Luminescent GaN semiconductor based on surface modification with lanthanide complexes through an ionic liquid bridge†
Qiu-Ping
Li
and
Bing
Yan
*
Department of Chemistry, Tongji University; State Key Lab of Water Pollution and Resource Reuse (Tongji University), Siping Road 1239, Shanghai 200092, China. E-mail: byan@tongji.edu.cn; Fax: +86-21-65981097; Tel: +86-21-65984663
First published on 18th September 2012
Abstract
A novel GaN-based luminescent hybrid material has been prepared by covalently functionalizing the GaN matrices with an ionic liquid (IL), and then exchanging the anion of the IL with lanthanide complexes. 1-Methyl-3-[3-(trimethoxysilyl)propyl]imidazolium chloride, a kind of multi-functional room temperature IL, is used to silanize the hydroxylated surfaces of the GaN sample. After that, an anion exchange measurement is performed to introduce the tetrakis β-diketonate europium(III) complex anion to the GaN matrices. The FTIR spectra, UV-vis diffuse reflection absorption spectra, scanning electron micrograph, XRD patterns and photoluminescent properties (luminescence, lifetime and quantum efficiency) for the resulting material were studied in detail. The results suggest that our method is an effective way for constructing novel functional GaN based hybrid materials.
During the last few years, gallium nitride (GaN) has attracted increasing interest as a substrate material for various applications because of its outstanding chemical stability under tough conditions, physical hardness, well electron mobility and good optical properties. The mass production of bulk GaN crystals presents opportunities for designing GaN-based functional materials,1 such as light-emitting diodes, chemical or biological sensors, power electronics and photovoltaic devices.2 Many works have been carried out to construct GaN-based luminescent materials by doping lanthanide elements to GaN matrices or attaching a luminescent phosphor to the surface of it.3 Before the attachment of the luminescent phosphors, the GaN must be pretreated to provide an oxidized and hydrogen-terminated surface for the further immobilization of luminescent molecules.4 It has been proven that the silanization of hydroxylated surfaces is an effective way to construct silane-based multifunctional materials, which have been investigated in numerous works during the last decades.5 The successful covalent functionalization of GaN surfaces with organosilanes has been demonstrated and confirmed by several reported literatures.6 Recent examples of this sort of materials are the GaN-based microsensors which are constructed by covalently functionalize the GaN surface with luminescent Ru2+ complexes or long-lived luminescent indicator dyes.7
Recently, room-temperature ionic liquids (IL) are gathering lots of interest as environmentally benign solvents for organic synthesis and separation,8 and some recent studies focus on their use in material science.9 The most common ILs include alkylammonium salts, alkylphosphonium salts, imidazolium salts as well as N-alkylpyridinium salts. Among which the imidazolium salts are widely applied in constructing functional materials and have found a variety of applications owing to their distinctive properties.10 One of the best-studied ways of using it is to immobilize the imidazolium salts on various supporting matrices, which have been published many times in recent years, such as silica-supported imidazolium salt derived catalyst, and silica-based imidazolium stationary phases used in liquid chromatography.11 Since the tetrakis β-diketonate lanthanide(III) complexes can be electrostatically coupled to an IL, it presents opportunities for constructing imidazolium salt-derived luminescent materials, which has been confirmed by several previous literature.12 But to our best knowledge, there are no previous reports about functionalizing GaN with an IL.
In this Communication, we report on the covalent functionalization of GaN surfaces with an imidazolium salt IL, which bears an organosilane group and can silanize the hydroxylated surfaces of GaN and then can adsorb the tetrakis β-diketonate europium complex anion through the electrostatically driven anion exchange reaction. The aim of the work described herein is to explore a way of constructing GaN-based luminescent materials using the IL and research their photophysical properties.
Fig. 1 shows the detailed synthetic pathways to obtain the luminescent materials. GaN powder is provided by Alfa Aesar, while all the other reagents and solvents are obtained from Aladdin and used without further purification. In a typical experiment, the GaN powder is treated with piranha solution (H2SO4/H2O2 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v)) for 30 min, and then washed with deionized water and dried under vacuum condition prior to silanization.7,13 The oxidized sample is then silalized by ultrosonicating it in the as-prepared 1-methyl-3-[3-(trimethoxysilyl)propyl]imidazolium chloride (room temperature IL, marked as TMOSIM+Cl−) toluene solution under 50 °C. After one hour, a uniformly IL-coated material is obtained, and the unbound IL is removed by washing with enough ethanol. At the same time the tetrakis β-diketonate europium(III) complex NEt4Eu(TTA)4 (TTA = thenoyltrifluoroacetone) was prepared by following a modified literature procedure.14 In order to anchor the luminescent phosphor [Eu(TTA)4]− onto the surface of GaN sample, we perform an anion exchange reaction, typically, NEt4Eu(TTA)4 is added to a dispersion of IL-bearing GaN powder in ethanol and stirred for 24 h at room temperature (RT). Finally, the GaN powder is rinsed with sufficient ethanol to remove any trace of NEt4Eu(TTA)4 and dried under vacuum conditions. The resulting material is denoted as GaN-IM+-[Eu(TTA)4]−.
