Structural characterization, solution stability, and potential health and environmental effects of the Nano-TiO2 bioencapsulation matrix and the model product of its biodegradation TiBALDH†
Nicole
Groenke
ab,
Gulaim A.
Seisenbaeva
a,
Vitaliy
Kaminskyy
c,
Boris
Zhivotovsky
c,
Benedikt
Kost
b and
Vadim G.
Kessler
*a
aDepartment of Chemistry, SLU BioCenter, Box 7015, 75007 Uppsala, Sweden. E-mail: vadim.kessler@slu.se
bDepartment of Plant Biology and Forest Pathology, SLU BioCenter, Box 7082, 75007 Uppsala, Sweden
cDepartment of Toxicology, Institute of Environmental Medicine, Karolinska Institutet, Box 210, 17177 Stockholm, Sweden
First published on 27th March 2012
Abstract
Dispersions of small TiO2 nanoparticles in alcohol, stabilized by surface complexation with protonated triethanolamine ligands, are proposed as bio-encapsulation matrices. They were characterized by TEM and EXAFS spectroscopy, and DLS was used to investigate the nanoparticles stability over time in their pure form and after insertion into aqueous medium. This study reveals unusual structural features that explain the recently demonstrated facile formation of dense encapsulates on the surface of biological objects. In the view of the potentially broad application of this already industrially available material in agriculture as an encapsulation matrix for biocontrol organisms, its potential health and environmental effects were characterized by employing a number of model systems. The potential health effects of the produced stable aqueous dispersions were studied in vitro using A549 and U1810 lung carcinoma cell lines. The nanotitania in the environment is partly bio-digested with the formation of citrate and lactate complexes. The effects on the growth of tobacco pollen grains by the nanotitania and of the ammonium lactato-oxo-titanate (TiBALDH, a product already broadly used in biomineralization studies) were investigated to gain insight into the impact of these materials on the environment and specifically on plant reproduction. TiBALDH was used as a model product of the bio-digestion and its structure has been probed in this work by X-ray powder diffraction and EXAFS spectroscopy. No acute negative bio-effects could be observed for the studied materials at significantly high concentrations, such as 50 μg ml−1 for the viability of human lung cancer cells and up to about 120 μg ml−1 for the growth of pollen grains, corresponding to the conditions of proposed field applications. This observation was contrasted by the apparently high toxicity of the LaAlO3 based nanophosphor which was applied as a positive control.
1. Introduction
Recently, increased interest in versatile and cheap nanostructured matrices for bio-encapsulation and drug delivery has urged the development and investigation of biocompatible inorganic nanomaterials. Some examples of materials proposed for these applications are calcium carbonate1 and calcium phosphate,2 silica,3,4 and, more recently, titanium dioxide.5,6 These materials are highly attractive, particularly due to their high versatility and ability to display sensitivity to the chemical conditions in surrounding media, which is applicable for regulated release of the encapsulated content. Examples of such behaviour are the facile dissolution of CaCO3 at decreased pH1 and the solubilisation (bio-digestion) of nanosized TiO2 when the concentration of chelating carboxylate ligands such as citrate or lactate is increased (as occurs in biological media subject to oxidative stress).5a,6 It is important to note that this bio-digestion can apparently be reversed by the action of enzymes, producing a new titania shell via biomineralization. This was recently demonstrated using an ammonium titanium lactate complex (TiBALDH) as the starting material.5bA number of studies have demonstrated that titanium dioxide is non-toxic for many species of microorganisms5 with limited effect on the biochemical balance in soil associated with removal of phosphorus and even of nitrogen.7a In fact, several types of microorganisms have skeletons that consist mostly of titania, while magnetic iron titanate, FeTiO3 ilmenite, is used within the organs of orientation in a variety of species.7b An attractive titania-based nanomaterial for environmental applications is CaptiGel, a solution of uniform TiO2 nanoparticles (4 ± 1 nm) stabilized by electrostatic charges from surface capping with triethanol-ammonium cations.8 It was demonstrated to be able to preserve biomaterials and microorganisms through the formation of dense coatings via aggregation of nanoparticles. The titania particles coalesce, producing dense membranes on the surface of living cells. It was originally supposed that the driving force in this process was the electrostatic interaction between the positive charges on the surface of the modified titania particles and the effective negative charges of phosphate head groups in the membrane lipids.5a It is important to get a better insight into its structure and trace the potential transformation of this material in aqueous medium, especially in view of the recent data that positively charged nanoparticles can damage cell membranes.9 We sought also to trace longer term effects that can be caused by this type of material on broader scale applications. We focus on two types of possible impact, the first on human health through exposure via the respiratory tract, and the second on plant reproduction by influencing viability and growth of the pollen grains. It also appeared important to identify the chemical nature of the bio-digestion products of nano-titania and evaluate their own potential environmental impact.
