Interstrand interactions on DNA duplexes modified by TTF units at the 3′ or 5′-ends†‡
Sónia
Pérez-Rentero
a,
Isaac
Gállego
a,
Álvaro
Somoza
b,
Rubén
Ferreira
a,
Jiří
Janoušek
c,
Martin
Bělohradský
c,
Irena G.
Stará
c,
Ivo
Starý
c and
Ramon
Eritja
*a
aInstitute for Research in Biomedicine (IRB Barcelona), IQAC-CSIC, CIBER-BBN, Baldiri Reixac 10, 08028, Barcelona, Spain. E-mail: recgma@cid.csic.es; Fax: 34 93 2049942; Tel: 34 93 4039942
bIMDEA-Nanociencia, E-28049 Madrid, Spain
cInstitute of Organic Chemistry and Biochemistry (IOCB), v.v.i. Academy of Sciences of the Czech Republic, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
First published on 16th March 2012
Abstract
Short DNA duplexes carrying TTF units at the same termini exhibit a high increase in melting temperature. When both TTF units were on opposite termini, salt-dependent aggregation is observed, yielding well defined spherical DNA supramolecular structures.
Tetrathiafulvalene (TTF) derivatives are strong electron-donating molecules which have been used for the development of organic conducting materials1 as well as building blocks in supramolecular chemistry.2 TTFs are frequently used as donor units in donor–acceptor (D–A) assemblies in molecular electronics.3 Upon oxidation, TTF derivatives are capable of forming stable cation radical and dication species.1 The incorporation of aliphatic and amphiphilic groups peripheral to the TTF unit allows for the generation of ordered arrays4,5 as well as oxidation-responsive organogels.6,7
We are interested in modifying oligonucleotides with functional π-electron systems, as their π–π or D–A interactions may provide additional binding energies without perturbing the specific recognition of complementary sequences. To this end we combine the base-pairing system of DNA with the properties of TTF in order to study the properties of the resulting hybrid systems. Previous to this work, the synthesis of a small RNA oligonucleotide carrying a uridine derivative with TTF was described.8 Later, TTF-labelled nucleotide triphosphates were incorporated into DNA by primer extension with DNA polymerases although a low incorporation efficiency was reported.9 Recently, the controlled incorporation of TTF units into internal positions of oligonucleotides was published.10,11 Fluorescence quenching in TTF/pyrene modified heteroduplexes was observed10 as well as the electrochemical properties of the TTF–oligonucleotides,11 indicating that the properties of TTF are preserved when inserted in oligonucleotides.
Based on these reports, we synthesized several oligonucleotides carrying a TTF unit functionalized with three aliphatic S-butyl groups at either the 3′ or 5′-end of the oligonucleotide. Their complementary oligonucleotide sequences carrying TTF groups were used to form DNA duplexes with TTF groups at the same or opposite termini of the duplex. The relative thermal stability was analyzed, demonstrating distinct behavior of the TTF oligonucleotide conjugates that is in agreement with the supramolecular properties of both TTF and oligonucleotides and is regulated by the salt concentration.
L-threoninol was selected as a linker to incorporate the TTF moiety into DNA or alternatively incorporate an ethyl group as a non-π-electron system for comparison purposes. Threoninol has been used previously to introduce several units such as Methyl Red moieties,12 photoactive azobenzenes,13 or TTF,11 into oligonucleotides.
The synthesis of the threoninol derivatives is described in the ESI.† Tetrathio-substituted TTF equipped with a carboxylic acid functional group was prepared analogously to the literature.14 The oligonucleotide sequences shown in Table 1 were prepared using a DNA synthesizer. Oligonucleotides 1 and 2 are self-complementary octamers without or with a single TTF unit at the 3′-end, respectively. Oligonucleotides 3 and 4 are pentadecamers consisting of complementary sequences. Oligonucleotides 5–8 are derivatives of 3 and 4 modified by a single TTF unit that is attached at the 3′ or 5′-end. Oligonucleotide 9 is the derivative of 3 carrying one ethyl group at the 3′-end. In general, oligonucleotides were obtained in good yields but those carrying the TTF unit were not entirely stable in the presence of ammonia. The degradation was reduced by shortening the period of deprotection (4 h at 55 °C). The desired oligonucleotides were easily separated from the degradation products by HPLC (ESI†). We also observed degradation during the phosphoramidite coupling to attach the TTF unit at the 5′-end. This instability of the TTF-modified compounds occurred primarily when the reaction period was prolonged from 30 s to 10 min. Accordingly, shorter coupling times were used. Mass spectrometry analyses of the major HPLC peaks were consistent with the desired structures (ESI†).
