DOI:
10.1039/C2RA20437A
(Paper)
RSC Adv., 2012,
2, 10072-10084
Received
9th March 2012
, Accepted 22nd August 2012
First published on 24th August 2012
Introduction
Zeolites are crystalline microporous materials, which are widely used as molecular sieves, ion exchangers, and solid acid catalysts.1–5 Zeolites are excellent solid acid catalytic materials due to their crystalline framework structures that are ordered at the atomic level.1,4 Structure directing agents (SDA) for the synthesis of zeolites are able to form zeolites with pore sizes below 15 Å. The microporous nature of zeolites restricts their use as molecular sieves or catalysts in processes that involve large reactant molecules.4,6 Thus, zeolites with controlled shape and large porosity are preferred to minimize diffusion constraints. To overcome these limitations, large pore mesoporous materials via a variety of supramolecular templating methods have been developed.7–10 Recently, several review articles have appeared, which highlight various strategies to create additional porosity in these microporous materials.2,5,11
Meso- and/or macropores can be created in the zeolite via either non-templating (through a post-synthesis treatment by steaming or partial leaching of zeolite lattice elements12,13) or templating synthesis procedures.2,5,11 Templating (hard/soft) procedures offer the opportunity to generate a controlled and possibly variable mesopore system, determined by the size and/or amount of the structure directing agents. Among the templating methods, hard templating has been first employed to create mesopores inside zeolites.14–17 Mesoporous carbon and carbon nanotubes have been used as hard templates to form mesoporous MFI.15,16,18,19 A truly intra-crystalline mesoporous MFI zeolite was obtained by using amphiphilic organosilane as the template.20 A mesoporous MFI zeolite with a mesoporous/microporous hierarchical structure shows a remarkably improved resistance to the deactivation of catalytic activities in various reactions.21 Another approach to improve the diffusion in the zeolite is to minimize the size of the zeolite crystals and thereby shorten the diffusion path.22 Mesoporous MFI nanoparticle aggregates (termed as nanocrystalline MFI) have been synthesized using various triethoxyalkylsilanes.23 Mesoporous MFI has also been synthesized using linear Gemini-type templates consisting of several bridged ammonium cations terminated by long alkyl chains.24,25 Most of these studies were focused on the synthesis of nanocrystalline MFI. Only a few efforts have been made to prepare nanocrystalline Beta and MTW.25–34 It has been reported that hierarchically porous Beta can be prepared by using 3,10-diazoniabicyclo[10.2.2]hexadeca-12,14,15-triene-3,3,10,10-tetramethyl-dichloride29,27 or by using a suitable silylating agent such as phenylaminopropyl-trimethoxysilane.28 Nanosized Beta with various particle sizes and high surface areas were synthesized with tetraethylammonium hydroxide (TEAOH) and cetyltrimethylammonium surfactants using a dry-gel method.29
The literature survey suggests that soft templates, which are cationic in nature, are able to form hierarchical zeolites of different framework structures. Our research is focused on the synthesis and applications of ionic liquids.30–35 The above mentioned observations stimulated us to design several quaternary ammonium salts of imidazole, piperidine and pyridine based SDA for the synthesis of zeolites. In this study, a variety of piperidine, imidazole, and pyridine based mono-quaternary and di-quaternary ammonium salts were prepared and utilized as a SDA for the synthesis of zeolites. In our preliminary communication, the synthesis of zeolite Beta using a few di-quaternary ammonium salts was reported.35 In this study, the work has been extended to other kinds of quaternary ammonium salts for the synthesis of zeolites of different framework structures. A detailed investigation of the influence of textural properties of the resultant materials was made by using different types of precursors for the zeolite synthesis, synthesis parameters, and the role of several new quaternary ammonium salt based SDAs.
Experimental
Since the quaternary ammonium salts of imidazole, piperidine and pyridine have ionic liquid-like properties, they have been designated as IL. IL2, IL4–IL9, and IL11–IL13 were synthesized by following previously reported procedures (see Scheme 1 and Scheme 2).30,35,36
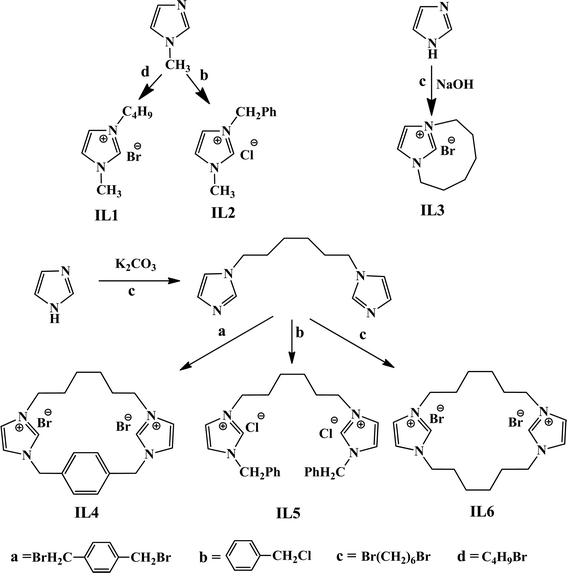 |
| | Scheme 1 Schematic representation for the synthesis of IL1–IL6. | |
For the synthesis of IL3, first the sodium salt of imidazole (NaIm) was synthesized according to the reported procedure.36 In a typical synthesis, imidazole (4 mmol) and NaOH (4 mmol) were mixed and stirred at 383 K for 4 h under solvent-free conditions (Scheme 1). After the reaction, the reaction mixture was cooled and the by-product H2O was removed under reduced pressure to obtain solid NaIm (yield= 94%). 1,6-Dibromohexane (5 mmol) was added to a solution of NaIm (5 mmol) and dry THF (30 mL) under nitrogen atmosphere. The reaction mixture was stirred for 12 h at room temperature. The obtained solid was filtered and the filtrate was concentrated under reduced pressure. The residue was subjected to column chromatography (silica gel (100–200 mesh), hexane/ethyl acetate (80/20) to afford 1-(6-bromo-hexyl)-imidazole as a colorless liquid (yield= 81%). The liquid was dissolved in acetonitrile (40 mL) and refluxed for 6 d. Upon completion of the reaction, the solvent was evaporated under vacuum. The residue was washed with ethyl acetate 3–4 times and then dried under vacuum at 353 K (yield= 87%).
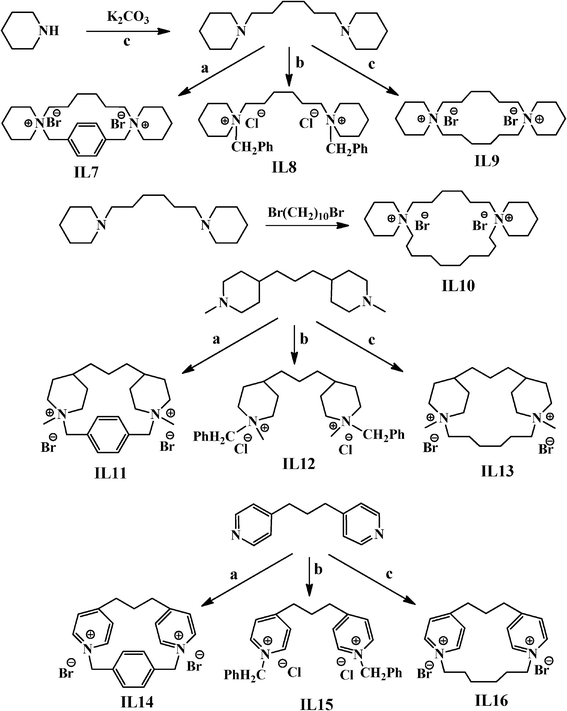 |
| | Scheme 2 Schematic representation for the synthesis of IL7-IL16. Structure of a, b, c is provided in Scheme 1. | |
IL10 was prepared using a similar procedure to that adopted for the synthesis of IL7. In a typical synthesis, N,N′-hexamethylenebis(piperidine) (10 mmol) was reacted with 1,10-dibromo-decane (10 mmol) to obtain IL10 (yield= 88%) (Scheme 2).
IL14–IL16 were prepared using a similar procedure to that adopted for the synthesis of IL11. For their synthesis, 4,4′-Trimethylene bis(pyridine) (10 mmol) was reacted with 1,1′-dibromo-p-xylene (10 mmol), benzyl chloride (20 mmol) or 1,6-dibromohexane (10 mmol) in toluene to obtain IL14 (yield= 89%), IL15 (yield= 92%) or IL16 (yield= 90%) (Scheme 2).
IL3.
IR (KBr, υ, cm−1) = 3400, 2936, 1626, 1560, 1452, 1327, 1160, 1043, 867, 643. 1H NMR (D2O) δ = 9.02 (s, 1H), 7.3 (s, 2H), 4.2 (t, 4H), 1.82 (m, 4H) 1.30 (m, 4H). 13C NMR δ = 135.6, 122.3, 49.4, 29.0, 24.8. Elemental analysis found: C, 46.37; H, 6.67; N, 12.07. Calc. for C9H15N2Br: C, 46.75; H, 6.49; N, 12.12%.
IL10.
IR (KBr, υ, cm−1) = 3397, 2938, 1619, 1425, 1052, 941, 892, 861, 830,734. 1H NMR (D2O) δ = 4.09–3.91 (m, 16H), 1.73–1.19 (m, 36H). 13C NMR δ = 59.1, 54.5, 29.0, 28.2, 27.9, 26.7, 22.6, 20.1. Elemental analysis found: C, 56.45; H, 9.61; N, 5.01. Calc. for C26H52N2Br2: C, 56.52; H, 9.49; N, 5.07%.
IL14.
IR (KBr, υ, cm−1) = 3415, 3037, 2948, 2880, 1633, 1469, 1292, 1158, 767. 1H NMR (D2O) δ = 8.7 (d, 4H), 7.25–7.78 (m, 8H), 5.12 (s, 4H), 2.51 (t, 4H), 1.88 (m, 2H). 13C NMR δ = 159.2, 145.0, 134.1, 131.2, 129.7, 129.1, 60.1, 35.6, 29.1. Elemental analysis found: C, 54.48; H, 4.97; N, 5.93. Calc. for C21H22N2Br2: C, 54.57; H, 4.8; N, 6.06%.
IL15.
IR (KBr, υ, cm−1) = 3398, 3040, 2949, 2882, 1633, 1470, 1294, 1159, 763. 1H NMR (D2O) δ = 8.75 (d, 4H), 7.20–7.82 (m, 14H), 5.92 (s, 4H), 2.64 (t, 4H), 1.90 (m, 2H). 13C NMR δ = 156.8, 144.9, 136.8, 131.1, 130.3, 128.9, 125.6, 62.7, 36.1, 28.2. Elemental analysis found: C, 71.72; H, 6.42; N, 6.14. Calc. for C27H28N2Cl2: C, 71.84; H, 6.25; N, 6.21%.
IL16.
IR (KBr, υ, cm−1) = 3398, 3039, 2948, 2882, 1633, 1474, 1293, 1158, 764. 1H NMR (D2O) δ = 8.8 (d, 4H), 7.89 (d, 4H), 4.01 (t, 4H), 2.60 (t, 4H), 1.99 (m, 2H), 1.87 (m, 4H), 1.31 (m, 4H). 13C NMR δ = 157.2, 143.5, 128.3, 60.9, 34.3, 30.2, 28.5, 24.8. Elemental analysis found: C, 51.41; H, 6.09; N, 5.84. Calc. for C19H26N2Br2: C, 51.6; H, 5.93; N, 6.33%.