2. Experimental
The titania colloids described in the literature as nano-TiO2 stabilized with surface-capping protonated tea-ligands5 were received from CaptiGel AB, Sweden, as an ethanol solution with a TiO2 concentration of 120 mg ml−1 and was diluted for further applications by either anhydrous ethanol or LiChrosolv water (Merck, pH = 7.00). The titanium(IV) lactate complex solution, with the commercial name TiBALDH, was at 50 wt% concentration in relation to the formula C6H18N2O8Ti and was diluted by water for further tests. LaAlO3 and Tb-doped LaAlO3 nanoparticles were produced from alkoxide precursors using the Bradley reaction with acetophenone as the solvent as described in ref. 10.The solutions were measured on a Malvern Zetasizer DLS to obtain information about size distribution and surface charge of the particles. Polymer electrolyte titrations were carried out at the Polymer Chemistry Department of the Royal Institute of Technology (KTH) in Stockholm. Electrospray mass-spectrometry measurements were carried out with a Bruker Esquire-LC.
The solutions were dried in air for 2 weeks under a hood and the produced solids were investigated with an optical MOTIC digital microscope and a TM-1000-μ-DeX SEM-EDS. TEM images were obtained with a TOPCON EM002B operating at 200 kV (at the Laboratory of Multimaterials and Interfaces, CNRS UMR 5615, University Claude Bernard Lyon-1). The samples were analyzed by X-ray powder diffraction using a Bruker SMART Apex-II diffractometer (sealed-tube Mo-Kα radiation, λ = 0.71073 Å) as-obtained and after thermal treatment at 500 °C in 1 h. Rietveld refinement of powder data was carried out using an EXPO 2009 program package.11 The thermal decomposition of the as-obtained dried samples was followed by TG-FTIR analysis using a Perkin-Elmer Pyris-1 Spectrum 100 combined installation, using a heating rate of 10 °C min−1 up to a final temperature of 600 °C. The phase composition of the heat-treated samples was established using a Bruker EVA program package.
EXAFS-Data collection. Titanium K edge X-ray absorption spectra were recorded at the wiggler beam line I811 at the MAXLab, Lund University, Sweden. The EXAFS station was equipped with a Si [111] double crystal monochromator. The data collection was performed in luminescence mode. Higher order harmonics were reduced by detuning the second monochromator to 30% of the maximum intensity at the end of the scans. The energy scale of the X-ray absorption spectra were calibrated by assigning the first inflection point of the K edge of a titanium foil to 4966 eV.12 The IFEFFIT program package13 was used for the data treatment.
Cell culture and treatments
Human non-small cell lung carcinoma (NSCLC) cell lines (A549 and U1810) were cultured in RPMI 1640 with 10% fetal calf serum (FCS), 2 mM glutamine, penicillin (100 μg ml−1) and streptomycin (100 μg ml−1). Cells were seeded in 6 well plates twenty four hours prior to treatment with titanium oxide (5–100 μg μl−1), doxorubicin (0.5 μg μl−1) or their combination. They were counted after 120 h using Beckman cell counter, according with manufacturers instruction.Immunoblotting
Cells were lysed in RIPA buffer (25 mM Tris·HCl pH 8, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS), supplemented with a protease inhibitor cocktail (Complete-M, Roche). The protein concentration was determined using a BCA protein assay (Pierce). The samples were mixed with Laemmli's buffer, boiled for 5 min, subjected to SDS-PAGE and blotted onto nitrocellulose membranes. The membranes were blocked for 1 h with 5% non-fat milk in PBS and probed with primary antibodies diluted in PBS containing 2% BSA and 0.05% Tween-20. The following antibodies were used: mouse anti-cleaved PARP (Cell Signaling Technology), p53 (Santa Cruz), rabbit anti-actin (Sigma-Aldrich). The recognized proteins were detected using horseradish peroxidase-labelled secondary antibodies: anti-mouse IgG or anti-rabbit IgG (all from Pierce), and an enhanced chemiluminescence kit (Western blot detection reagent, GE Healthcare UK Limited).MTS assay
Cells were cultured at a density of 2 × 103 cells per well in 96-well plates and some CellTiter 96® AQueos One Solution Reagent (Promega, Madison, WI) was added to each well according to the manufacturer's instructions. After 48 of treatment with titanium nanoparticles the cell viability was determined by measuring the absorbance at 490 nm using a Labsystems Multiskan MCC/340 plate reader.Plant culture and treatments
For the preparation of the growth medium 3.2 ml of 0.5 M H3BO3, 1 ml of 1 M CaCl2·2H2O, 2 ml of 0.5 M Ca(NO3)2·4H2O, 2 ml of 0.5 M MgSO4·7H2O and 100 g sucrose were added to a flask with 500 ml water. The pH value was subsequently adjusted to 6.5 with 2 M potassium hydroxide. Afterwards the growth medium was filtered to make it sterile and stored at 4 °C.Mature stamens covered with pollen were collected from flowering N. tabacum plants, which were kept in growth chambers under standard conditions (16 h illumination per day at 23 °C; night temperature: 20 °C). The stamens were transferred into a growth medium and stirred to release pollen. The pollen suspension was filtered through a grid to remove anthers and mixed with different concentrations of CaptiGel or TiBALDH to test the toxicity. 400 μl of the pollen suspension with different particles concentrations were added to a plate with 24 wells. The cells were kept at room temperature in the dark for 2.5 h before analysis. Then the pollen tubes were imaged using an epifluorescence microscope. Microscope pictures were taken with 2.5× magnification and afterwards the length of the pollen tubes was measured. To look more carefully at the pollen some pictures were also taken at 10× magnification. For the TiBALDH-containing samples it was also possible to count the number of germinated pollen after 1.5 h. Addition of CaptiGel produced in the growth medium a non-transparent gel, so the following treatment was applied to produce samples permitting observation: 1 ml of TiO2 solution was added to 1 ml CaCl2 (1M) and 8 ml water in a tube and mixed. A TiO2 gel was formed, giving a white suspension. The evolved clumps were centrifuged for 15 min (1800 rpm) and the supernatant, which was a transparent solution, was poured out. The precipitate was washed three times with 10 ml water and ground mechanically with a spatula. After pouring out the supernatant the precipitate was mixed with 10 ml water and subjected to ultrasound treatment to get a suspension of TiO2 particles for observation under the optical microscope.
