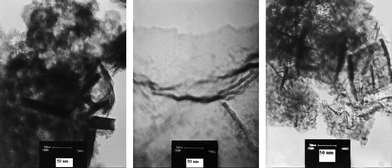 | ||
| Fig. 2 TEM micrographs of layered zinc (left), aluminium (middle) or calcium (right) manganese oxides (scale bar 50 nm). | ||
The XRD data for the compounds are of very poor resolution (Fig. 3). However, the peak near θ ∼19 (2.4 Å) which corresponds to the typical Mn–Mn observed in all octahedrally coordinated manganese oxide materials, and the peak near θ ∼5.5 (7.0 Å), found in most layered materials such as birnessite, are observed in the XRD patterns of this compound and are related to the previously reported structures of similar compounds (Fig. 3 and Fig. S1, ESI†).11,17
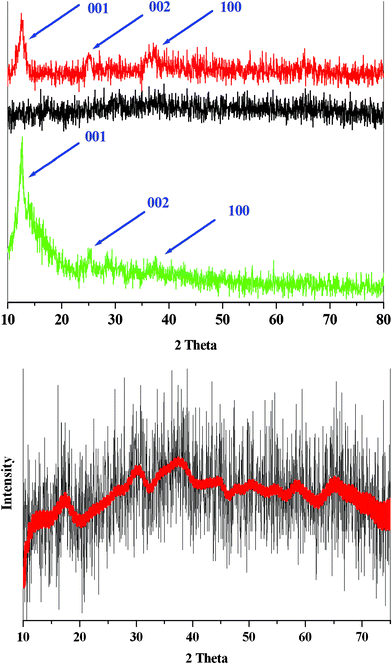 | ||
| Fig. 3 XRD data for the zinc (red), Ca (black) or aluminium (green) manganese oxide (Top). XRD patterns of the obtained of calcium-manganese oxide (black) and FFT Filter Smoothing of XRD patterns of the compound (red) (Bottom).18 | ||
Dynamic light scattering (DLS) showed the average size of the particles for zinc, aluminium or calcium–manganese oxide: ∼225, 215 and 127 nm in solution under sonicated conditions.
Recently, the structure of the layered calcium–manganese oxide was carefully studied19 by X-ray absorption near-edge structure (XANES) and extended X-Ray absorption fine structure (EXAFS). Those results show that CaMnIV1.6MnIII0.4O4.5(OH)0.5 is a better formula for the oxide, and the mean oxidation number for manganese is 3.8.19
In this structure, similarly to layered manganese oxides, neighboring manganese ions are extensively interconnected by two bridging oxygens. However, in this case, two different calcium-containing motifs were identified. One of them results in the formation of a Mn3CaO4 cube. Other calcium ions likely interconnect oxide-layer fragments (Scheme 1).19 Regarding SEM, IR and XRD results, similar structures are suggested for zinc and aluminium–manganese oxides.
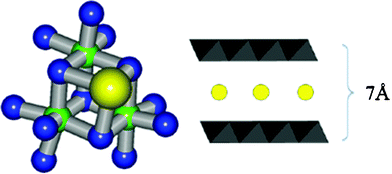 | ||
| Scheme 1 Motif of edge-sharing MnO6 octahedra (di-μ-oxido bridging) in the manganese–calcium oxides (manganese: green, oxygen: blue, calcium: yellow) (left). Schematic representations of layered manganese oxides and calcium ions (yellow circle) between these layers (right). | ||
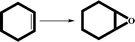
These compounds show efficient activity towards the epoxidation of olefins in the presence of anhydrous TBHP as an oxidant. The catalytic performances of CaMn2O4·H2O (1), Zn0.15MnO2·H2O (2) and Al0.1MnO2·H2O (3) were studied in the epoxidation of five olefins, styrene, cyclohexene, cyclooctene, indene and 1-hexene as substrate, and TBHP as the oxygen donor. In a series of experiments, we evaluated the influence of several factors. Table 1 shows different reaction conditions and conversion results of the catalyst. Conversion percent was found to be dependent on the amount of catalyst and oxidant. According to the results in Table 1, changing the catalyst amount from 0.01 mmol to 0.02 mmol and 0.05 mmol reflect an increase of the conversion percent and changing the amount of TBHP from 0.1 mmol to 0.2 mmol has a positive effect on the catalytic activity. Generally, excellent epoxide yields and full selectivity were observed for all substrates in a short time and rather low temperature. The efficiency of catalysts could be related to the open structure and small particle size of these oxides.14 Compared with nanocrystalline Mn3O4 catalyst that shows similar activity in epoxidation of olefins, these catalysts could be prepared more easily without using any ultrasound power, organic solvent or organic compounds. The layered and the open structure of these compounds could be used to replace other cations to synthesize varied catalysts.15
| Entry | Epoxidation reactiona | Catalyst loading (mmol) | Conversion (%)b | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
a The reactions were performed in (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture of CH3OH/CH2Cl2 (1.2 mL) under reflux (at 70 °C) for a period of 2 h.
b The GC conversions (%) are measured relative to the starting substrate.