| Oligonucleotide | Sequence (5′–3′)a |
|---|---|
| a TTF: tetrathiafulvalene, Et: ethyl | |
| 1 | CCAATTGG |
| 2 | CCAATTGG-TTF |
| 3 | TAGAGGCTCCATTGC |
| 4 | GCAATGGAGCCTCTA |
| 5 | TAGAGGCTCCATTGC-TTF |
| 6 | GCAATGGAGCCTCTA-TTF |
| 7 | TTF-TAGAGGCTCCATTGC |
| 8 | TTF-GCAATGGAGCCTCTA |
| 9 | TAGAGGCTCCATTGC-Et |
Thermal denaturation curves of the duplexes formed by the self-complementary octamer 1 or 2 were recorded at the high salt concentration (1 M NaCl, 10 mM sodium phosphate at pH 7, Fig. 1). The unmodified octamer 1 exhibited a normal sigmoidal curve with hyperchromicity at 260 nm, giving a melting temperature of Tm = 36.1 °C (Fig. 1A). On the contrary, the octamer 2 showed an unusual denaturation curve (Fig. 1B). The small increase in absorbance at 260 nm was due to an anomalous UV spectrum of the octamer 2 at low temperature (10 °C, Fig. 1D). The absorption bands were broad, showing light scattering due to the formation of aggregates. This effect disappeared upon heating to 80 °C (Fig. 1D). By lowering the salt concentration from 1 M to 50 mM NaCl, the melting curves returned to the standard shapes below 200 mM NaCl (Fig. 1B). The UV spectra at low temperatures and the low salt concentration showed no light scattering (Fig. 1C).
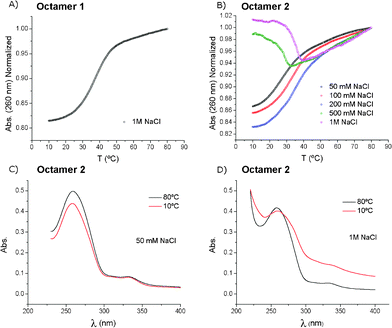 | ||
| Fig. 1 The denaturation curves of the self-complementary octamers 1 and 2. A) Melting profile of the octamer 1 at 1 M NaCl (Tm = 36.1 °C). B) The TTF–oligonucleotide 2 at different NaCl concentrations (50 mM: Tm = 27.3 °C; 100 mM: Tm = 33.8 °C; 200 mM: Tm = 35.8 °C). C) Absorption spectra at 10 °C and 80 °C of the TTF–oligonucleotide 2 at 50 mM NaCl. D) the same as in (C) but at 1 M NaCl. | ||
Since the larger changes in the absorbance spectrum of the octamer 2 at the 1 M NaCl concentration were observed at λ ≥ 300 nm, the melting experiments were also recorded at 300 nm to monitor the aggregation process using two different gradients of temperature (1 °C min−1 and 0.1 °C min−1). A large hysteresis was observed (Fig. S3, ESI†), indicating that the dissociation of the aggregate was faster than the formation of the aggregate. A similar experiment was performed with a 50 mM NaCl, 10 mM sodium cacodylate and 10 mM MgCl2 buffer (pH 7) using a 1 °C min−1 gradient (Fig. S3B†). Compared with the melting profile measured with the 1 M NaCl solution, we can conclude that magnesium ions are promoting aggregation more effectively than sodium ions as only the 10 mM magnesium concentration is needed to observe aggregation (Fig. S3, Table S1†).
The formation of the TTF–oligonucleotide aggregates at high salt concentration and low temperature was confirmed by dynamic light scattering (DLS) and transmission electron microscopy (TEM). The mean size obtained at 15 °C by DLS with 1 M NaCl solutions of octamer 2 after incubation at 4 °C for 24 h and 48 h were similar (Fig. S10, ESI†). The averaged diameter after 24 h was 216 ± 32 nm with an averaged polydispersity index (PDI) of 0.23 ± 0.04 and after 48 h was 221 ± 31 nm with an averaged PDI of 0.24 ± 0.03. Heating to 80 °C or lowering the salt concentration to 50 mM NaCl induced a large reduction of the particle size to 10–15 nm with an increase of the PDI. TEM visualization of the octamer 2 formed at 1 M NaCl at 24 h and 48 h showed that the aggregates are mainly spherical structures of sizes ranging from 90 to 210 nm with a mean diameter of about 150 nm (Fig. 2,Fig. S11†). The difference in size obtained from both techniques could be explained by the drying process needed for TEM visualisation (ESI†).