The introduction of LaAlO3 nanoparticles was made by shooting a portion of the suspension onto a filter with collected pollen grains with subsequent desorption and dilution until the desired concentration was achieved (see ESI†).
3. Results and discussion
Characterization of applied inorganic materials
According to DLS measurements, the initial ethanol solutions of nano-TiO2 stabilized by surface complexation with protonated triethanolamine (CaptiGel) contain uniform 4 ± 1 nm particles. The colour of the solution changes on storage from light yellow to orange, but no change in the particle size was observed even after 24 months of storage. Immersion of CaptiGel into a 10-fold excess of water produced clear solutions that were stable in a closed vessel for at least 2 weeks without the appearance of either opalescence or precipitation. DLS measurements for these aqueous solutions were carried out in triplicate with high reproducibility and showed the average particle size was about 60 nm (complex particle size distribution, see Table TS1 in the ESI† with major peaks at 140, 15 and 5 nm). The size decreased to about 24 nm after 15 min of ultrasound treatment through redistribution between the fractions in favour of the 5 nm sized fraction, which corresponded to single non-aggregated particles. Only a marginal decrease in size was observed after 48 h of storage. The particles in the aqueous medium are negatively charged with an average ζ potential of −11.1 mV, indicating complete loss of the tri-ethanol-ammonium ligand (clearly detectable by ESI-MS in the produced medium) and re-charging of the originally positively charged particles, see ref. 5. Titration with poly-dimethylammonium chloride gives a charge value of 0.006 equivalents per gram in relation to TiO2. This confirms the negative surface charge of the particles, which is important for decreasing the threat of membrane damage for encapsulated biomaterials commonly caused by positively charged particles.9The stability of the suspensions produced by immersion of CaptiGel into a 10-fold excess of isotonic NaCl solution is much lower: considerable opalescence appears after 2 h and complete gelation of the whole solution occurs within 24 h. Gelation in the presence of a CaCl2 solution occurs within 15 min. The reason for this behaviour is apparently charge compensation through adsorption of cations.
According to TEM observations (see Fig. 1) the non-heat-treated gels consist of strongly aggregated TiO2 particles with a size of about 5 nm. The material is amorphous under X-rays, but the distance between fringes in the high-resolution TEM and the selected area diffraction patterns (see ref. 5 for the latter) indicate that the particles have an anatase structure in the core, but are covered with an amorphous shell. The small size of the structurally coherent region precludes obtainment of the X-ray diffraction pattern. The air-dried gel contains high amounts of organic stabilizer, tri-ethanolamine and tri-ethanol ammonium salt, and residual water (weight loss of 55% upon heat treatment, see Fig. FS1†). Heat treatment at 500 °C provides a mixture of anatase and rutile phases (Fig. FS2†) indicating typical behavior for strongly aggregated small anatase particles that facilitates phase transition into rutile.14 When the gels were prepared using isotonic NaCl instead of water, the resulting xerogels contained relatively large, 30–50 nm, single crystals of NaCl. Hindered nucleation of salts in inorganic gels, resulting in the formation of single crystals was reported earlier for the drying of silica gel droplets.15
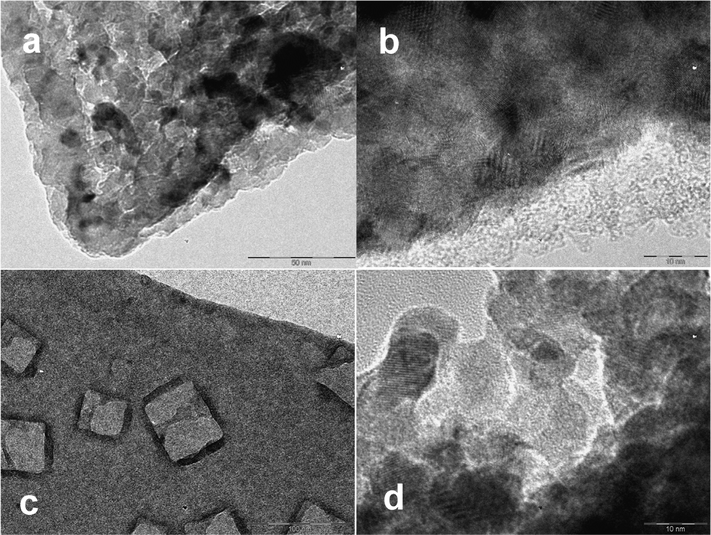 | ||
| Fig. 1 Air-dried gel produced from CaptiGel diluted by water (a, b) and via gelation in isotonic NaCl solution (c, d). The cubic NaCl crystals are visible in C as phase-separated inclusions. | ||
The EXAFS study of the CaptiGel xerogel reveals a striking difference between this material, bulk, and well-crystallized nano-sized anatase16 (see Fig. FS3†). The spectrum of anatase can be distinguished as a weaker contributing component. The strongest signals, however, indicate a shortened Ti–O distance (testifying decreased average coordination number) and also drastically weakened signals in the area corresponding to Ti–Ti interactions, which are apparently quenched by the truly amorphous structure. It is probably the latter that is behind the ability of CaptiGel particles to coalesce and form dense TiO2-shells on the surface of biological objects.5 The ability to adhere to the surface of biomembranes may also be explained by the enhanced strength of surface complexation of phosphate entities in phospholipids with the amorphous titania.6,17