For the synthesis of zeolites using sodium-silicate, first a sodium silicate solution was made using Ludox (40% aqueous solution, Aldrich) as the silica source. The silica source was diluted with a dilute NaOH solution yielding a sodium silicate solution with a molar ratio of 100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30Na2O. Two kinds of aluminum source, Al2(SO4)3 and Al(NO3)3, were investigated in this study. The aluminum source was dissolved in dilute sulfuric acid (x
30Na2O. Two kinds of aluminum source, Al2(SO4)3 and Al(NO3)3, were investigated in this study. The aluminum source was dissolved in dilute sulfuric acid (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) z
z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500, Aluminum source
1500, Aluminum source![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2SO4
H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O). The two solutions were mixed via vigorous stirring at room temperature. The resultant mixture was a clear solution. When IL was added slowly into this solution, under vigorous stirring at room temperature, the mixture gelled immediately. The molar gel composition can be represented as 30Na2O
H2O). The two solutions were mixed via vigorous stirring at room temperature. The resultant mixture was a clear solution. When IL was added slowly into this solution, under vigorous stirring at room temperature, the mixture gelled immediately. The molar gel composition can be represented as 30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xAl2O3
xAl2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) yIL
yIL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zH2SO4
zH2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O (where 0 ≤ x ≤ 2.5, 0 ≤ y ≤ 10, z = 15 or 20). After aging for 6 h at room temperature, the reaction mixture converted to a free flow liquid gel (less viscous), which was transferred to a Teflon-coated stainless-steel autoclave (100 mL capacity) and heated at 443 K with stirring. Precipitates were filtered, washed with deionised water, dried at 383 K, and calcined at 823 K for 4 h under flowing air. For the synthesis of Beta using tetraethylorthosilicate (TEOS), the required amount of H2SO4, followed by IL11 was added to a conventional alkaline mixture containing NaOH, TEOS, and distilled water to make the molar composition 30Na2O:2.5Al2O3:100SiO2:10IL11:15H2SO4:6000H2O. When TEOS was used as silica source, ethanol formed was removed (after 6 h of reaction) during the hydrolysis of TEOS, before being loaded into autoclave. Conventional MFI34 and Beta37 were synthesized by following the reported procedure.
6000H2O (where 0 ≤ x ≤ 2.5, 0 ≤ y ≤ 10, z = 15 or 20). After aging for 6 h at room temperature, the reaction mixture converted to a free flow liquid gel (less viscous), which was transferred to a Teflon-coated stainless-steel autoclave (100 mL capacity) and heated at 443 K with stirring. Precipitates were filtered, washed with deionised water, dried at 383 K, and calcined at 823 K for 4 h under flowing air. For the synthesis of Beta using tetraethylorthosilicate (TEOS), the required amount of H2SO4, followed by IL11 was added to a conventional alkaline mixture containing NaOH, TEOS, and distilled water to make the molar composition 30Na2O:2.5Al2O3:100SiO2:10IL11:15H2SO4:6000H2O. When TEOS was used as silica source, ethanol formed was removed (after 6 h of reaction) during the hydrolysis of TEOS, before being loaded into autoclave. Conventional MFI34 and Beta37 were synthesized by following the reported procedure.
Characterization
X-ray diffraction (XRD) patterns were recorded in the 2θ range of 5–50° with a scan speed of 2° min−1 on a PANalytical X'PERT PRO diffractometer using Cu Kα radiation (λ = 0.1542 nm, 40 kV, 40 mA) and a proportional counter detector. Nitrogen adsorption measurements at 77 K were performed by Autosorb-IQ Quantachrome Instruments volumetric adsorption analyzer. The sample was out-gassed at 573 K for 2 h in the degas port of the adsorption apparatus. The specific surface area was determined by BET method using the data points of P/P0 in the range of about 0.05–0.3. The pore diameter was estimated using the Barret–Joyner–Halenda (BJH) model. The Transmission electron microscopy (TEM) investigation was carried out using a FEI, TECNAI G2 20, S-TWIN microscope operating at 200 kV, equipped with a GATAN CCD camera. The calcined sample was dispersed in ethanol using an ultrasonic bath, mounted on a carbon coated Cu grid, dried, and then used for the TEM measurement. Scanning electron microscopy (SEM) measurements were carried out on a JEOL JSM-6610LV to investigate the morphology.
Density function theory (DFT) calculation
The minimum-energy geometries of the quaternary ammonium salts were obtained by performing DFT geometry optimizations at the B3LYP/6-31G level using the Gaussian09 program.38 Vibrational analysis was performed to ensure the absence of negative frequencies, which verify the existence of a true minimum. Theoretical studies were also performed by considering temperature in the calculations. The Born–Oppenheimer molecular dynamics (BOMD) simulation model was used to ensure the stability of the geometrically optimized structures of quaternary ammonium salts at the zeolite synthesis temperature (443 K) by using the method of Nose and Hoover.
Result and discussion
In this study, several mono-quaternary and di-quaternary ammonium salts of imidazole, piperidine and pyridine were synthesized using a multi-step synthetic route (Scheme 1 and Scheme 2). The quaternary ammonium salts investigated in this study are classified in five categories, they are: (1) imidazole based mono-cationic quaternary ammonium salts (IL1, IL2, and IL3), (2) imidazole based di-quaternary ammonium salts (IL4, IL5, and IL6), (3) piperidine based di-quaternary ammonium salts (IL7, IL8, IL9, and IL10), (4) 4,4′-trimethylenebis(1-methylpiperidine) based di-quaternary ammonium salts (IL11, IL12, and IL13), and (5) 4,4′-trimethylenebis(pyridine) based di-quaternary ammonium salts (IL14, IL15 and IL16). The quaternary ammonium salts were utilized as SDAs for the preparation of zeolites under hydrothermal conditions using sodium silicate and aluminum sulfate as the zeolite precursors. The effects of synthetic variables such as Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio, amount of sulfuric acid, role of silica and aluminum source, and hydrothermal temperatures were investigated. Zeolites were synthesized under strong basic conditions using the molar composition 30Na2O
Al molar ratio, amount of sulfuric acid, role of silica and aluminum source, and hydrothermal temperatures were investigated. Zeolites were synthesized under strong basic conditions using the molar composition 30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xAl2O3
xAl2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) yIL
yIL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zH2SO4
zH2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O, where 0 ≤ x ≤ 2.5, y = 0–10, z = 15 or 20. We are interested in obtaining the synthesis conditions by which zeolite materials having Beta, MFI, and MTW framework structures with high surface area and mesoporosity are obtained. The XRD patterns of the as synthesized form of the zeolites (Beta, MFI and MTW) obtained using IL are similar to the XRD patterns of the calcined materials. The XRD patterns match well with the reported XRD pattern of zeolites (Beta, MFI and MTW), which clearly indicates the high purity of the obtained zeolite materials. CHN analysis confirms that only a negligible amount of organic content (<0.1%) is present in the calcined form of the zeolite.
First, the mono-cationic quaternary ammonium salts were explored for the synthesis of zeolites under our synthesis conditions. The investigation was started using commercially available 1-butyl,3-methyl-imidazolium bromide (IL1), which is known to form zeolite Beta and MFI.39 However, under our synthesis conditions, an unknown crystalline silicate phase was observed (Fig. S1, ESI†). Using IL2, no crystalline phase was obtained under a variety of the synthesis conditions investigated in this study. IL3 was able to form zeolite MFI [designated as MFI-IL3-1 (when 15H2SO4 was used), and MFI-IL3-2 (when 20H2SO4 was used)] under the synthesis composition 30Na2O
6000H2O, where 0 ≤ x ≤ 2.5, y = 0–10, z = 15 or 20. We are interested in obtaining the synthesis conditions by which zeolite materials having Beta, MFI, and MTW framework structures with high surface area and mesoporosity are obtained. The XRD patterns of the as synthesized form of the zeolites (Beta, MFI and MTW) obtained using IL are similar to the XRD patterns of the calcined materials. The XRD patterns match well with the reported XRD pattern of zeolites (Beta, MFI and MTW), which clearly indicates the high purity of the obtained zeolite materials. CHN analysis confirms that only a negligible amount of organic content (<0.1%) is present in the calcined form of the zeolite.
First, the mono-cationic quaternary ammonium salts were explored for the synthesis of zeolites under our synthesis conditions. The investigation was started using commercially available 1-butyl,3-methyl-imidazolium bromide (IL1), which is known to form zeolite Beta and MFI.39 However, under our synthesis conditions, an unknown crystalline silicate phase was observed (Fig. S1, ESI†). Using IL2, no crystalline phase was obtained under a variety of the synthesis conditions investigated in this study. IL3 was able to form zeolite MFI [designated as MFI-IL3-1 (when 15H2SO4 was used), and MFI-IL3-2 (when 20H2SO4 was used)] under the synthesis composition 30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.625Al2O3
0.625Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL3
10IL3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 or 20H2SO4
15 or 20H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O (Fig. 1a). N2-adsorption studies show that both materials exhibited type IV isotherms with H4 hysteresis (Fig. 1b), which shows that the material contains both micropores and mesopores. The isotherm of MFI-IL3-1 clearly evidences capillary condensation in the mesopores of the material, which is reflected in their textural properties (Table 1). The morphology of MFI-IL3-1 and MFI-IL3-2 was found to be spheroidal (Fig. 1c). The particle sizes of MFI-IL3-1 were found to be smaller than those of MFI-IL3-2. Agglomeration of the small particles is reflected in the properties of the obtained materials. Due to the relatively small particle size, the surface area of MFI-IL3-1 was found to be larger when compared to MFI-IL3-2. The agglomeration of the small particles in MFI-IL3-1 is reflected in its higher mesopore volume compared to MFI-IL3-2. Mesoporous void spaces are formed due to the crystal packing of these small particles. It is, further, interesting to note that when the synthesis was performed in the absence of Al (30Na2O
6000H2O (Fig. 1a). N2-adsorption studies show that both materials exhibited type IV isotherms with H4 hysteresis (Fig. 1b), which shows that the material contains both micropores and mesopores. The isotherm of MFI-IL3-1 clearly evidences capillary condensation in the mesopores of the material, which is reflected in their textural properties (Table 1). The morphology of MFI-IL3-1 and MFI-IL3-2 was found to be spheroidal (Fig. 1c). The particle sizes of MFI-IL3-1 were found to be smaller than those of MFI-IL3-2. Agglomeration of the small particles is reflected in the properties of the obtained materials. Due to the relatively small particle size, the surface area of MFI-IL3-1 was found to be larger when compared to MFI-IL3-2. The agglomeration of the small particles in MFI-IL3-1 is reflected in its higher mesopore volume compared to MFI-IL3-2. Mesoporous void spaces are formed due to the crystal packing of these small particles. It is, further, interesting to note that when the synthesis was performed in the absence of Al (30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0Al2O3
0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL3
10IL3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O), MTW was obtained (designated as MTW-IL3, Fig. 1a). MFI-IL3-1 exhibited significantly larger surface area and mesopore volume compared to conventional MFI, which signifies that MFI-IL3-1 is nanocrytsalline in nature. One structure directing agent is able to form zeolites of two different framework structures (MFI and MTW). The structure of IL3 was optimized using density functional theory (DFT) method employing B3LYP with 6-31G basis set (Fig. 1d and Table 2).38 The molecular dimension of IL3 is larger than the pore apertures of the MFI and MTW zeolites (maximum diameters of a sphere that can be included in MFI and MTW framework structure are 6.36 and 6.08 Å, respectively). Hence, theoretical study confirms that the size of the SDA is not the important factor for the synthesis of MFI and MTW structures. Very recently, it has been reported that for the formation of zeolite Beta, the molecular dimension of the SDA should be less than the pore channel of the 12 member ring of Beta (pore dimensions: 6.6 × 6.7 Å for [100] and 5.6 × 5.6 Å for [001]).26 The molecular size of the IL3 is less than the pore aperture of zeolite Beta, but it was unable to form zeolite Beta. Therefore, it can be concluded that the critical size of the SDA is not the only necessary condition for the formation of zeolite Beta.
6000H2O), MTW was obtained (designated as MTW-IL3, Fig. 1a). MFI-IL3-1 exhibited significantly larger surface area and mesopore volume compared to conventional MFI, which signifies that MFI-IL3-1 is nanocrytsalline in nature. One structure directing agent is able to form zeolites of two different framework structures (MFI and MTW). The structure of IL3 was optimized using density functional theory (DFT) method employing B3LYP with 6-31G basis set (Fig. 1d and Table 2).38 The molecular dimension of IL3 is larger than the pore apertures of the MFI and MTW zeolites (maximum diameters of a sphere that can be included in MFI and MTW framework structure are 6.36 and 6.08 Å, respectively). Hence, theoretical study confirms that the size of the SDA is not the important factor for the synthesis of MFI and MTW structures. Very recently, it has been reported that for the formation of zeolite Beta, the molecular dimension of the SDA should be less than the pore channel of the 12 member ring of Beta (pore dimensions: 6.6 × 6.7 Å for [100] and 5.6 × 5.6 Å for [001]).26 The molecular size of the IL3 is less than the pore aperture of zeolite Beta, but it was unable to form zeolite Beta. Therefore, it can be concluded that the critical size of the SDA is not the only necessary condition for the formation of zeolite Beta.