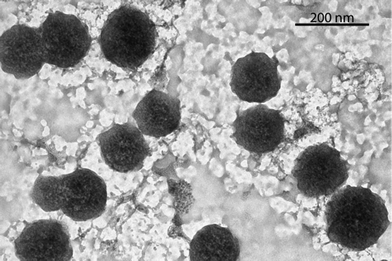 | ||
| Fig. 2 TEM micrograph of the DNA spherical structures of the self-complementary octamer 2. The structures were imaged after 48 h of incubation at 4 °C, adsorbed over the grid and stained with uranyl acetate. | ||
Next, melting experiments with duplexes consisting of the oligonucleotides 3–8, which are longer and non self-complementary, were performed at high and low salt concentrations (Table 2, Fig. S6–S9, ESI†). At the 50 mM NaCl concentration, the unmodified duplex 3 + 4 melted at 55.8 °C. Duplexes with a TTF unit, such as 3 + 6 and 5 + 4, showed higher Tm than unmodified duplex 3 + 4 (ΔTm 2.8 and 1.4). The duplexes 5 + 6 and 7 + 8 with two TTF units, each one at the different ends of the duplex, exhibit a higher stability. This is in agreement with the additive effect of two TTF units (ΔTm 3.1 and 3.4). Surprisingly, the duplexes 7 + 6 and 5 + 8 with two TTF units at the same end of the duplex had an extraordinarily high thermal stability (ΔTm 11.4 and 10.9). Such a high increase in Tm is indicative of a strong intermolecular interaction between the TTF moieties.
| Oligonucleotides | T m (°C) (50 mM NaCl) | T m (°C) (1 M NaCl) |
|---|---|---|
| a Aggr. Indicates that a broad UV spectrum with a large light scattering was observed. b The data in parentheses show the difference in Tm between the non modified duplex and modified duplexes under otherwise the same conditions. The arrows indicate the oligonucleotide orientation 5′→3′. | ||
3 + 4 |
55.8 | 67.4 |
6

|
— | Aggr.a |
3 + 6 |
58.6 (+2.8)b | 69.5 (+2.1)b |
5 + 4 |
57.2 (+1.4) | 68.2 (+0.8) |
5 + 6 |
58.9 (+3.1) | Aggr. |
7 + 8 |
59.2 (+3.4) | Aggr. |
7 + 6 |
67.2 (+11.4) | Aggr./81.6 (+14.2) |
5 + 8 |
66.7 (+10.9) | 86.2 (+18.8) |
9 + 6 |
56.9 (+1.1) | 68.7 (+1.3) |
9 + 8 |
57.1 (+1.3) | 68.1 (+0.7) |
A similar behavior was also observed at the 1 M NaCl concentration when an aggregation could also be monitored. As for the octamer 2, the aggregation was mainly seen at the duplexes 5 + 6 and 7 + 8 with two TTF units at different ends of the duplex. This indicated an intermolecular interaction between TTFs, which resulted in aggregation rather than the duplex stabilization. In addition, aggregation was also observed at the single-strand TTF oligonucleotide 6 and at the pair 6 + 7 (Fig. S5–S7, ESI†). In the latter case, the duplex-to-random coil transition was so high that it could be observed after the aggregation process and Tm was measured. At 1 M NaCl duplexes 7 + 6 and 5 + 8 had the highest thermal stability (ΔTm 14.2 and 18.8). As expected, the duplexes carrying the ethyl group (9 + 6 and 9 + 8) showed similar melting temperatures compared with the unmodified duplex (3 + 4).
In summary, we described the striking effect of a TTF moiety linked to the 5′ or 3′-end of oligonucleotides on the stability of the corresponding DNA duplexes. If two TTF units are attached, then either aggregation, strong duplex stabilization or both can be observed depending on the relative position of the TTF units within the duplex (being bound to the same or opposite ends) and salt concentration. Such remarkable changes in assembly properties of the TTF-modified oligonucleotides have not yet been reported.15 It is worth mention that salt-induced aggregation of DNA–porphyrin conjugates have been observed by long-ranged exciton-coupled circular dichroism.16 Moreover, DNA micelles can be obtained from lipid–DNA conjugates with increased cell permeability.17 The strong oligonucleotide duplex stabilization and the salt-induced formation of aggregates in diluted solutions of TTF–DNA conjugates may provide a basis for the development of applications like the design of novel delivery systems or the development of salt-responsive self-assembled materials.