It was previously reported that nano-titania is bio-digestible and dissolves in the presence of chelating carboxylate ligands such as citrate or lactate. The reaction in the presence of an excess of such ligands should lead to tris-carboxylato-titanate species that are reported and well structurally characterized in literature for environmentally relevant conditions.18 However, application of the CaptiGel material is proposed for field conditions with relatively low concentrations of lactic acid, e.g. in soil, and should lead to a product with lower Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactate ratio. Such a compound is commercially available under the trivial name TiBALDH, which stands for titanium bis-ammonium lactato-dihydroxide. It appeared logical to use this product as a model for investigating the potential environmental effects of bio-digested nano-titania. We felt that it needed further chemical characterization since a tentative chemical formula from following from its common name was apparently erroneous. In spite of the relatively broad application of this product in the synthesis of titanium dioxide19 and even drawings of it's “structure” in quite serious journals20 the latter has never before been investigated. Moreover, it is well-known that titanium cations do not form stable hydroxide species in aqueous media and not a single reliably defined structure of titanium(IV) species with two hydroxide groups attached to one and the same Ti atom can be found in the Cambridge Structural Database.
lactate ratio. Such a compound is commercially available under the trivial name TiBALDH, which stands for titanium bis-ammonium lactato-dihydroxide. It appeared logical to use this product as a model for investigating the potential environmental effects of bio-digested nano-titania. We felt that it needed further chemical characterization since a tentative chemical formula from following from its common name was apparently erroneous. In spite of the relatively broad application of this product in the synthesis of titanium dioxide19 and even drawings of it's “structure” in quite serious journals20 the latter has never before been investigated. Moreover, it is well-known that titanium cations do not form stable hydroxide species in aqueous media and not a single reliably defined structure of titanium(IV) species with two hydroxide groups attached to one and the same Ti atom can be found in the Cambridge Structural Database.
A DLS study of TiBALDH indicated the presence of well-defined nanoparticles with a size of about 10 nm. Slow drying the 50 wt% solution in air provided a material that looked like a combination of a polycrystalline material and an opalescent gel/glassy solid under the microscope (Fig. 2a). The TGA study indicated weight loss of 71 wt% (Fig. FS4†), which reasonably correlated with the proposed C6H18N2O8Ti formula. The Rietveld refinement of the obtained X-ray powder data (Fig. 2b) is in agreement with a monoclinic unit cell a = 11.8685(2), b = 13.2396(2), c = 19.9578(2) Å, β = 107.718(3)°, Space group P21/c. These data closely resemble the unit cell parameters and symmetry settings for the ammonium titanyl oxalate, (NH4)8[Ti4O4(C2O4)8](H2O)4 (a = 13.473(2), b = 11.329(1), c = 17.646(2) Å, β = 126.66(1)°, Space group P21/n), which also has an analogous chemical composition.21 The phase-corrected Fourier-transformed EXAFS spectrum of dried crystalline TiBALDH reveals relatively strong peaks in the range corresponding to the Ti–Ti distance (about 3.5 Å), confirming the oligo-nuclear nature of this species (Fig. 2c). With the view that in principle all other known titanium carboxylates with a Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratio lower than 1
L ratio lower than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 are oxo-species with bridging oxygen atoms,22 this provides a very strong indication that TiBALDH has to be formulated as ammonium titanyl lactate, i.e. ammonium oxo-lactato-titanate, (NH4)8[Ti4O4(C3H5O3)8](H2O)4 (Fig. 2d). The commercial samples apparently contain a minor admixture of titania nanoparticle solutions stabilized by adsorbed lactate ligands. Thermal treatment of TiBALDH results in a pure anatase phase of TiO2 (see Fig. FS5†), which is consistent with random nucleation in decomposition of a molecular precursor.
3 are oxo-species with bridging oxygen atoms,22 this provides a very strong indication that TiBALDH has to be formulated as ammonium titanyl lactate, i.e. ammonium oxo-lactato-titanate, (NH4)8[Ti4O4(C3H5O3)8](H2O)4 (Fig. 2d). The commercial samples apparently contain a minor admixture of titania nanoparticle solutions stabilized by adsorbed lactate ligands. Thermal treatment of TiBALDH results in a pure anatase phase of TiO2 (see Fig. FS5†), which is consistent with random nucleation in decomposition of a molecular precursor.