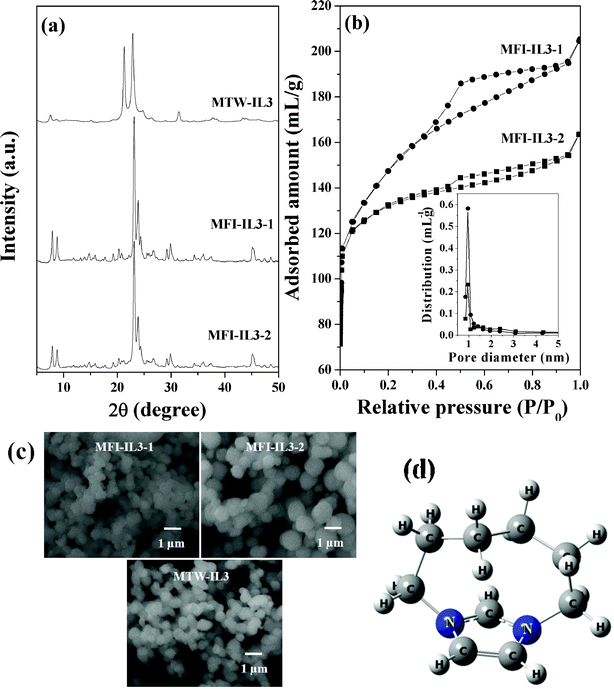 |
| | Fig. 1 (a) XRD patterns of MTW-IL3, MFI-IL3-1 and MFI-IL3-2, (b) N2-adsorption isotherms of MFI-IL3-1 and MFI-IL3-2 (inset shows pore size distributions), (c) SEM images of MFI-IL3-1, MFI-IL3-2 and MTW-IL3 and (d) Optimized structure of IL3 using B3LYP/6-31G. | |
Table 1 Textural properties of various zeolites (except Beta) synthesized using a variety of quaternary ammonium salt based structure directing agents. Unless otherwise stated, sodium silicate and Al2(SO4)3 were used as the silica and aluminium sources, respectively
| Zeolite |
Molar composition |
S
BET (m2 g−1) |
V
micro (mL g−1) |
V
meso (mL g−1) |
| MFI-IL3-1 |
30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.625Al2O3 0.625Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2 100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL3 10IL3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4 15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O 6000H2O |
497 |
0.14 |
0.24 |
| MFI-IL3-2 |
30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.625Al2O3 0.625Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2 100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL3 10IL3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20H2SO4 20H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O 6000H2O |
326 |
0.12 |
0.10 |
| MFI |
30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.625Al2O3 0.625Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2 100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10TPABr 10TPABr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20H2SO4 20H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O 6000H2O |
328 |
0.13 |
0.08 |
| MTW-IL3 |
30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0Al2O3 0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2 100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL3 10IL3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4 15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O 6000H2O |
204 |
0.10 |
0.04 |
| MTW-IL6-1 |
30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.625Al2O3 0.625Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2 100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL3 10IL3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20H2SO4 20H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O 6000H2O |
280 |
0.11 |
0.16 |
| MTW-IL6-2 |
30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.625Al2O3 0.625Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2 100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL3 10IL3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4 15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O 6000H2O |
256 |
0.10 |
0.13 |
| MTW-IL11-1 |
30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0Al2O3 0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2 100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL11 10IL11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4 15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O 6000H2O |
301 |
0.11 |
0.12 |
| MTW-IL11-2 |
30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.625Al2O3 0.625Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2 100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL11 10IL11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20H2SO4 20H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O 6000H2O |
230 |
0.10 |
0.06 |
| MTW-IL11-3 |
30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0Al2O3 0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2 100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL11 10IL11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20H2SO4 20H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O 6000H2O |
287 |
0.11 |
0.11 |
| MTW-IL13 |
30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.63Al2O3 0.63Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2 100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL16 10IL16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4 15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O 6000H2O |
298 |
0.12 |
0.11 |
| Quaternary ammonium salt |
Dipole moment (Debye) |
Energy (a.u) |
Molecular size (Å) |
| IL1 |
5.82 |
−423.06 |
10.21 × 4.3 × 3.38 |
| IL2 |
7.60 |
−536.15 |
10.1 × 4.42 × 4.33 |
| IL3 |
2.32 |
−460.62 |
6.46 × 5.02 × 3.74 |
| IL4 |
1.29 |
−995.36 |
10.46 × 7.6 × 4.62 |
| IL5 |
5.52 |
−1227.60 |
21.0 × 7.76 × 5.23 |
| IL6 |
0.82 |
−921.58 |
10.88 × 8.24 × 3.82 |
| IL7 |
0.17 |
−1047.34 |
16.34 × 5.52 × 5.89 |
| IL8 |
3.27 |
−1279.59 |
13.84 × 5.80 × 5.30 |
| IL9 |
1.04 |
−973.57 |
13.99 × 6.84 × 4.63 |
| IL10 |
4.79 |
−1130.80 |
15.3 × 8.33 × 5.41 |
| IL11 |
4.16 |
−1008.06 |
12 × 5 × 6.8 |
| IL12 |
2.33 |
−1240.30 |
16.49 × 8.3 × 6.82 |
| IL13 |
4.01 |
−934.27 |
12.16 × 8.1 × 4.78 |
| IL14 |
1.37 |
−922.21 |
7.11 × 6.91 × 4.54 |
| IL15 |
2.07 |
−1154.48 |
21.0 × 4.75 × 4.81 |
| IL16 |
0.62 |
−848.44 |
8.3 × 7.9 × 4.63 |
Imidazole based di-quaternary ammonium salts (IL4–IL6) were investigated as SDAs for the synthesis of zeolites. MTW was obtained using all the imidazole based quaternary ammonium salts with molar composition (30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xAl2O3
xAl2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL
10IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 or 20H2SO4
15 or 20H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O; 75 < Si/Al < ∞). It was observed that MTW was formed under low Al content and this is consistent with the reported literature, which states that MTW is thermodynamically favored under high-silica synthesis conditions.40 In this manuscript, physico-chemical data is provided only for MTW synthesized using IL6 (MTW-IL6-1, MTW-IL6-2) (Fig. 2a and Table 1).
6000H2O; 75 < Si/Al < ∞). It was observed that MTW was formed under low Al content and this is consistent with the reported literature, which states that MTW is thermodynamically favored under high-silica synthesis conditions.40 In this manuscript, physico-chemical data is provided only for MTW synthesized using IL6 (MTW-IL6-1, MTW-IL6-2) (Fig. 2a and Table 1).
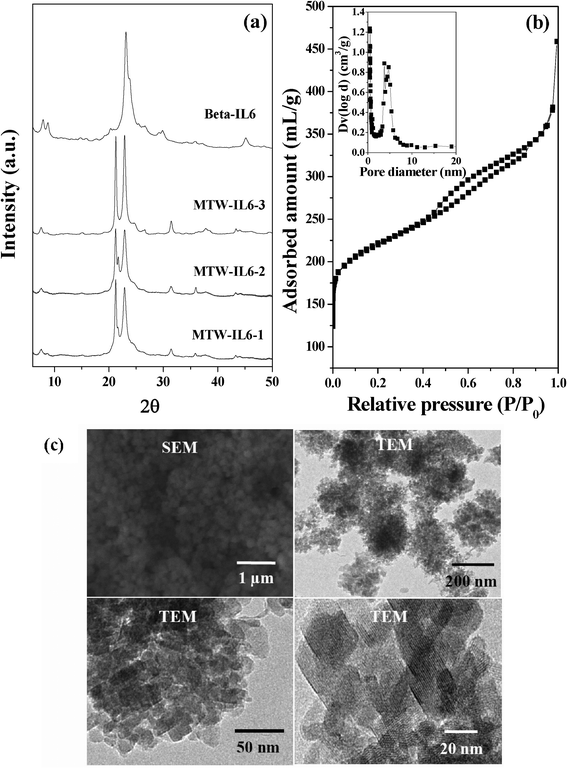 |
| | Fig. 2 (a) XRD patterns of BETA-IL6, MTW-IL6-1, MTW-IL6-2 and MTW-IL6-3, (b) N2-adsorption isotherm of BETA-IL6 (inset shows pore size distribution) and (c) SEM and TEM images of BETA-IL6. | |
It is very interesting to note that zeolite Beta was obtained using IL6 (designated as Beta-IL6), when synthesis was performed using the gel composition 30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5Al2O3
2.5Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL6
10IL6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O (Fig. 2a), whereas IL4 and IL5 were unable to form any crystalline phase under this synthesis condition. The broad nature of the XRD peak of Beta-IL6 clearly shows that its crystal sizes are smaller than conventional Beta (Table 3). Zeolite Beta obtained in this condition is not the traditional zeolite Beta but a mixture of polymorphs of A and B phases. Traditional zeolite Beta is highly disordered. It is made of the random intergrowth of polymorph A and polymorph B in the ratio of ca. 45
6000H2O (Fig. 2a), whereas IL4 and IL5 were unable to form any crystalline phase under this synthesis condition. The broad nature of the XRD peak of Beta-IL6 clearly shows that its crystal sizes are smaller than conventional Beta (Table 3). Zeolite Beta obtained in this condition is not the traditional zeolite Beta but a mixture of polymorphs of A and B phases. Traditional zeolite Beta is highly disordered. It is made of the random intergrowth of polymorph A and polymorph B in the ratio of ca. 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55.41,42 Both the polymorphs A and B are constructed from the same centrosymmetric, tertiary building unit (TBU) arranged in layers successively, which interconnect in either a left handed (L) or right handed (R) manner. Polymorph A represents an uninterrupted sequence of either left handed or right handed, whereas the polymorph B has an alternative stacking sequence. TBU has no preference for either mode of connection, which leads to an almost equal possibility of each in the structure of Beta, which enhances the growth of a faulty structure. It is reported in the literature that if a mixture of polymorph A and polymorph B of zeolite beta is formed then the broad peak at low angle splits into two sharp peaks of different intensity showing their percentage composition in the structure.41,42 Based on the XRD obtained for Beta-IL6, one can say that Beta-IL6 consists of mixture of polymorph B and polymorph A. The N2-adsorption study shows that Beta-IL6 exhibited a type IV isotherm with a hysteresis loop corresponding to capillary condensation in the mesopores (Fig. 2b). Furthermore, the zeolite exhibited a bi-modal distribution of mesopores in the range of 3.1 to 6.3 nm. N2-adsorption studies show that the material has very high surface area and mesopore volume compared to conventional Beta (Table 3). SEM and TEM analysis showed that Beta-IL6 is composed of zeolite nanocrystals of 16-18 nm of diameter (Fig. 2c).
55.41,42 Both the polymorphs A and B are constructed from the same centrosymmetric, tertiary building unit (TBU) arranged in layers successively, which interconnect in either a left handed (L) or right handed (R) manner. Polymorph A represents an uninterrupted sequence of either left handed or right handed, whereas the polymorph B has an alternative stacking sequence. TBU has no preference for either mode of connection, which leads to an almost equal possibility of each in the structure of Beta, which enhances the growth of a faulty structure. It is reported in the literature that if a mixture of polymorph A and polymorph B of zeolite beta is formed then the broad peak at low angle splits into two sharp peaks of different intensity showing their percentage composition in the structure.41,42 Based on the XRD obtained for Beta-IL6, one can say that Beta-IL6 consists of mixture of polymorph B and polymorph A. The N2-adsorption study shows that Beta-IL6 exhibited a type IV isotherm with a hysteresis loop corresponding to capillary condensation in the mesopores (Fig. 2b). Furthermore, the zeolite exhibited a bi-modal distribution of mesopores in the range of 3.1 to 6.3 nm. N2-adsorption studies show that the material has very high surface area and mesopore volume compared to conventional Beta (Table 3). SEM and TEM analysis showed that Beta-IL6 is composed of zeolite nanocrystals of 16-18 nm of diameter (Fig. 2c).