Acknowledgements
This research was supported by the European Commission under Grant No. FP7-FUNMOL 213382, by the Spanish Ministry of Education (CTQ2010-20541), the Generalitat de Catalunya (2009/SGR/208), the Czech Science Foundation (P207/10/2214) and the Institute of Organic Chemistry and Biochemistry AS CR (this work is part of the Research Project Z4 055 0506). Dr Radek Pohl is acknowledged for the NMR spectra measurement as well as the electron cryo-microscopy unit from CCiT of University of Barcelona for the use of the EM facility.References
- J. Yamada and T. Sugimoto, in TTF Chemistry, Fundamentals and applications of tetrathiafulvalene, Springer, Berlin 2004 Search PubMed.
- J. L. Segura and N. Martin, Angew. Chem., Int. Ed., 2001, 40, 1372 CrossRef CAS.
- M. Bendikov, F. Wudl and D. F. Perepichka, Chem. Rev., 2004, 104, 4891 CrossRef CAS.
- M. M. S. Abdel-Mottaleb, E. Gomar-Nadal, M. Suri, H. Uji-I, W. Mamdouh, J. Veciana, V. Lemaur, C. Rovira, J. Cornil, R. Lazzaroni, D. B. Amabilino, S. De Feyter and F. C. De Schryver, J. Mater. Chem., 2005, 15, 4601 RSC.
- D. R. Talham, Chem. Rev., 2004, 104, 5479 CrossRef CAS.
- J. Puigmartí, V. Laukhin, A. V. del Pino, J. Vidal-Gancedo, C. Rovira, E. Laukhina and D. B. Amabilino, Angew. Chem., Int. Ed., 2007, 46, 238 CrossRef.
- C. Wang, D. Zhang and D. Zhu, J. Am. Chem. Soc., 2005, 127, 16372 CrossRef CAS.
- O. Neilands, V. Liepinsh and B. Turovska, Org. Lett., 1999, 1, 2065 CrossRef CAS.
- J. Riedl, P. Horáková, P. Sebest, R. Pohl, L. Havran, M. Fojta and M. Hocek, Eur. J. Org. Chem., 2009, 3519 CrossRef CAS.
- N. Bouquin, V. L. Malinovskii, X. Guégano, S. X. Liu, S. Decurtins and R. Häner, Chem.–Eur. J., 2008, 14, 5732 CrossRef CAS.
- M. Schnippering, A. Zahn, S. X. Liu, C. Leumann and S. Decurtins, Chem. Commun., 2009, 5552 RSC.
- H. Asanuma, K. Shirasuka, T. Takarada, H. Kashida and M. Komiyama, J. Am. Chem. Soc., 2003, 125, 2217 CrossRef CAS.
- H. Kashida, X. Liang and H. Asanuma, Curr. Org. Chem., 2009, 13, 1065 CAS.
- A. Zhao, B.T. Blesa, M.J. Le Derfa, F. Caneveta, D. Benhaoua, C. Mazari, M. Allaina and M. Sallé, Tetrahedron, 2007, 63, 10768 CrossRef.
- J. Tuma, W. H. Connors, D. H. Stitelman and C. Richert, J. Am. Chem. Soc., 2002, 124, 4236 CrossRef CAS.
- A. Mammana, G. Pescitelli, T. Asakawa, S. Jockusch, A. G. Petrovic, R. R. Monaco, R. Purello, N. J. Turro, K. Nakanishi, G. A. Ellestad, M. Balaz and N. Berova, Chem.–Eur. J., 2009, 15, 11853 CrossRef CAS.
- H. Liu, Z. Zhu, H. Kang, Y. Wu, K. Stefan and W. Tan, Chem.–Eur. J., 2010, 16, 3791 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: Experimental section, HPLC profiles, melting curves, DLS, EM and NMR spectra. See DOI: 10.1039/c2ra20477k/ |
| ‡ In memory of Professor Har Gobind Khorana (1922−2011), acknowledging his legacy to the scientific community. |
| This journal is © The Royal Society of Chemistry 2012 |