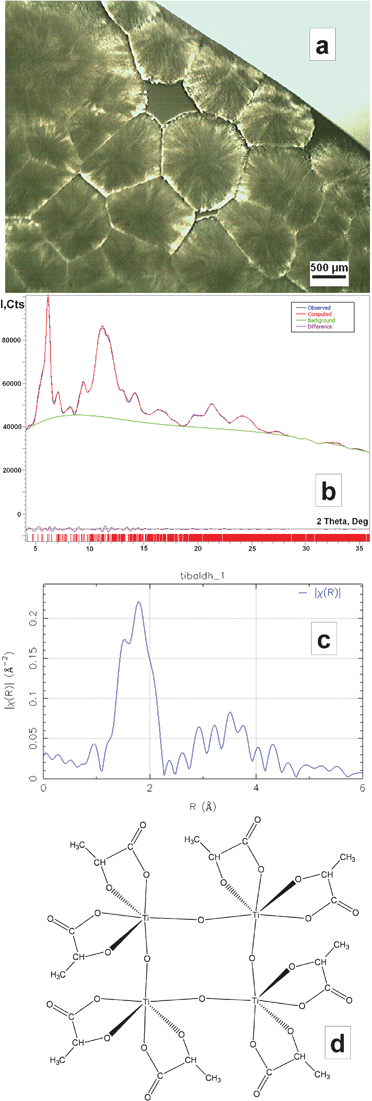 | ||
| Fig. 2 Microscopic image of the air-dried TiBALDH sample (a), the fit of the Rietveld refinement of the powder X-ray pattern for this material (b), its phase-corrected Fourier transformed EXAFS spectrum (c), and the model of the centrosymmetric tetranuclear oxo-lactato-titanate anion (d). | ||
Health effects on lung cells
In our study we intended to get insight into the possible effects of exposure to titania bio-encapsulation matrices on different types of lung cells and analyse the toxic effect of this compound . Two model cell lines were chosen for in vitro studies: adenocarcinomic human alveolar basal epithelial cells A549, which have become a common model today for screening the health effects of components in air pollution,23 and also non-small lung cell carcinoma cells U1810, which have been broadly investigated as a model for potential effects of medicines and chemicals on lung cancer cells.24 The cells were treated as described above using different concentrations of nanoparticles based on titanium oxide. Treatment of U1810 cells that express mutant p53 protein with nanoparticles did not induce cleavage of caspase-related substrate PARP-1, indicating that these nanoparticles do not activate apoptotic cell death, identified to be the typical mechanism in cytotoxic effect of small nanoparticles on the living cells.25 The nanoparticles did not induce expression of p53 proteins in A549 wild type cells that express p53 and, importantly, we were also unable to detect apoptosis in these cells, as measured by the level of cleaved form of PARP-1 (Fig. 3).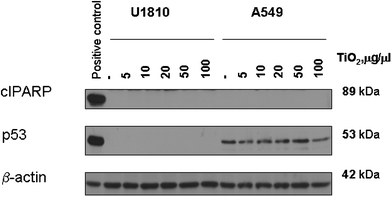 | ||
| Fig. 3 Western blot analysis of PARP-1 cleavage and p53 expression in A549 and U1810 cells treated with different concentrations of titanium oxide-based nanoparticles | ||
To further explore the effect of nanoparticles on the studied cells, their effect on cell viability was measured. For this purpose, the cells were treated with titanium oxide for 48 h at concentrations of 1, 5, 10 and 50 μg μl−1. The results for the highest concentration, 50 μg μl−1, presented in Fig. 4 shows the absence of cytotoxicity of titanium oxide nanoparticles.
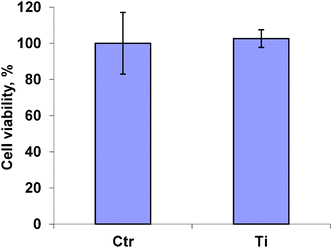 | ||
| Fig. 4 Viability of A549 cells treated with titanium oxide nanoparticles | ||
In addition, cell growth was measured in cells treated for 120 h with titanium oxide nanoparticles and the DNA damaging drug, doxorubicin. The results in Fig. 5 show a 16-fold increase in cell growth in untreated cells and the same fold increase in the number of cells observed after the treatment of cancer cells with the nanoparticles. We also examined if the nanoparticles had an additive effect in combination with doxorubicin (0.5 μg μl−1). As shown in Fig. 5, doxorubicin inhibited cell growth, but its combination with the nanoparticles had no additive inhibitory effect on cell growth. Therefore, based on these experiments we can conclude that titanium oxide-derived nanoparticles are nontoxic to the studied cells.