Table 3 Textural properties of zeolite Beta synthesized using a variety of quaternary ammonium salts based structure directing agents with molar composition 30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xAl2O3
xAl2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) yIL
yIL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zH2SO4
zH2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O
6000H2O
| Zeolite |
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) z z |
Time (d) |
Temp. (K) |
Crystallite size (Å) |
SBET (m2 g−1) |
Vmicro (mL g−1) |
Vmeso (mL g−1) |
|
Synthesized by following the reported procedure,
TEOS was used as silica source,
Al2(NO3)3 was used aluminum source.
|
| Betaa |
— |
— |
— |
29 |
495 |
0.18 |
0.23 |
| Beta-IL6 |
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
2 |
443 |
13.6 |
670 |
0.18 |
0.63 |
| Beta-IL11-1 |
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
2 |
443 |
13.5 |
685 |
0.18 |
0.65 |
| Beta-IL11-2 |
0.625![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
2 |
443 |
17.5 |
645 |
0.17 |
0.62 |
| Beta-IL11-3 |
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
4 |
443 |
16.3 |
663 |
0.18 |
0.62 |
| Beta-IL11-4 |
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10:15 10:15 |
7 |
403 |
15.4 |
672 |
0.18 |
0.63 |
| Beta-IL11-5b |
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10:15 10:15 |
2 |
443 |
24.0 |
528 |
0.17 |
0.32 |
| Beta-IL11-6c |
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10:15 10:15 |
2 |
443 |
16.9 |
648 |
0.18 |
0.62 |
| Beta IL12 |
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10:15 10:15 |
4 |
443 |
23.1 |
480 |
0.17 |
0.21 |
| Beta-IL13 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10:15 10:15 |
2 |
443 |
16.3 |
590 |
0.18 |
0.5 |
To understand why only IL6 is able to form zeolite Beta, structures of IL4–IL6 were optimized (Fig. 3) and structural parameters are provided in Table 2. The molecular dimensions of IL4–IL6 were found to be larger than the pore aperture of MTW and Beta (maximum diameter of a sphere that can be included in Beta framework structure is 6.68 Å). Formation of zeolite Beta using IL6 confirms that the molecular size is not a prerequisite for the formation of zeolite Beta. The optimized structures reveal that the imidazole rings in IL4–IL6 are situated at different planes (Fig. 3). Two imidazole rings in IL4, IL5, and IL6 make an angle of 58°, 73°, and 110°, respectively. Based on the experimental and theoretical studies, one can conclude that Beta can be obtained using imidazole containing cyclic SDA, when two imidazole rings make an angle of 110°. Such an observation can lead us to conclude that structural rigidity is an important factor, which leads to the formation of a zeolite of suitable framework structure. Since MTW is formed by IL4–IL6, hence it can be concluded that such structural rigidity is not a prerequisite for the formation of the MTW framework.
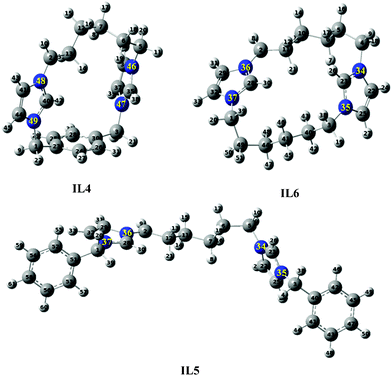 |
| | Fig. 3 Optimized structure of IL4–IL6 using B3LYP/6-31G. | |
 |
| | Fig. 4 Optimized structures of IL7 and IL8 using B3LYP/6-31G. | |
 |
| | Fig. 5 Optimized structures of IL9 and IL10 using B3LYP/6-31G. | |
Under the synthesis composition 30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xAl2O3
xAl2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL11
10IL11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O (with Si/Al < 100), zeolite Beta (Beta-IL11-1 and Beta-IL11-2) was obtained, when IL11 was used as the SDA (Fig. 6a, Table 3). The N2-adsorption study shows that Beta-IL11-1 exhibited a type IV isotherm with H2 hysteresis loop corresponding to capillary condensation in the mesopores (Fig. 6b). Furthermore, the zeolite exhibited a broad distribution of mesopore diameters (2.7 to 8.3 nm). The crystallite size of Beta-IL11-1 calculated from XRD was found to be 13.5 Å, which matches well with the TEM investigation (Fig. 6c). Crystallite size, surface area, and pore volume of Beta-IL11-2 were found to be 17.5 Å, 645 m2 g−1, and 0.79 mL g−1, respectively (Table 3). The influence of the amount of IL11 in the synthesis was investigated. It was found that, when the synthesis was performed using SDA
6000H2O (with Si/Al < 100), zeolite Beta (Beta-IL11-1 and Beta-IL11-2) was obtained, when IL11 was used as the SDA (Fig. 6a, Table 3). The N2-adsorption study shows that Beta-IL11-1 exhibited a type IV isotherm with H2 hysteresis loop corresponding to capillary condensation in the mesopores (Fig. 6b). Furthermore, the zeolite exhibited a broad distribution of mesopore diameters (2.7 to 8.3 nm). The crystallite size of Beta-IL11-1 calculated from XRD was found to be 13.5 Å, which matches well with the TEM investigation (Fig. 6c). Crystallite size, surface area, and pore volume of Beta-IL11-2 were found to be 17.5 Å, 645 m2 g−1, and 0.79 mL g−1, respectively (Table 3). The influence of the amount of IL11 in the synthesis was investigated. It was found that, when the synthesis was performed using SDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 = 1
SiO2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 instead of 1
20 instead of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Beta (designated as Beta-IL11-3) synthesis took a longer time (4 d). Crystallite size of the Beta-IL11-3 calculated from XRD was found to be larger (16.3 Å) than Beta-IL11-1, whereas surface area and pore volume of Beta-IL11-3 was found to be less compared to Beta-IL11-1 (Table 3). When synthesis was performed using SDA
10, Beta (designated as Beta-IL11-3) synthesis took a longer time (4 d). Crystallite size of the Beta-IL11-3 calculated from XRD was found to be larger (16.3 Å) than Beta-IL11-1, whereas surface area and pore volume of Beta-IL11-3 was found to be less compared to Beta-IL11-1 (Table 3). When synthesis was performed using SDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 = 1
SiO2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, Beta did not form even after 4 d of hydrothermal treatment. Based on the above investigation, it can be concluded that a good quality Beta sample can be obtained when SDA
40, Beta did not form even after 4 d of hydrothermal treatment. Based on the above investigation, it can be concluded that a good quality Beta sample can be obtained when SDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 = 1
SiO2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. When the synthesis was carried out at 403 K instead of 443 K, 7 d are required to form zeolite Beta (Beta-IL11-4) (Fig. 6a, Table 3). Based on these investigations one can conclude that either by decreasing the amount of SDA or by decreasing the synthesis temperature, the synthesis of Beta required a longer time. An interesting result was observed when the synthesis was performed using TEOS, which required 4 d to form zeolite Beta (Beta-IL11-5) (Fig. 7a). The crystallite size of Beta-IL11-5 was found to be comparatively larger (24 Å) than Beta-IL11-1. The N2-sorption study shows that Beta-IL11-5 exhibited a type IV isotherm with H3 hysteresis loop corresponding to capillary condensation in mesopores (Fig. 6b). Capillary condensation in Beta-IL11-5 was less prominent compared to Beta-IL11-1. Although zeolite Beta was obtained using Al(NO3)2 in 2 d (designated as Beta-IL11-6), it exhibits slightly less surface area and pore volume as compared to Beta-IL11-1 (Table 3). After evaluating all the synthesis parameters, it can be concluded that a high quality Beta sample can be obtained by using sodium silicate as the silica source and Al2(SO4)3 as the alumina source with the synthesis composition (30Na2O
10. When the synthesis was carried out at 403 K instead of 443 K, 7 d are required to form zeolite Beta (Beta-IL11-4) (Fig. 6a, Table 3). Based on these investigations one can conclude that either by decreasing the amount of SDA or by decreasing the synthesis temperature, the synthesis of Beta required a longer time. An interesting result was observed when the synthesis was performed using TEOS, which required 4 d to form zeolite Beta (Beta-IL11-5) (Fig. 7a). The crystallite size of Beta-IL11-5 was found to be comparatively larger (24 Å) than Beta-IL11-1. The N2-sorption study shows that Beta-IL11-5 exhibited a type IV isotherm with H3 hysteresis loop corresponding to capillary condensation in mesopores (Fig. 6b). Capillary condensation in Beta-IL11-5 was less prominent compared to Beta-IL11-1. Although zeolite Beta was obtained using Al(NO3)2 in 2 d (designated as Beta-IL11-6), it exhibits slightly less surface area and pore volume as compared to Beta-IL11-1 (Table 3). After evaluating all the synthesis parameters, it can be concluded that a high quality Beta sample can be obtained by using sodium silicate as the silica source and Al2(SO4)3 as the alumina source with the synthesis composition (30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5Al2O3
2.5Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL14
10IL14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O) in 2 d of hydrothermal treatment at 443 K. Though it was difficult to differentiate the morphology and particle sizes of various Beta samples prepared in this study using SEM, a clear differentiation can be obtained for the sample synthesized using TEOS and samples synthesized using sodium silicates (compare SEM images in Fig. 7b with Fig. 6c). A large particle size of about 1 μm was observed in the SEM micrograph of Beta-IL11-5, which is made up of several small nanocrystals. TEM investigation gives clear evidence that Beta-IL11-5 synthesized using TEOS is made up of small nanocrystals of about 25–28 nm (Fig. 7b), whereas the Beta-IL11-1 mesoporous framework is composed of zeolite nanocrystals of 14–15 nm in diameter (Fig. 6c). These diameters are in agreement with the crystallite sizes calculated from XRD by using the Scherrer equation.
6000H2O) in 2 d of hydrothermal treatment at 443 K. Though it was difficult to differentiate the morphology and particle sizes of various Beta samples prepared in this study using SEM, a clear differentiation can be obtained for the sample synthesized using TEOS and samples synthesized using sodium silicates (compare SEM images in Fig. 7b with Fig. 6c). A large particle size of about 1 μm was observed in the SEM micrograph of Beta-IL11-5, which is made up of several small nanocrystals. TEM investigation gives clear evidence that Beta-IL11-5 synthesized using TEOS is made up of small nanocrystals of about 25–28 nm (Fig. 7b), whereas the Beta-IL11-1 mesoporous framework is composed of zeolite nanocrystals of 14–15 nm in diameter (Fig. 6c). These diameters are in agreement with the crystallite sizes calculated from XRD by using the Scherrer equation.