c The amount of TBHP is 0.1 mmol. 1) mixture of CH3OH/CH2Cl2 (1.2 mL) under reflux (at 70 °C) for a period of 2 h.
b The GC conversions (%) are measured relative to the starting substrate.
c The amount of TBHP is 0.1 mmol.
|
|||||
| 1 |
|
0.01 | 53(25)c | ||
| 0.02 | 66(31) | ||||
| 0.05 | 88(48) | 57 | 31 | ||
| 2 |
|
0.01 | 41(24) | ||
| 0.02 | 59(31) | ||||
| 0.05 | 83(45) | 60 | 29 | ||
| 3 |
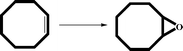
|
0.01 | 37(19) | ||
| 0.02 | 58(25) | ||||
| 0.05 | 79(38) | 55 | 27 | ||
| 4 |

|
0.01 | 55(25) | ||
| 0.02 | 69(41) | ||||
| 0.05 | 93(66) | 78 | 33 | ||
| 5 |

|
0.01 | 38(13) | ||
| 0.02 | 51(27) | ||||
| 0.05 | 68(39) | 49 | 11 | ||
Similar experiments with bulk MnO2, Mn2O3 and Mn3O4 show trace activity towards epoxidation of these olefins with anhydrous TBHP as the oxidant.
The recovery and recyclability of CaMn2O4·H2O have been also examined. The catalyst was tested for reactivity in the oxidation of styrene, five recycling steps were needed and a weak progressive decrease of conversion was noted (from 88% to 71%). However, the yield markedly decreased to 49% after the eighth run. The mechanism of epoxidation of olefins is not known but Lewis-acid-catalyzed activation of the terminal oxygen of TBHP proposed by Adam et al. and Collman et al. is suggested as a proposal mechanism (Scheme 2).20 It could be suggested that calcium, aluminium or zinc has no specific role in epoxidation, and we conclude that manganese ions are the only species involved directly in epoxidation of olefins. Compared with other manganese oxides, the efficiency of the layered manganese oxides towards the epoxidation of olefins could be related to the open structure and small particle size of these oxides because of the incorporation of calcium, aluminum and zinc in the structure.
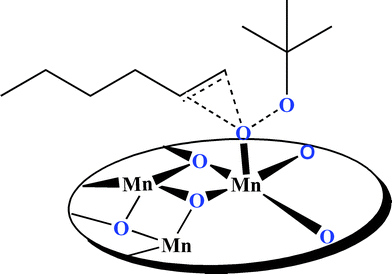 | ||
| Scheme 2 Proposal mechanism for epoxidation of olefins by manganese oxides. | ||
The authors are grateful to Institute for Advanced Studies in Basic Sciences and Sharif University of Technology for funding.
References
- (a) Z. Tian, W. Tong, J. Wang, N. Duan, V. V. Krishnan and S. L. Suib, Science, 1997, 276, 926 CrossRef CAS; (b) H. Cao and S. L. Suib, J. Am. Chem. Soc., 1994, 116, 5334 CrossRef CAS.
- (a) A. Harriman, I. J. Pickering, J. M. Thomas and P. A. Christensen, J. Chem. Soc., Faraday Trans. 1, 1988, 84, 2795 RSC; (b) V. Y. Shafirovich and A. E. Shilov, Kinetika i Kataliz, 1979, 20, 1156 CAS.
- T. Yamashita and A. Vannice, Appl. Catal., B, 1997, 13, 141 CrossRef CAS.
- R. B. Anderson, K. C. Stein, J. J. Feenan and L. J. E. Hofer, Ind. Eng. Chem., 1961, 53, 809 CrossRef CAS.
- B. Dmuchovsky, M. C. Freerks and F. B. Zienty, J. Catal., 1965, 4, 577 CrossRef CAS.
- G. S. Kumar, M. Palanichamy, M. Hartmann and V. Murugesan, Catal. Commun., 2007, 8, 493 CrossRef CAS.
- S. A. C. Carabineiro, S. S. T. Bastos, J. J. M. Órfão, M. F. R. Pereira, J. J. Delgado and J. L. Figueiredo, Catal. Lett., 2010, 134, 217 CrossRef CAS.
- (a) F. G. Duran, B. P. Barbero, L. E. Cadus, C. Rojas, M. A. Centeno and J. A. Odriozola, Appl. Catal., B, 2009, 92, 194 CrossRef CAS; (b) V. V. Krishnan and S. L. Suib, J. Catal., 1999, 184, 305 CrossRef CAS; (c) H. Zhou, J. Y. Wang, X. Chen, C. L. O'Young and S. L. Suib, Microporous Mesoporous Mater., 1998, 21, 315 CrossRef CAS; (d) X. Chen, Y. F. Shen, S. L. Suib and C. L. O'Young, J. Catal., 2001, 197, 292 CrossRef CAS; (e) J. Y. Wang, G. G. Xia, Y. G. Yin and S. L. Suib, Chem. Ind., 1998, 75, 621 CAS; (f) J. Y. Wang, G. G. Xia, Y. G. Yin, S. L. Suib and C. L. O'Young, J. Catal., 1998, 176, 275 CrossRef CAS; (g) Y. C. Son, V. D. Makwana, A. R. Howell and S. L. Suib, Angew. Chem., Int. Ed., 2002, 40(22), 4280 CrossRef; (h) R. Ghosh, Y. C. Son, V. D. Makwana and S. L. Suib, J. Catal., 2004, 224, 288 CrossRef CAS.