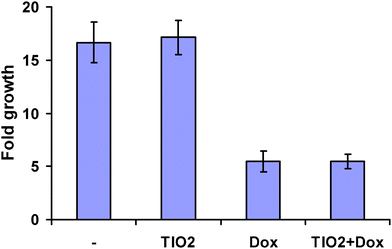 | ||
| Fig. 5 Growth of the cells treated with titanium oxide nanoparticles (50 μg μl−1), doxorubicin (0.5 μg μl−1) or their combination. | ||
Effects on pollen grain survival and growth
The environmental impact and potential effects on plant reproduction were followed by visual observation of the growth of pollen tubes. Either washed titanium dioxide gel obtained from CaptiGel or TiBALDH diluted by growth medium were added to freshly collected grains. In view of the different form of application, suspension vs. solution of an individual water soluble complex species, the concentrations of the produced mixtures are expressed as mM in relation to the Ti(IV) content. Converted into weight/volume content of TiO2 this gives 120 μg ml−1 for 1.5 mM Ti(IV) content. As can be seen from Fig. 6b, no measurable effect on pollen tube growth could be observed for this relatively high concentration of titania gel produced from CaptiGel. At higher concentrations the growth of pollen tubes was sterically hindered as the solution was “crowded” by clumps of the gel. Observations at much higher concentrations were possible for TiBALDH, up to about 30 mM. No measurable effects could be observed at concentrations as high as 15 mM (1.2 mg ml−1 TiO2 equivalent—a truly huge concentration that is hardly achievable even in a direct field application of the formulated biomaterials). This permits us to conclude that neither titanium formulation matrices nor model products of their bio-digestion display any measurable toxicity.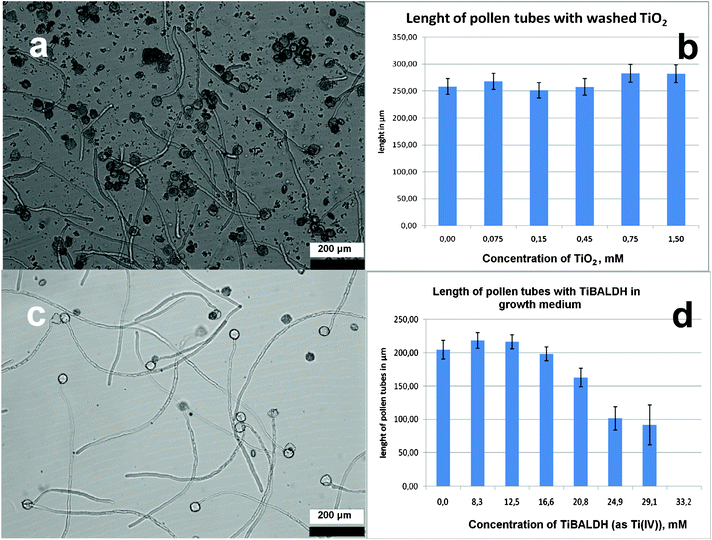 | ||
| Fig. 6 Growth of pollen tubes in a 100 μg ml−1 suspension of titania gel produced from CaptiGel after 3 h of exposure (a). Length of pollen tubes measured in solutions with different concentrations of titania gel (b). Growth of pollen tubes in a 600 μg ml−1 solution of TiBALDH (c). Length of pollen tubes measured in solutions with different concentrations of TiBALDH (d). | ||
The reliability of the applied test model was evaluated using a LaAlO3 nanophosphor as a positive control. It was surface-modified by adenosine tri-phosphate (ATP) according to a procedure described in literature17 for improved solution stability (to permit it to be applied in controlled concentrations). The epifluorescence microscope images (Fig. 7a and 7b) show enhanced autofluorescence of pollen grains, supposedly caused by interaction of membrane proteins with metal oxide nanoparticles (Fig. 7a and 7b) and thus indicates the interaction of particles with the grains. The increased autoluminescence was recently observed to be a general feature in the interaction of plant cells with oxide particles smaller than 15 nm.26 It is important to note that the solution stability of the LaAlO3 suspension was rather limited and aggregation (glowing clumps) can be seen even in the images of fresh samples. Precipitation of aggregates is apparent after 1 h and the negative effect of this material is clearly visible in the areas where the precipitate is concentrated. Even at rather low total concentrations of about 10 μg ml−1 the pollen grains were dead within 4 h in the proximity of the precipitated nanomaterials (see Fig. 7c). This was probably caused by the evolution of Al3+ cations, which are known to be highly toxic for plant cells, upon partial dissolution of the nanoparticles.
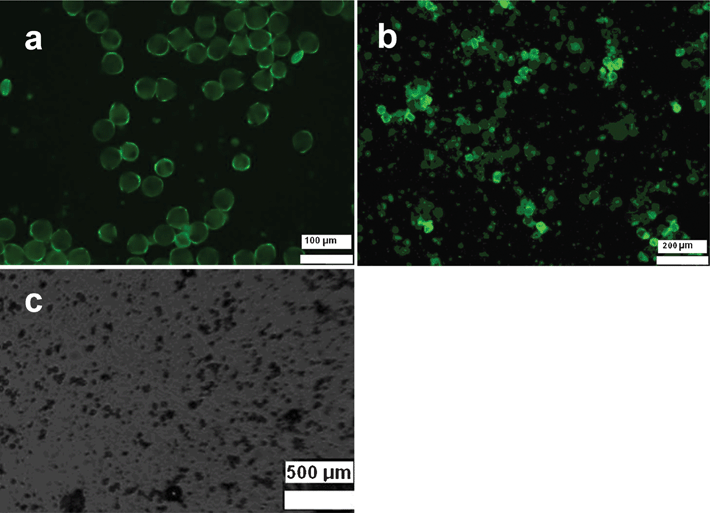 | ||
| Fig. 7 Pollen grains incubated with LaAlO3 nanophosphor: as-produced (a), after 2 h (b) and after 4 h (c). | ||
Acknowledgements
This research was supported by the Swedish Research Council grant “Molecular Precursors and Molecular Models of Nanoporous Materials”, and the Swedish Research Council FORMAS grant “Particle Impurities in Air: Express-analysis and Health Effects”, by the Swedish and Stockholm Cancer Societies. Vitaliy Kaminskyy was supported by a fellowship from the Swedish Institute and Karolinska Institutet. Julia Netrval (now at the Bruker-AXS Sweden) is gratefully acknowledged for assistance with the ζ potential measurements and polyelectrolyte titrations. We express our gratitude to Suresh Gohil for the help with electrospray mass-spectrometry studies and to Prof. Ingmar Persson for the help with EXAFS experiments. We thank also Prof. Stephane Parola and Dr Fernand Chassagneux at the University Claude Bernard Lyon-1 for help with TEM experiments and continuous fruitful discussions.References
- M. Fujiwara, K. Shiokawa, K. Morigaki, Y. C. Zhu and Y. Nakahara, Calcium carbonate microcapsules encapsulating biomacromolecules, Chem. Eng. J., 2008, 137(1), 14–22 CrossRef CAS.