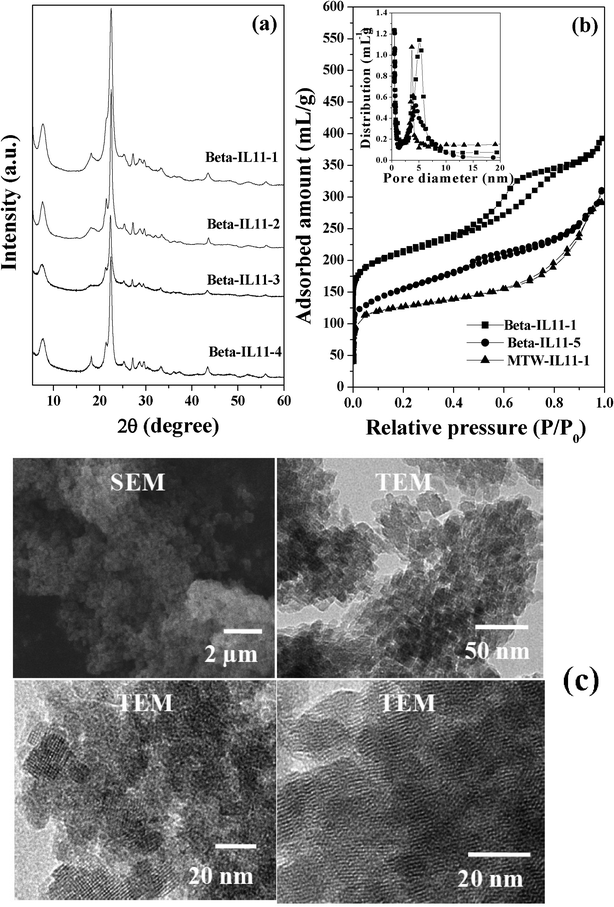 |
| | Fig. 6 (a) XRD patterns of various Beta samples synthesized using IL11 under different conditions, (b) N2-adsorption isotherms of Beta-IL11-1, Beta-IL11-5 and MTW-IL11-1 (inset shows pore size distribution), (c) SEM and TEM images of Beta-IL11-1. | |
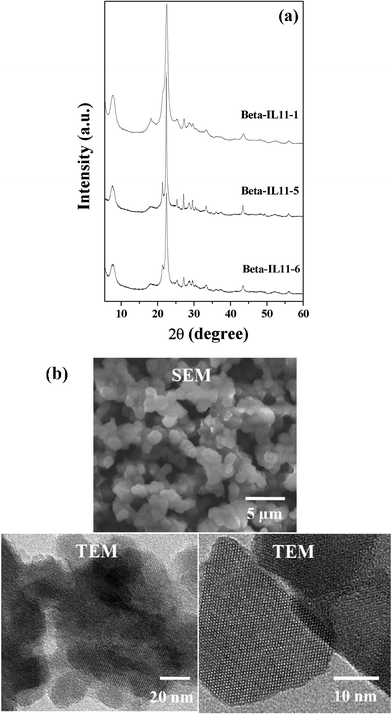 |
| | Fig. 7 (a) XRD patterns of Beta-IL11-1, Beta-IL11-5 and Beta-IL11-6, and (b) SEM and TEM images of Beta-IL11-5. | |
Under a variety of synthesis conditions, IL11 leads to the formation of zeolite Beta. However, when the synthesis was performed by using a large amount of H2SO4, or under high Si/Al condition (Si/Al > 100), it resulted in the formation of zeolite MTW. For example: under the same synthesis composition but without Al [(30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0Al2O3
0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL14
10IL14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O), IL11 forms MTW (MTW-IL11-1)] (Fig. 8a). When the amount of H2SO4 was increased from 15H2SO4 to 20H2SO4, then zeolite MTW was obtained (0 < Si/Al < ∞) (designated as MTW-IL11-2 and MTW-IL11-3). SEM investigations confirm that the morphology of samples is varied by varying the synthesis composition. MTW-IL11-2 exhibits nanowire like morphology, whereas other two samples of this series exhibited spheroidal morphology (Fig. 8c). The N2-adsorption study of MTW-IL11-1 shows that the material exhibited a type IV isotherm with a broad distribution of mesopore diameters (3 to 10 nm) (Fig. 6b). The hysteresis loop of MTW-IL11-1 confirms that the material has less mesopore volume compared to Beta-IL11-1. Textural properties obtained from the N2-adsorption studies are summarized in Table 1, which confirms that the MTW materials have less surface area and lower pore volumes compared to Beta samples synthesized using IL11. The formation of MTW zeolite at low Al content in the present work is consistent with the reported literature.40
6000H2O), IL11 forms MTW (MTW-IL11-1)] (Fig. 8a). When the amount of H2SO4 was increased from 15H2SO4 to 20H2SO4, then zeolite MTW was obtained (0 < Si/Al < ∞) (designated as MTW-IL11-2 and MTW-IL11-3). SEM investigations confirm that the morphology of samples is varied by varying the synthesis composition. MTW-IL11-2 exhibits nanowire like morphology, whereas other two samples of this series exhibited spheroidal morphology (Fig. 8c). The N2-adsorption study of MTW-IL11-1 shows that the material exhibited a type IV isotherm with a broad distribution of mesopore diameters (3 to 10 nm) (Fig. 6b). The hysteresis loop of MTW-IL11-1 confirms that the material has less mesopore volume compared to Beta-IL11-1. Textural properties obtained from the N2-adsorption studies are summarized in Table 1, which confirms that the MTW materials have less surface area and lower pore volumes compared to Beta samples synthesized using IL11. The formation of MTW zeolite at low Al content in the present work is consistent with the reported literature.40
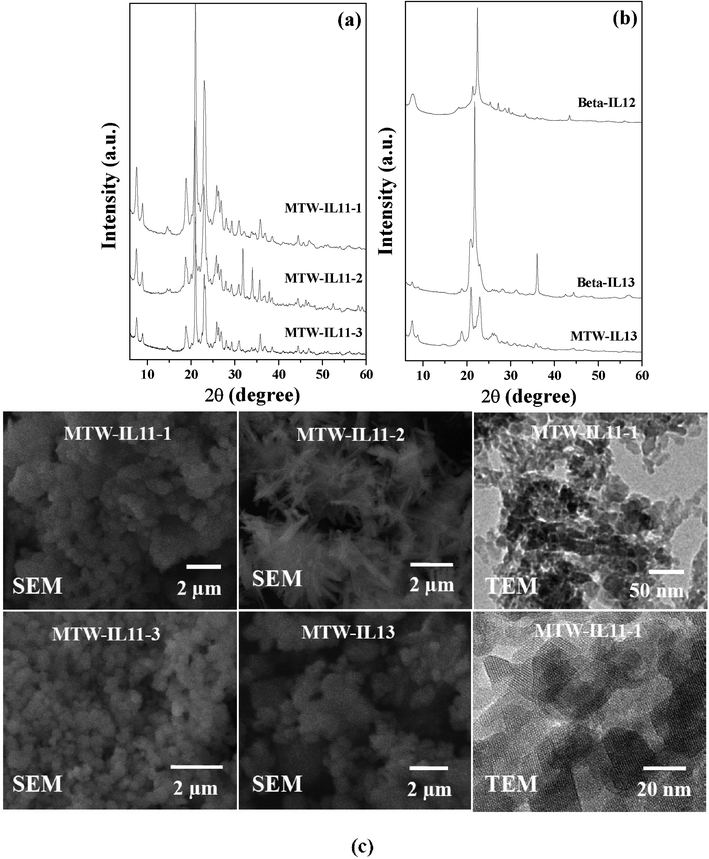 |
| | Fig. 8 (a) XRD patterns of MTW-IL11-1, MTW-IL11-2 and MTW-IL11-3, (b) XRD patterns of Beta-IL12, Beta-IL13 and MTW-IL13, (c) SEM images of MTW-IL11-1, MTW-IL11-2, MTW-IL11-3 and MTW-IL13 and TEM images of MTW-IL11-1. | |
Under the synthesis composition 30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xAl2O3
xAl2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL12
10IL12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O (with Si/Al < 50) using IL12, zeolite Beta was obtained (Beta-IL12). Either increasing the Si/Al or increasing the amount of H2SO4 leads to the formation of a mixture of zeolites (BETA + MTW). Beta (designated as Beta-IL13) was obtained using IL13 using the synthesis composition 30Na2O
6000H2O (with Si/Al < 50) using IL12, zeolite Beta was obtained (Beta-IL12). Either increasing the Si/Al or increasing the amount of H2SO4 leads to the formation of a mixture of zeolites (BETA + MTW). Beta (designated as Beta-IL13) was obtained using IL13 using the synthesis composition 30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0Al2O3
0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL13
10IL13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
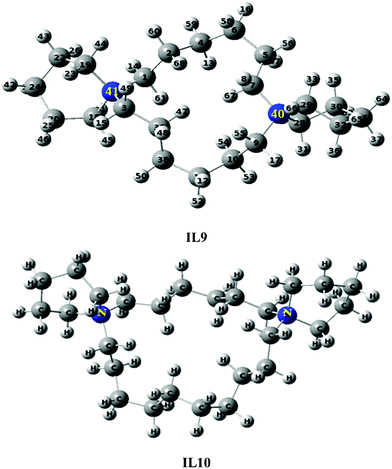
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O, in the absence of Al. Under high Al content (Si/Al < 50) or using a high concentration of H2SO4, no zeolite phase was obtained using IL13. Under low aluminium content (70 < Si/Al < ∞), MTW was obtained using IL13 (MTW-IL13). The textural properties obtained from the N2-adsorption studies are provided in Table 1 and Table 3. The structures of IL11–IL13 were optimized (Fig. 9) and their structural properties are provided in Table 2.
6000H2O, in the absence of Al. Under high Al content (Si/Al < 50) or using a high concentration of H2SO4, no zeolite phase was obtained using IL13. Under low aluminium content (70 < Si/Al < ∞), MTW was obtained using IL13 (MTW-IL13). The textural properties obtained from the N2-adsorption studies are provided in Table 1 and Table 3. The structures of IL11–IL13 were optimized (Fig. 9) and their structural properties are provided in Table 2.
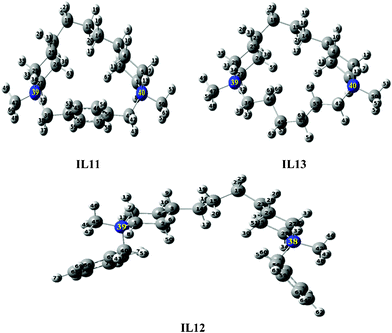 |
| | Fig. 9 Optimized structures of IL11–IL13 using B3LYP/6-31G. | |
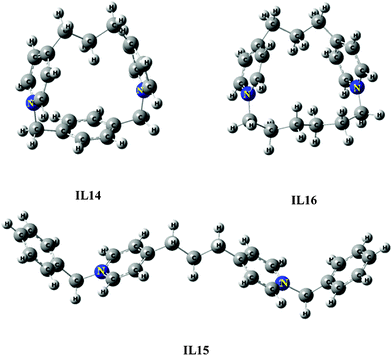 |
| | Fig. 10 Optimized structures of IL14–IL16 using B3LYP/6-31G. | |
Control experiments reveal that the initial gel phase (0 h) contained only 2.5 wt% of TEA+ and the final zeolite phase contained 7.7 wt% TEA+, when zeolite Beta was synthesized using conventional TEA+ as the SDA. This demonstrates that when using TEA+ as the SDA, Na+ was the predominant cation in the initial gel, and this was replaced with TEA+ after prolonged hydrothermal crystallization. The similar composition of IL11 in the initial phase (16.5%) and final zeolite phase (19.2%) suggests that the initial phase consists of zeolite like organized silicate species around IL11. Such a pre-organized zeolite like assembly is responsible for the facile synthesis of zeolite Beta using IL11. It is highly possible that the di-quaternary ammonium salts are able to generate a large number of zeolite seeds when compared to their mono-ammonium analogues TEA+ or 1-butyl,3-methyl-imidazolium cation.39 This was confirmed from the XRD analysis of the products obtained using IL11, which show that a pre-organized zeolite like assembly was formed during gelation (0 h, before crystallization) (Fig. 11). This process reduced the nucleation time and further crystal growth took place rapidly to form nanocrystalline zeolite Beta. The synthesis of nanocrystalline zeolites using di-quaternary ammonium salts indicates that the presence of two ammonium groups in the same molecule could be the most important factor for the generation of nanocrystalline morphologies and mesoporosity.