- M. M. Mohamed, I. Othman and R. M. Mohamed, J. Photochem. Photobiol., A, 2007, 191, 153 CrossRef CAS.
- J. Jiang and L. Li, Mater. Lett., 2007, 61, 4894 CrossRef CAS.
- (a) M. M. Najafpour, S. Nayeri and B. Pashaei, Dalton Trans., 2011, 40, 9374 RSC; (b) D. M. Robinson, Y. B. Go, M. Greenblatt and G. C. Dismukes, J. Am. Chem. Soc., 2010, 132, 11467 CrossRef CAS; (c) M. M. Najafpour, Dalton Trans., 2011, 40, 3805 RSC; (d) V. B. Ram Boppana and F. Jiao, Chem. Commun., 2011, 47, 8973 RSC; (e) F. Jiao and H. Frei, Chem. Commun., 2010, 46, 2920–2922 RSC; (f) Y. Zhao, C. Li, F. Li, Z. Shi and S. Feng, Dalton Trans., 2011, 40, 583 RSC; (g) Y. Gorlin and T. F. Jaramillo, J. Am. Chem. Soc., 2010, 132, 13612 CrossRef CAS; (h) F. Jiao and H. Frei, Angew. Chem., Int. Ed., 2009, 48, 1841 CrossRef CAS; (i) F. Jiao and H. Frei, Energy Environ. Sci., 2010, 3, 1018 RSC.
- S. T. Oyama, Mechanisms in Homogeneous and Heterogeneous Epoxidation Catalysis, ELSEVIER, 2008 Search PubMed.
- (a) L. Liu, H. Li, L. Wan, Z. Ren, H. Wang and J. Lang, Chem. Commun., 2011, 47, 11146 RSC; (b) L. Mahmoudi, D. Mohajer, R. Kissner and W. H. Koppenol, Dalton Trans., 2011, 40, 8695 RSC; (c) M. Wu, B. Wang, S. Wang, C. Xia and W. Sun, Org. Lett., 2010, 12(8), 1892 CrossRef CAS; (d) H. R. Khavasi, K. Sasan, M. Pirouzmand and S. Nejad Ebrahim, Inorg. Chem., 2009, 48(13), 5593 CrossRef CAS.
- (a) R. Ghosh, X. Shen, J. C. Villegas, Y. Ding, K. Malinger and S. L. Suib, J. Phys. Chem. B, 2006, 110(14), 7592 CrossRef CAS; (b) R. Ghosh, Y. C. Son, V. D. Makwana and S. L. Suib, J. Catal., 2004, 224, 288 CrossRef CAS; (c) L. Espinal, S. L. Suib and J. F. Rusling, J. Am. Chem. Soc., 2004, 126, 7676 CrossRef CAS.
- A. Askarinejad, M. Bagherzadeh and A. Morsali, Appl. Surf. Sci., 2010, 256, 6678 CrossRef CAS.
- (a) M. M. Najafpour, T. Ehrenberg, M. Wiechen and P. Kurz, Angew. Chem., Int. Ed., 2010, 49, 2233 CrossRef CAS; (b) M. M. Najafpour, J. Photochem. Photobiol., B, 2011, 104, 111 CrossRef CAS; (c) M. M. Najafpour, Origins Life Evol. Biosphere, 2011, 41, 237 CrossRef CAS; (d) M. M. Najafpour, Dalton Trans., 2011, 40, 3793 RSC; (e) D. Shevela, S. Koroidov, M. M. Najafpour, J. Messinger and P. Kurz, Chem.–Eur. J., 2011, 17, 5415 CrossRef CAS; (f) M. M. Najafpour, Chem. Commun., 2011, 47, 11724 RSC; (g) M. M. Najafpour and S. I. Allakhverdiev, Int. J. Hydrogen Energy, 2012, 02, 075, DOI:10.1016/j.ijhydene; (h) M. M. Najafpour, S. Nayeri and B. Pashaei, Dalton Trans. 10.1039/C2DT12189A; (i) M. M. Najafpour, S. Nayeri and B. Pashaei, Dalton Trans. 10.1039/C2DT12189A.
- H. Caot and S. L. Suib, J. Am. Chem. Soc., 1994, 116, 5334 CrossRef.
- FFT Filter Smoothing is a signal processing technique typically used to remove noise from signals. For more information see: http://www.originlab.com/www/helponline/Origin/en/Category/Smoothing.html.
- I. Zaharieva, M. M. Najafpour, M. Wiechen, M. Haumann, P. Kurz and H. Dau, Energy Environ. Sci., 2011, 4, 2400 CAS.
- For a review see: E. M. McGarrigle and D. G. Gilheany, Chem. Rev., 2005, 105(5), 1563 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20297b/ |
| ‡ The third and fourth authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2012 |