- M. Epple, K. Ganesan, R. Heumann, J. Klesing, A. Kovtun, S. Neumann and V. Sokolova, Application of calcium phosphate nanoparticles in biomedicine, J. Mater. Chem., 2010, 20(1), 18–23 RSC.
- N. Nassif, O. Bouvet, M. N.Rager, C. Roux, T. Coradin and J. Livage, Living bacteria in silica gels, Nat. Mater., 2002, 1(1), 42–44 CrossRef CAS.
- M. Perullini, M. Amoura, C. Roux, T. Coradin, J. Livage, M. L. Japas, M. Jobbagy and S. A. Bilmes, Improving silica matrices for encapsulation of Escherichia coli using osmoprotectors, J. Mater. Chem., 2011, 21(12), 4546–4552 RSC.
- (a) V. G. Kessler, G. A. Seisenbaeva, S. Håkansson and M. Unell, Chemically triggered biodelivery using metal–organic sol–gel synthesis, Angew. Chem., Int. Ed., 2008, 47(44), 8506–8509 CrossRef CAS; (b) S. H. Yang, E. H. Ko and I. S. Choi, Cytocompatible Encapsulation of Individual Chlorella Cells within Titanium Dioxide Shells by a Designed Catalytic Peptide, Langmuir, 2012, 28(4), 2151–2155 CrossRef CAS.
- G. A. Seisenbaeva, M. P. Moloney, R. Tekoriute, A. Hardy-Dessource, J. M. Nedelec, Y. K. Gun'ko and V. G. Kessler, Biomimetic synthesis of hierarchically porous metal oxide microparticles–potential scaffolds for drug delivery and catalysis, Langmuir, 2010, 26(12), 9809–9817 CrossRef CAS.
- (a) X. Zheng, Y. Chen and R. Wu, Long-Term Effects of Titanium Dioxide Nanoparticles on Nitrogen and Phosphorus Removal from Wastewater and Bacterial Community Shift in Activated Sludge, Environ. Sci. Technol., 2011, 45(17), 7284–7290 CAS; (b) K. M. Buettner and A. M. Valentine, Bioinorganic Chemistry of Titanium, Chem. Rev., 2012, 112, 1863 CrossRef CAS.
- V. G. Kessler, G. A. Seisenbaeva, S. Håkansson and M. Unell, Metal oxide hydrogels and hydrosols, their preparation and use, WO, 07145573 Search PubMed.
- (a) G. Orr, D. J. Panther, K. J. Cassens, J. L. Phillips, B. J. Tarasevich and J. G. Pounds, Syndecan-1 mediates the coupling of positively charged submicrometer amorphous silica particles with actin filaments across the alveolar epithelial cell membrane, Toxicol. Appl. Pharmacol., 2009, 236(2), 210–220 CrossRef CAS; (b) G. Orr, D. J. Panther, J. L. Phillips, B. J. Tarasevich, A. Dohnalkova, D. H. Hu, J. G. Teeguarden and J. G. Poundsl, Submicrometer and nanoscale inorganic particles exploit the actin machinery to be propelled along microvilli-like structures into alveolar cells, ACS Nano, 2007, 1(5), 463–475 CrossRef CAS.
- (a) R. Pazik, G. A. Seisenbaeva, R. Wiglusz, L. Kepinski and V. G. Kessler, Crystal structure and morphology evolution in the LaXO3, X-Al, Ga, In nano-oxide series. Consequences for the synthesis of luminescent phosphors, Inorg. Chem., 2011, 50(7), 2966–2974 CrossRef CAS; (b) R. Pazik, G. A. Seisenbaeva, S. Gohil, R. Wiglusz, L. Kepinski, W. Strek and V. G. Kessler, Simple and Efficient Synthesis of a Nd:LaAlO3 NIR Nanophosphor from Rare Earth Alkoxo-Monoaluminates Ln2Al2(OiPr)12(iPrOH)2 Single Source Precursors by Bradley Reaction, Inorg. Chem., 2010, 49(6), 2684–2691 CrossRef CAS.
- A. Altomare, M. Camalli, C. Cuocci, C. Giacovazzo, A. Moliterni and R. Rizzi, EXPO2009: structure solution by powder data in direct and reciprocal space, J. Appl. Crystallogr., 2009, 42, 1197–1202 CrossRef CAS.