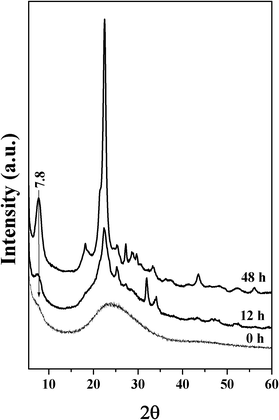 |
| | Fig. 11 XRD patterns of the product obtained during the crystallization of zeolite Beta using IL11. | |
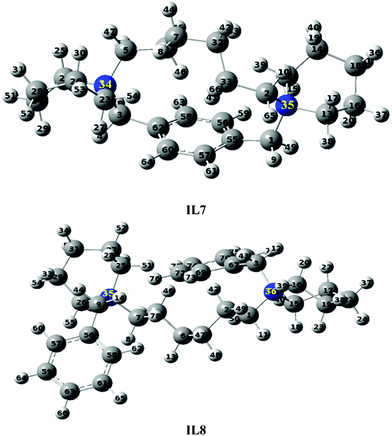
DFT calculations helped us to understand the ability and inability of the quaternary ammonium salts investigated in this study to form zeolite Beta. Very recently, it has been reported that for the formation of zeolite Beta the molecular dimension of the SDA should be less than the pore channel of the 12 member ring of Beta.26 However, our results, and previous reports26 clearly demonstrate that this may not be the necessary condition for the formation of Beta. To understand the reason behind our observations, the geometrically optimized structures were carefully analyzed. The piperidine rings of IL7–IL10 exist in twist-boat conformation, whereas the piperidine rings of IL11–IL13 exist in chair conformation. Since only IL11–IL13 are able to form zeolite Beta and MTW, we can conclude that a suitable conformation (chair form) is required for the formation of Beta and MTW. Such a limited conformational freedom is known as an important prerequisite for the selective structure direction of zeolites.50 The BOMD simulation method of Nose and Hoover indicates that conformational change by thermal rotation and vibration is greatly suppressed, even at zeolite synthesis temperature (443 K), due to rigid cyclic geometry. For instance, results of the simulation model for IL11 are provided in the ESI section (Fig. S2†). The molecular structure of IL11 (Fig. S3, ESI†) at different stages of the potential energy diagram confirms that the chair confirmation is stable even at the zeolite synthesis temperature (443 K). In the above section, it has been described that among the imidazole based SDA, only IL6 was able to form zeolite beta, in which two imidazole rings make an angle of 110°. However, all imidazole based SDA (IL4–IL6) were able to form zeolite MTW. Further, pyridine based SDA (IL14–IL16) were unable to form Beta or MTW. Based on these observations, one can conclude that structural rigidity varies from ring to ring and therefore their ability to form a suitable kind of zeolite framework also varies. After analyzing all the results, it can be concluded that the size of the SDA (investigated in this study) is not an important factor to modulate the crystallization process for a suitable kind of framework structure, but it requires a suitable special orientation of imidazole and piperidine rings, which enables the formation of a zeolite of a particular framework structure.
Conclusion
A variety of mono-quaternary and di-quaternary ammonium salts of imidazole, piperidine and pyridine were utilized as structure directing agents for the synthesis of zeolite Beta, MFI, and MTW. 4,4′-trimethylenebis(1-methylpiperidine) and imidazole based di-quaternary ammonium salts were found to be very effective SDAs for zeolites having a 12-membered pore channel, i.e., Beta and MTW, whereas the mono-quaternary ammonium salts of the imidazole based cyclic compounds generated MTW and nanocrystalline MFI zeolites. The zeolites thus obtained were shown to be nanocrystalline materials with high surface areas and large mesopore volumes. The presence of the mesopores between adjacent nanocrystals is known to be an important factor, leading to significantly improved molecular diffusion and hence catalytic performance (e.g., lifetime, activity and selectivity). The synthetic cost of Beta is very high due to the high cost for the preparation of organic SDAs in the hydroxide form. In this study, Beta was synthesized using low cost structure directing agents (in halide form), sodium silicate as the silica source, and aluminum sulphate as the aluminum source. Experimental evidence and DFT calculations suggest that structural rigidity along with a specific spatial orientation of imidazole and piperidine rings of quaternary ammonium salts are important factors for delivering zeolites of a particular framework structure.
Acknowledgements
The authors are thankful to the Department of Science and Technology, New Delhi, for financial assistance (DST grant SR/S1/PC/31/2009). We are grateful to Dr T. J. Dhilip Kumar, Chemistry Department, IIT Ropar for providing Gaussian 09 software and valuable suggestions. The authors are also thankful to Prof. M. K. Surappa, Director IIT Ropar for his constant encouragement.
References
- A. Corma, Chem. Rev., 1997, 97, 2373 CrossRef CAS.
- Y. Tao, H. Kanoh, L. Abrams and K. Kaneko, Chem. Rev., 2006, 106, 896 CrossRef CAS.
- G. Basca, Chem. Rev., 2007, 107, 5366 CrossRef.
- R. Rinaldi and F. Schüth, Energy Environ. Sci., 2009, 2, 610 CAS.
- C. S. Cundy and P. A. Cox, Chem. Rev., 2003, 103, 663 CrossRef CAS.
- J. S. Beck, J. C. Vartuli, G. J. Kennedy, C. T. Kresge, W. J. Roth and S. E. Schramm, Chem. Mater., 1994, 6, 1816 CrossRef CAS.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Frederickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS.
- P. T. Tanev and T. J. Pinnavaia, Science, 1995, 267, 865 CAS.
- T. W. Kim, F. Klitz, B. Paul and R. Ryoo, J. Am. Chem. Soc., 2005, 127, 7601 CrossRef CAS.
- J. Pérez-Ramírez, C. H. Christensen, K. Egeblad, C. H. Christensen and J. C. Groen, Chem. Soc. Rev., 2008, 37, 2530 RSC.
- A. H. Janssen, A. J. Koster and K. P. de Jong, Angew. Chem., Int. Ed., 2001, 40, 1102 CrossRef CAS.
- R. Srivastava, N. Iwasa, S-I. Fujita and M. Ara, Catal. Lett., 2009, 130, 655 CrossRef CAS.
- B. T. Holland, L. Abrams and A. Stein, J. Am. Chem. Soc., 1999, 121, 4308 CrossRef CAS.
- C. J. H. Jacobsen, C. Madsen, J. Houzvicka, I. Schmidt and A. Carlsson, J. Am. Chem. Soc., 2000, 122, 7116 CrossRef CAS.
- Y. S. Tao, H. Kanoh and K. Kaneko, J. Am. Chem. Soc., 2003, 125, 6044 CrossRef CAS.
- K. Egeblad, H. Christina Christensen, M. Kustova and C. H. Christensen, Chem. Mater., 2008, 20, 946 CrossRef CAS.
- A. Sakthivel, S. J. Huang, W. H. Chen, Z. H. Lan, K. H. Chen, T. W. Kim and R. Ryoo, Chem. Mater., 2004, 16, 3168 CrossRef CAS.
- I. Schmidt, A. Boisen, E. Gustavsson, K. Stáhl, S. Pehrson, S. Dahl, A. Carlsson and C. J. H. Jacobsen, Chem. Mater., 2001, 13, 4416 CrossRef CAS.
- M. Choi, H. S. Cho, R. Srivastava, C. Venkatesan, D. H. Choi and R. Ryoo, Nat. Mater., 2006, 5, 718 CrossRef CAS.
- R. Srivastava, M. Choi and R. Ryoo, Chem. Commun., 2006, 4489 RSC.
-
R. Srivastava, N. Iwasa, S-I. Fujita and M. Arai, in Progress in porous media Research, ed. K. S. Tian and H. J. Shu, Nova Science Publisher, 2009, Chapter 1, 1-53 Search PubMed.
- R. Srivastava, N. Iwasa, S. I. Fujita and M. Arai, Chem.–Eur. J., 2008, 14, 9507 CrossRef CAS.
- K. Na, C. Jo, J. Kim, K. Cho, J. Jung, Y. Seo, R. J. Messinger, B. F. Chmelka and R. Ryoo, Science, 2011, 333, 328 CrossRef CAS.
- W. Park, D. Yu, K. Na, K. E. Jelfs, B. Slater, Y. Sakamoto and R. Ryoo, Chem. Mater., 2011, 23, 5131 CrossRef CAS.
- M. Choi, K. Na and R. Ryoo, Chem. Commun., 2009, 2845 RSC.
- K. Na, M. Choi and R. Ryoo, J. Mater. Chem., 2009, 19, 6713 RSC.
- D. P. Serrano, J. Aguado, J. M. Escola, J. M. Rodrìguez and A. Peral, Chem. Mater., 2006, 18, 2462 CrossRef CAS.
- A. Sakthivel, A. Iida, K. Komura, Y. Sugi and K. V. R. Chary, Microporous Mesoporous Mater., 2009, 119, 322 CrossRef CAS.
- R. Kore and R. Srivastava, J. Mol. Catal. A: Chem., 2011, 345, 117 CrossRef CAS.
- R. Kore and R. Srivastava, Catal. Commun., 2011, 12, 1420 CrossRef CAS.
- R. Kore and R. Srivastava, Tetrahedron Lett., 2012, 53, 3245 CrossRef CAS.
- R. Kore, T. J. D. Kumar and R. Srivastava, J. Mol. Catal. A: Chem., 2012, 360, 61 CrossRef CAS.
- R. Kore and R. Srivastava, Catal. Commun., 2012, 18, 11 CrossRef CAS.
- R. Kore, B. Satpati and R. Srivastava, Chem.–Eur. J., 2011, 17, 14360 CrossRef CAS.
- J. E. Bara, Ind. Eng. Chem. Res., 2011, 50, 13614 CrossRef CAS.
- M. A. Camblor and J. P. Pariente, Zeolites, 1991, 11, 202 CrossRef CAS.
-
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford CT, Revision A.1, 2009 Search PubMed.
- L. M. Marcelo, O. S. Michele, B. C. P. Sibele, F. S. Roberto and B. G. Katia, Appl. Catal., A, 2010, 374, 26 CrossRef.
- P. M. Piccione, S. Yang, A. Navrotsky and M. E. Davis, J. Phys. Chem. B, 2002, 106, 3629 CrossRef CAS.
- A. Corma, M. Moliner, A. Cantin, M. J. Diaz-Cabanas, J. L. Jorda, D. Zhang, J. Sun, K. Jansson, S. Hovmoller and X. Zou, Chem. Mater., 2008, 20, 3218 CrossRef CAS.
- M. D. Kadgaonkar, M. W. Kasture, D. S. Bhange, P. N. Joshi, V. Ramaswamy and R. Kumar, Microporous Mesoporous Mater., 2007, 101, 108 CrossRef CAS.
- M. Antonietti, D. B. Kuang, B. Smarsly and Z. Yong, Angew. Chem., Int. Ed., 2004, 43, 4988 CrossRef CAS.
- A. K. Boal, F. Ilhan, J. E. DeRouchey, T. T. Albrecht, T. P. Russell and V. M. Rotello, Nature, 2000, 404, 746 CrossRef CAS.
- J. Jin, T. Iyoda, C. Cao, Y. Song, L. Jiang, T. Li and D. Zhu, Angew. Chem., Int. Ed., 2001, 40, 2135 CrossRef CAS.
- R. P. Andres, J. D. Biefield, J. I. Henderson, D. B. Janes, V. R. Kolagunta, C. P. Kubiak, W. J. Mahoney and R. G. Osifchin, Science, 1996, 273, 1690 CrossRef CAS.
- B. G. Trewyn, C. M. Whitman and V. S. Y. Lin, Nano Lett., 2004, 4, 2139 CrossRef CAS.
- Y. Zhou, J. H. Schattka and M. Antonietti, Nano Lett., 2004, 4, 477 CrossRef CAS.
- Y. Liu, J. Li, M. Wang, Z. Li, H. Liu, P. He, X. Yang and J. Li, Cryst. Growth Des., 2005, 5, 1643 CAS.
- Y. Kubota, M. M. Helmkamp, S. I. Zones and M. E. Davis, Microporous Mater., 1996, 6, 213 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20437a |
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30Na2O. Two kinds of aluminum source, Al2(SO4)3 and Al(NO3)3, were investigated in this study. The aluminum source was dissolved in dilute sulfuric acid (x
30Na2O. Two kinds of aluminum source, Al2(SO4)3 and Al(NO3)3, were investigated in this study. The aluminum source was dissolved in dilute sulfuric acid (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) z
z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500, Aluminum source
1500, Aluminum source![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2SO4
H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O). The two solutions were mixed via vigorous stirring at room temperature. The resultant mixture was a clear solution. When IL was added slowly into this solution, under vigorous stirring at room temperature, the mixture gelled immediately. The molar gel composition can be represented as 30Na2O
H2O). The two solutions were mixed via vigorous stirring at room temperature. The resultant mixture was a clear solution. When IL was added slowly into this solution, under vigorous stirring at room temperature, the mixture gelled immediately. The molar gel composition can be represented as 30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xAl2O3
xAl2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) yIL
yIL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zH2SO4
zH2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O (where 0 ≤ x ≤ 2.5, 0 ≤ y ≤ 10, z = 15 or 20). After aging for 6 h at room temperature, the reaction mixture converted to a free flow liquid gel (less viscous), which was transferred to a Teflon-coated stainless-steel autoclave (100 mL capacity) and heated at 443 K with stirring. Precipitates were filtered, washed with deionised water, dried at 383 K, and calcined at 823 K for 4 h under flowing air. For the synthesis of Beta using tetraethylorthosilicate (TEOS), the required amount of H2SO4, followed by IL11 was added to a conventional alkaline mixture containing NaOH, TEOS, and distilled water to make the molar composition 30Na2O:2.5Al2O3:100SiO2:10IL11:15H2SO4:6000H2O. When TEOS was used as silica source, ethanol formed was removed (after 6 h of reaction) during the hydrolysis of TEOS, before being loaded into autoclave. Conventional MFI34 and Beta37 were synthesized by following the reported procedure.
6000H2O (where 0 ≤ x ≤ 2.5, 0 ≤ y ≤ 10, z = 15 or 20). After aging for 6 h at room temperature, the reaction mixture converted to a free flow liquid gel (less viscous), which was transferred to a Teflon-coated stainless-steel autoclave (100 mL capacity) and heated at 443 K with stirring. Precipitates were filtered, washed with deionised water, dried at 383 K, and calcined at 823 K for 4 h under flowing air. For the synthesis of Beta using tetraethylorthosilicate (TEOS), the required amount of H2SO4, followed by IL11 was added to a conventional alkaline mixture containing NaOH, TEOS, and distilled water to make the molar composition 30Na2O:2.5Al2O3:100SiO2:10IL11:15H2SO4:6000H2O. When TEOS was used as silica source, ethanol formed was removed (after 6 h of reaction) during the hydrolysis of TEOS, before being loaded into autoclave. Conventional MFI34 and Beta37 were synthesized by following the reported procedure.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio, amount of sulfuric acid, role of silica and aluminum source, and hydrothermal temperatures were investigated. Zeolites were synthesized under strong basic conditions using the molar composition 30Na2O
Al molar ratio, amount of sulfuric acid, role of silica and aluminum source, and hydrothermal temperatures were investigated. Zeolites were synthesized under strong basic conditions using the molar composition 30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xAl2O3
xAl2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) yIL
yIL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zH2SO4
zH2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O, where 0 ≤ x ≤ 2.5, y = 0–10, z = 15 or 20. We are interested in obtaining the synthesis conditions by which zeolite materials having Beta, MFI, and MTW framework structures with high surface area and mesoporosity are obtained. The XRD patterns of the as synthesized form of the zeolites (Beta, MFI and MTW) obtained using IL are similar to the XRD patterns of the calcined materials. The XRD patterns match well with the reported XRD pattern of zeolites (Beta, MFI and MTW), which clearly indicates the high purity of the obtained zeolite materials. CHN analysis confirms that only a negligible amount of organic content (<0.1%) is present in the calcined form of the zeolite.
6000H2O, where 0 ≤ x ≤ 2.5, y = 0–10, z = 15 or 20. We are interested in obtaining the synthesis conditions by which zeolite materials having Beta, MFI, and MTW framework structures with high surface area and mesoporosity are obtained. The XRD patterns of the as synthesized form of the zeolites (Beta, MFI and MTW) obtained using IL are similar to the XRD patterns of the calcined materials. The XRD patterns match well with the reported XRD pattern of zeolites (Beta, MFI and MTW), which clearly indicates the high purity of the obtained zeolite materials. CHN analysis confirms that only a negligible amount of organic content (<0.1%) is present in the calcined form of the zeolite.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.625Al2O3
0.625Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL3
10IL3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 or 20H2SO4
15 or 20H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O (Fig. 1a). N2-adsorption studies show that both materials exhibited type IV isotherms with H4 hysteresis (Fig. 1b), which shows that the material contains both micropores and mesopores. The isotherm of MFI-IL3-1 clearly evidences capillary condensation in the mesopores of the material, which is reflected in their textural properties (Table 1). The morphology of MFI-IL3-1 and MFI-IL3-2 was found to be spheroidal (Fig. 1c). The particle sizes of MFI-IL3-1 were found to be smaller than those of MFI-IL3-2. Agglomeration of the small particles is reflected in the properties of the obtained materials. Due to the relatively small particle size, the surface area of MFI-IL3-1 was found to be larger when compared to MFI-IL3-2. The agglomeration of the small particles in MFI-IL3-1 is reflected in its higher mesopore volume compared to MFI-IL3-2. Mesoporous void spaces are formed due to the crystal packing of these small particles. It is, further, interesting to note that when the synthesis was performed in the absence of Al (30Na2O
6000H2O (Fig. 1a). N2-adsorption studies show that both materials exhibited type IV isotherms with H4 hysteresis (Fig. 1b), which shows that the material contains both micropores and mesopores. The isotherm of MFI-IL3-1 clearly evidences capillary condensation in the mesopores of the material, which is reflected in their textural properties (Table 1). The morphology of MFI-IL3-1 and MFI-IL3-2 was found to be spheroidal (Fig. 1c). The particle sizes of MFI-IL3-1 were found to be smaller than those of MFI-IL3-2. Agglomeration of the small particles is reflected in the properties of the obtained materials. Due to the relatively small particle size, the surface area of MFI-IL3-1 was found to be larger when compared to MFI-IL3-2. The agglomeration of the small particles in MFI-IL3-1 is reflected in its higher mesopore volume compared to MFI-IL3-2. Mesoporous void spaces are formed due to the crystal packing of these small particles. It is, further, interesting to note that when the synthesis was performed in the absence of Al (30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0Al2O3
0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL3
10IL3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O), MTW was obtained (designated as MTW-IL3, Fig. 1a). MFI-IL3-1 exhibited significantly larger surface area and mesopore volume compared to conventional MFI, which signifies that MFI-IL3-1 is nanocrytsalline in nature. One structure directing agent is able to form zeolites of two different framework structures (MFI and MTW). The structure of IL3 was optimized using density functional theory (DFT) method employing B3LYP with 6-31G basis set (Fig. 1d and Table 2).38 The molecular dimension of IL3 is larger than the pore apertures of the MFI and MTW zeolites (maximum diameters of a sphere that can be included in MFI and MTW framework structure are 6.36 and 6.08 Å, respectively). Hence, theoretical study confirms that the size of the SDA is not the important factor for the synthesis of MFI and MTW structures. Very recently, it has been reported that for the formation of zeolite Beta, the molecular dimension of the SDA should be less than the pore channel of the 12 member ring of Beta (pore dimensions: 6.6 × 6.7 Å for [100] and 5.6 × 5.6 Å for [001]).26 The molecular size of the IL3 is less than the pore aperture of zeolite Beta, but it was unable to form zeolite Beta. Therefore, it can be concluded that the critical size of the SDA is not the only necessary condition for the formation of zeolite Beta.
6000H2O), MTW was obtained (designated as MTW-IL3, Fig. 1a). MFI-IL3-1 exhibited significantly larger surface area and mesopore volume compared to conventional MFI, which signifies that MFI-IL3-1 is nanocrytsalline in nature. One structure directing agent is able to form zeolites of two different framework structures (MFI and MTW). The structure of IL3 was optimized using density functional theory (DFT) method employing B3LYP with 6-31G basis set (Fig. 1d and Table 2).38 The molecular dimension of IL3 is larger than the pore apertures of the MFI and MTW zeolites (maximum diameters of a sphere that can be included in MFI and MTW framework structure are 6.36 and 6.08 Å, respectively). Hence, theoretical study confirms that the size of the SDA is not the important factor for the synthesis of MFI and MTW structures. Very recently, it has been reported that for the formation of zeolite Beta, the molecular dimension of the SDA should be less than the pore channel of the 12 member ring of Beta (pore dimensions: 6.6 × 6.7 Å for [100] and 5.6 × 5.6 Å for [001]).26 The molecular size of the IL3 is less than the pore aperture of zeolite Beta, but it was unable to form zeolite Beta. Therefore, it can be concluded that the critical size of the SDA is not the only necessary condition for the formation of zeolite Beta.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.625Al2O3
0.625Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL3
10IL3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O
6000H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.625Al2O3
0.625Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL3
10IL3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20H2SO4
20H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O
6000H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.625Al2O3
0.625Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10TPABr
10TPABr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20H2SO4
20H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O
6000H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0Al2O3
0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL3
10IL3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O
6000H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.625Al2O3
0.625Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL3
10IL3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20H2SO4
20H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O
6000H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.625Al2O3
0.625Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL3
10IL3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O
6000H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0Al2O3
0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL11
10IL11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O
6000H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.625Al2O3
0.625Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL11
10IL11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20H2SO4
20H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O
6000H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0Al2O3
0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL11
10IL11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20H2SO4
20H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O
6000H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.63Al2O3
0.63Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL16
10IL16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O
6000H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xAl2O3
xAl2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL
10IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 or 20H2SO4
15 or 20H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O; 75 < Si/Al < ∞). It was observed that MTW was formed under low Al content and this is consistent with the reported literature, which states that MTW is thermodynamically favored under high-silica synthesis conditions.40 In this manuscript, physico-chemical data is provided only for MTW synthesized using IL6 (MTW-IL6-1, MTW-IL6-2) (Fig. 2a and Table 1).
6000H2O; 75 < Si/Al < ∞). It was observed that MTW was formed under low Al content and this is consistent with the reported literature, which states that MTW is thermodynamically favored under high-silica synthesis conditions.40 In this manuscript, physico-chemical data is provided only for MTW synthesized using IL6 (MTW-IL6-1, MTW-IL6-2) (Fig. 2a and Table 1).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5Al2O3
2.5Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL6
10IL6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O (Fig. 2a), whereas IL4 and IL5 were unable to form any crystalline phase under this synthesis condition. The broad nature of the XRD peak of Beta-IL6 clearly shows that its crystal sizes are smaller than conventional Beta (Table 3). Zeolite Beta obtained in this condition is not the traditional zeolite Beta but a mixture of polymorphs of A and B phases. Traditional zeolite Beta is highly disordered. It is made of the random intergrowth of polymorph A and polymorph B in the ratio of ca. 45
6000H2O (Fig. 2a), whereas IL4 and IL5 were unable to form any crystalline phase under this synthesis condition. The broad nature of the XRD peak of Beta-IL6 clearly shows that its crystal sizes are smaller than conventional Beta (Table 3). Zeolite Beta obtained in this condition is not the traditional zeolite Beta but a mixture of polymorphs of A and B phases. Traditional zeolite Beta is highly disordered. It is made of the random intergrowth of polymorph A and polymorph B in the ratio of ca. 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55.41,42 Both the polymorphs A and B are constructed from the same centrosymmetric, tertiary building unit (TBU) arranged in layers successively, which interconnect in either a left handed (L) or right handed (R) manner. Polymorph A represents an uninterrupted sequence of either left handed or right handed, whereas the polymorph B has an alternative stacking sequence. TBU has no preference for either mode of connection, which leads to an almost equal possibility of each in the structure of Beta, which enhances the growth of a faulty structure. It is reported in the literature that if a mixture of polymorph A and polymorph B of zeolite beta is formed then the broad peak at low angle splits into two sharp peaks of different intensity showing their percentage composition in the structure.41,42 Based on the XRD obtained for Beta-IL6, one can say that Beta-IL6 consists of mixture of polymorph B and polymorph A. The N2-adsorption study shows that Beta-IL6 exhibited a type IV isotherm with a hysteresis loop corresponding to capillary condensation in the mesopores (Fig. 2b). Furthermore, the zeolite exhibited a bi-modal distribution of mesopores in the range of 3.1 to 6.3 nm. N2-adsorption studies show that the material has very high surface area and mesopore volume compared to conventional Beta (Table 3). SEM and TEM analysis showed that Beta-IL6 is composed of zeolite nanocrystals of 16-18 nm of diameter (Fig. 2c).