- A. Thompson, D. Attwood, E. Gullikson, M. Howells, K.-J. Kim, J. Kirz, J. Kortright, I. Lindau, P. Pianatta, A. Robinson, J. Scofield, J. Underwood, D. Vaughan, G. Williams and H. Winick, X-ray Data Booklet, Berkeley, California 94720, 2001 Search PubMed.
- M. Newville, J. Synchrotron Radiat., 2001, 8, 322–324 CrossRef CAS.
- Y. Djaoued, S. Badilescu, P. V. Ashrit, D. Bersani, P. P. Lottici and J. Robichaud, Study of Anatase to Rutile Phase Transition in Nanocrystalline Titania Films, J. Sol-Gel Sci. Technol., 2002, 24(3), 255–264 CrossRef CAS.
- X. M. Jiang and C. J. Brinker, J. Am. Chem. Soc., 2006, 128(14), 4512–4513 CrossRef CAS.
- R. Bouchet, A. Weibel, P. Knauth, G. Mountjoy and A. V. Chadwick, Chem. Mater., 2003, 15, 4996–5002 CrossRef CAS.
- R. Pazik, R. Andersson, L. Kepinski, V. G. Kessler, J. M. Nedelec and G. A. Seisenbaeva, Surface Functionalization of Metal Oxide Nanoparticles with Biologically Active Molecules Containing Phosphonate Moieties, J. Phys. Chem. C, 2011, 115(20), 9850–9860 CAS.
- (a) M. Kakihana, K. Tomita, V. Petrykin, M. Tada, S. Sasaki and Y. Nakamura, Chelating of Titanium by Lactic Acid in the Water-Soluble Diammonium Tris(2-hydroxypropionato)-titanate(IV), Inorg. Chem., 2004, 43(15), 4546–4548 CrossRef CAS; (b) J. M. Collins, R. Uppal, C. D. Incarvito and A. M. Valentine, Titanium(IV) Citrate Speciation and Structure under Environmentally and Biologically Relevant Conditions, Inorg. Chem., 2005, 44(10), 3431–3440 CrossRef CAS.
- (a) H. Möckel, M. Giersig and F. Willig, Formation of uniform size anatase nanocrystals from bis(ammonium lactato)titanium dihydroxide by thermohydrolysis, J. Mater. Chem., 1999, 9, 3051–3056 RSC; (b) G. P. Smith, K. J. Baustian, C. J. Ackerson and D. L. Feldheim, Metal oxide formation by serine and cysteine proteases, J. Mater. Chem., 2009, 19, 8299–8306 RSC.
- N. M. Kisinger, A. Wong, D. S. Li, F. Villalobos and D. Kisailus, Nucleation and Crystal Growth of Nanocrystalline Anatase and Rutile Phase TiO2 from a Water-Soluble Precursor, Cryst. Growth Des., 2010, 10(12), 5254–5261 Search PubMed.
- G. M. H. van de Velde, S. Harkema and P. J. Gellings, The crystal and molecular structure of ammonium titanyl oxalate, Inorg. Chim. Acta, 1974, 11, 243–245 CrossRef CAS.
- (a) M. Kakihana, M. Tada, M. Shiro, V. Petrykin, M. Osada and Y. Nakamura, Structure and Stability of Water Soluble (NH4)8[Ti4(C6H4O7)4(O2)4]·8H2O, Inorg. Chem., 2001, 40(5), 891–894 CrossRef CAS; (b) A. Fester, W. Bensch and M. Tromel, Crystal Structure of Cesium-bis(-oxalato)oxo-Titanate (IV) Hydrate, Inorg. Chim. Acta, 1992, 193, 99–103 CrossRef CAS.
- K. E. Wilkinson, L. Palmberg, E. Witasp, M. Kupczuk, N. Feliu, P. Gerde, G. Seisenbaeva, B. Fadeel, S. E. Dahlen and V. G. Kessler, Solution Engineered Pd Nanoparticles: Models for Health Effect Studies of Automotive Particulate Polution, ACS Nano, 2011, 5(7), 5312–5324 CrossRef CAS.
- T. H. Hemström, B. Joseph, G. Schulte, R. Lewensohn and B. Zhivotovsky, PKC412 sensitizes U1810 non-small lung cancer cells to DNA damage, Exp. Cell Res., 2005, 305(1), 200–213 CrossRef.
- R. Colognato, M. V. D. Z. Park, P. Wick and W. H. De Jong, Interactions with the Human Body, in Adverse Effects of Engineered Nanomaterials, ed. B. Fadeel, A. Pietroiusti and A.A. Shvedova, Elsevier, 2012, pp. 4–24 Search PubMed.
- R. Pazik, R. Tekoriute, S. Håkansson, R. Wiglusz, W. Strek, G. A. Siesenbaeva, Y. K. Gun'ko and V. G. Kessler, Precursor and solvent effects in the non-hydrolytic synthesis of complex oxide nanoparticles for bio-imaging applications by the ether elimination (Bradley) reaction, Chem.–Eur. J., 2009, 15(28), 6820–6826 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TG curves for thermal decomposition of air-dried materials, XPD patterns for heat-treated materials, tables summarising results of DLS measurements, EXAFS spectra of CaptiGel in comparison with literature data on anatase, and details of shooting nanoparticles suspension using Bio-rad instrument. See DOI: 10.1039/c2ra20388j |
| This journal is © The Royal Society of Chemistry 2012 |