55.41,42 Both the polymorphs A and B are constructed from the same centrosymmetric, tertiary building unit (TBU) arranged in layers successively, which interconnect in either a left handed (L) or right handed (R) manner. Polymorph A represents an uninterrupted sequence of either left handed or right handed, whereas the polymorph B has an alternative stacking sequence. TBU has no preference for either mode of connection, which leads to an almost equal possibility of each in the structure of Beta, which enhances the growth of a faulty structure. It is reported in the literature that if a mixture of polymorph A and polymorph B of zeolite beta is formed then the broad peak at low angle splits into two sharp peaks of different intensity showing their percentage composition in the structure.41,42 Based on the XRD obtained for Beta-IL6, one can say that Beta-IL6 consists of mixture of polymorph B and polymorph A. The N2-adsorption study shows that Beta-IL6 exhibited a type IV isotherm with a hysteresis loop corresponding to capillary condensation in the mesopores (Fig. 2b). Furthermore, the zeolite exhibited a bi-modal distribution of mesopores in the range of 3.1 to 6.3 nm. N2-adsorption studies show that the material has very high surface area and mesopore volume compared to conventional Beta (Table 3). SEM and TEM analysis showed that Beta-IL6 is composed of zeolite nanocrystals of 16-18 nm of diameter (Fig. 2c).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xAl2O3
xAl2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) yIL
yIL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zH2SO4
zH2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O
6000H2O
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y
y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) z
z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10:15
10:15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10:15
10:15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10:15
10:15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10:15
10:15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10:15
10:15


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xAl2O3
xAl2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL11
10IL11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O (with Si/Al < 100), zeolite Beta (Beta-IL11-1 and Beta-IL11-2) was obtained, when IL11 was used as the SDA (Fig. 6a, Table 3). The N2-adsorption study shows that Beta-IL11-1 exhibited a type IV isotherm with H2 hysteresis loop corresponding to capillary condensation in the mesopores (Fig. 6b). Furthermore, the zeolite exhibited a broad distribution of mesopore diameters (2.7 to 8.3 nm). The crystallite size of Beta-IL11-1 calculated from XRD was found to be 13.5 Å, which matches well with the TEM investigation (Fig. 6c). Crystallite size, surface area, and pore volume of Beta-IL11-2 were found to be 17.5 Å, 645 m2 g−1, and 0.79 mL g−1, respectively (Table 3). The influence of the amount of IL11 in the synthesis was investigated. It was found that, when the synthesis was performed using SDA
6000H2O (with Si/Al < 100), zeolite Beta (Beta-IL11-1 and Beta-IL11-2) was obtained, when IL11 was used as the SDA (Fig. 6a, Table 3). The N2-adsorption study shows that Beta-IL11-1 exhibited a type IV isotherm with H2 hysteresis loop corresponding to capillary condensation in the mesopores (Fig. 6b). Furthermore, the zeolite exhibited a broad distribution of mesopore diameters (2.7 to 8.3 nm). The crystallite size of Beta-IL11-1 calculated from XRD was found to be 13.5 Å, which matches well with the TEM investigation (Fig. 6c). Crystallite size, surface area, and pore volume of Beta-IL11-2 were found to be 17.5 Å, 645 m2 g−1, and 0.79 mL g−1, respectively (Table 3). The influence of the amount of IL11 in the synthesis was investigated. It was found that, when the synthesis was performed using SDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 = 1
SiO2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 instead of 1
20 instead of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Beta (designated as Beta-IL11-3) synthesis took a longer time (4 d). Crystallite size of the Beta-IL11-3 calculated from XRD was found to be larger (16.3 Å) than Beta-IL11-1, whereas surface area and pore volume of Beta-IL11-3 was found to be less compared to Beta-IL11-1 (Table 3). When synthesis was performed using SDA
10, Beta (designated as Beta-IL11-3) synthesis took a longer time (4 d). Crystallite size of the Beta-IL11-3 calculated from XRD was found to be larger (16.3 Å) than Beta-IL11-1, whereas surface area and pore volume of Beta-IL11-3 was found to be less compared to Beta-IL11-1 (Table 3). When synthesis was performed using SDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 = 1
SiO2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, Beta did not form even after 4 d of hydrothermal treatment. Based on the above investigation, it can be concluded that a good quality Beta sample can be obtained when SDA
40, Beta did not form even after 4 d of hydrothermal treatment. Based on the above investigation, it can be concluded that a good quality Beta sample can be obtained when SDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 = 1
SiO2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. When the synthesis was carried out at 403 K instead of 443 K, 7 d are required to form zeolite Beta (Beta-IL11-4) (Fig. 6a, Table 3). Based on these investigations one can conclude that either by decreasing the amount of SDA or by decreasing the synthesis temperature, the synthesis of Beta required a longer time. An interesting result was observed when the synthesis was performed using TEOS, which required 4 d to form zeolite Beta (Beta-IL11-5) (Fig. 7a). The crystallite size of Beta-IL11-5 was found to be comparatively larger (24 Å) than Beta-IL11-1. The N2-sorption study shows that Beta-IL11-5 exhibited a type IV isotherm with H3 hysteresis loop corresponding to capillary condensation in mesopores (Fig. 6b). Capillary condensation in Beta-IL11-5 was less prominent compared to Beta-IL11-1. Although zeolite Beta was obtained using Al(NO3)2 in 2 d (designated as Beta-IL11-6), it exhibits slightly less surface area and pore volume as compared to Beta-IL11-1 (Table 3). After evaluating all the synthesis parameters, it can be concluded that a high quality Beta sample can be obtained by using sodium silicate as the silica source and Al2(SO4)3 as the alumina source with the synthesis composition (30Na2O
10. When the synthesis was carried out at 403 K instead of 443 K, 7 d are required to form zeolite Beta (Beta-IL11-4) (Fig. 6a, Table 3). Based on these investigations one can conclude that either by decreasing the amount of SDA or by decreasing the synthesis temperature, the synthesis of Beta required a longer time. An interesting result was observed when the synthesis was performed using TEOS, which required 4 d to form zeolite Beta (Beta-IL11-5) (Fig. 7a). The crystallite size of Beta-IL11-5 was found to be comparatively larger (24 Å) than Beta-IL11-1. The N2-sorption study shows that Beta-IL11-5 exhibited a type IV isotherm with H3 hysteresis loop corresponding to capillary condensation in mesopores (Fig. 6b). Capillary condensation in Beta-IL11-5 was less prominent compared to Beta-IL11-1. Although zeolite Beta was obtained using Al(NO3)2 in 2 d (designated as Beta-IL11-6), it exhibits slightly less surface area and pore volume as compared to Beta-IL11-1 (Table 3). After evaluating all the synthesis parameters, it can be concluded that a high quality Beta sample can be obtained by using sodium silicate as the silica source and Al2(SO4)3 as the alumina source with the synthesis composition (30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5Al2O3
2.5Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL14
10IL14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O) in 2 d of hydrothermal treatment at 443 K. Though it was difficult to differentiate the morphology and particle sizes of various Beta samples prepared in this study using SEM, a clear differentiation can be obtained for the sample synthesized using TEOS and samples synthesized using sodium silicates (compare SEM images in Fig. 7b with Fig. 6c). A large particle size of about 1 μm was observed in the SEM micrograph of Beta-IL11-5, which is made up of several small nanocrystals. TEM investigation gives clear evidence that Beta-IL11-5 synthesized using TEOS is made up of small nanocrystals of about 25–28 nm (Fig. 7b), whereas the Beta-IL11-1 mesoporous framework is composed of zeolite nanocrystals of 14–15 nm in diameter (Fig. 6c). These diameters are in agreement with the crystallite sizes calculated from XRD by using the Scherrer equation.
6000H2O) in 2 d of hydrothermal treatment at 443 K. Though it was difficult to differentiate the morphology and particle sizes of various Beta samples prepared in this study using SEM, a clear differentiation can be obtained for the sample synthesized using TEOS and samples synthesized using sodium silicates (compare SEM images in Fig. 7b with Fig. 6c). A large particle size of about 1 μm was observed in the SEM micrograph of Beta-IL11-5, which is made up of several small nanocrystals. TEM investigation gives clear evidence that Beta-IL11-5 synthesized using TEOS is made up of small nanocrystals of about 25–28 nm (Fig. 7b), whereas the Beta-IL11-1 mesoporous framework is composed of zeolite nanocrystals of 14–15 nm in diameter (Fig. 6c). These diameters are in agreement with the crystallite sizes calculated from XRD by using the Scherrer equation.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0Al2O3
0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL14
10IL14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O), IL11 forms MTW (MTW-IL11-1)] (Fig. 8a). When the amount of H2SO4 was increased from 15H2SO4 to 20H2SO4, then zeolite MTW was obtained (0 < Si/Al < ∞) (designated as MTW-IL11-2 and MTW-IL11-3). SEM investigations confirm that the morphology of samples is varied by varying the synthesis composition. MTW-IL11-2 exhibits nanowire like morphology, whereas other two samples of this series exhibited spheroidal morphology (Fig. 8c). The N2-adsorption study of MTW-IL11-1 shows that the material exhibited a type IV isotherm with a broad distribution of mesopore diameters (3 to 10 nm) (Fig. 6b). The hysteresis loop of MTW-IL11-1 confirms that the material has less mesopore volume compared to Beta-IL11-1. Textural properties obtained from the N2-adsorption studies are summarized in Table 1, which confirms that the MTW materials have less surface area and lower pore volumes compared to Beta samples synthesized using IL11. The formation of MTW zeolite at low Al content in the present work is consistent with the reported literature.40
6000H2O), IL11 forms MTW (MTW-IL11-1)] (Fig. 8a). When the amount of H2SO4 was increased from 15H2SO4 to 20H2SO4, then zeolite MTW was obtained (0 < Si/Al < ∞) (designated as MTW-IL11-2 and MTW-IL11-3). SEM investigations confirm that the morphology of samples is varied by varying the synthesis composition. MTW-IL11-2 exhibits nanowire like morphology, whereas other two samples of this series exhibited spheroidal morphology (Fig. 8c). The N2-adsorption study of MTW-IL11-1 shows that the material exhibited a type IV isotherm with a broad distribution of mesopore diameters (3 to 10 nm) (Fig. 6b). The hysteresis loop of MTW-IL11-1 confirms that the material has less mesopore volume compared to Beta-IL11-1. Textural properties obtained from the N2-adsorption studies are summarized in Table 1, which confirms that the MTW materials have less surface area and lower pore volumes compared to Beta samples synthesized using IL11. The formation of MTW zeolite at low Al content in the present work is consistent with the reported literature.40
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xAl2O3
xAl2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL12
10IL12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O (with Si/Al < 50) using IL12, zeolite Beta was obtained (Beta-IL12). Either increasing the Si/Al or increasing the amount of H2SO4 leads to the formation of a mixture of zeolites (BETA + MTW). Beta (designated as Beta-IL13) was obtained using IL13 using the synthesis composition 30Na2O
6000H2O (with Si/Al < 50) using IL12, zeolite Beta was obtained (Beta-IL12). Either increasing the Si/Al or increasing the amount of H2SO4 leads to the formation of a mixture of zeolites (BETA + MTW). Beta (designated as Beta-IL13) was obtained using IL13 using the synthesis composition 30Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0Al2O3
0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100SiO2
100SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10IL13
10IL13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15H2SO4
15H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000H2O, in the absence of Al. Under high Al content (Si/Al < 50) or using a high concentration of H2SO4, no zeolite phase was obtained using IL13. Under low aluminium content (70 < Si/Al < ∞), MTW was obtained using IL13 (MTW-IL13). The textural properties obtained from the N2-adsorption studies are provided in Table 1 and Table 3. The structures of IL11–IL13 were optimized (Fig. 9) and their structural properties are provided in Table 2.
6000H2O, in the absence of Al. Under high Al content (Si/Al < 50) or using a high concentration of H2SO4, no zeolite phase was obtained using IL13. Under low aluminium content (70 < Si/Al < ∞), MTW was obtained using IL13 (MTW-IL13). The textural properties obtained from the N2-adsorption studies are provided in Table 1 and Table 3. The structures of IL11–IL13 were optimized (Fig. 9) and their structural properties are provided in Table 2.