1 v/v)) for 30 min, and then washed with deionized water and dried under vacuum condition prior to silanization.7,13 The oxidized sample is then silalized by ultrosonicating it in the as-prepared 1-methyl-3-[3-(trimethoxysilyl)propyl]imidazolium chloride (room temperature IL, marked as TMOSIM+Cl−) toluene solution under 50 °C. After one hour, a uniformly IL-coated material is obtained, and the unbound IL is removed by washing with enough ethanol. At the same time the tetrakis β-diketonate europium(III) complex NEt4Eu(TTA)4 (TTA = thenoyltrifluoroacetone) was prepared by following a modified literature procedure.14 In order to anchor the luminescent phosphor [Eu(TTA)4]− onto the surface of GaN sample, we perform an anion exchange reaction, typically, NEt4Eu(TTA)4 is added to a dispersion of IL-bearing GaN powder in ethanol and stirred for 24 h at room temperature (RT). Finally, the GaN powder is rinsed with sufficient ethanol to remove any trace of NEt4Eu(TTA)4 and dried under vacuum conditions. The resulting material is denoted as GaN-IM+-[Eu(TTA)4]−.
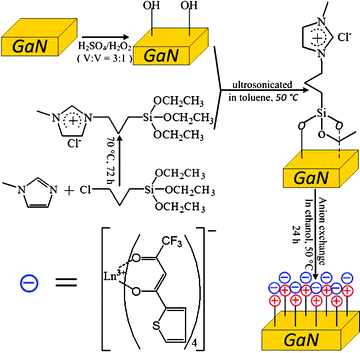 | ||
| Fig. 1 Synthetic scheme for the preparation of lanthanide complex-functionalized GaN. | ||
The success of surface functionalization for GaN has been proven by the compare of IR spectra between the neat GaN and the resulted material GaN-IM+-[Eu(TTA)4]− presented in Fig. 2. As shown in the IR spectrum of GaN-IM+-[Eu(TTA)4]−, the weak broad band centered around 3110 cm−1 can be assigned to the vibration of (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H). The IR band for υ(C
C–H). The IR band for υ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) vibrations of the mono-deprotonated ligand TTA appears in a lower frequency region (centered around 1625 cm−1), which can be ascribed to the complexation of the Eu3+ ion with the oxygen atom of the C
O) vibrations of the mono-deprotonated ligand TTA appears in a lower frequency region (centered around 1625 cm−1), which can be ascribed to the complexation of the Eu3+ ion with the oxygen atom of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The characteristic absorbance of the imidazole ring bend is found at 1577 cm−1.15 Besides, the absorption bands centered at 1309 cm−1 and 1137 cm−1 can be assigned to the symmetric and asymmetric stretching vibrations of the –CF3 group. In addition, the absorption peaks appearing at 621, 580 and 561 cm−1 are ascribed to the GaN matrices, it can be observed in both the spectra. Compare the IR spectrum of GaN-IM+-[Eu(TTA)4]− to the one of GaN, all the newly arisen absorption peaks which we have discussed above suggest that the europium complexes have been chemically immobilized on the surface of GaN.
O. The characteristic absorbance of the imidazole ring bend is found at 1577 cm−1.15 Besides, the absorption bands centered at 1309 cm−1 and 1137 cm−1 can be assigned to the symmetric and asymmetric stretching vibrations of the –CF3 group. In addition, the absorption peaks appearing at 621, 580 and 561 cm−1 are ascribed to the GaN matrices, it can be observed in both the spectra. Compare the IR spectrum of GaN-IM+-[Eu(TTA)4]− to the one of GaN, all the newly arisen absorption peaks which we have discussed above suggest that the europium complexes have been chemically immobilized on the surface of GaN.
![Infrared spectra of pure GaN and GaN-IM+-[Eu(TTA)4]−.](/image/article/2012/RA/c2ra20582c/c2ra20582c-f2.gif) | ||
| Fig. 2 Infrared spectra of pure GaN and GaN-IM+-[Eu(TTA)4]−. | ||
The X-ray photoelectron spectroscopy (XPS) analysis of samples before and after anion exchange reaction is provided as a complement to FTIR. As seen in the left part of Fig. 3, the emerging peak of F 1s appearing at 692 eV in GaN-IM+-[Eu(TTA)4]− is positive evidence for the success of anion exchange. According to the previous report, the O 1s core-level XPS spectra of IL modified GaN could be attributed to the O–H, O–Ga and O–Si constituent.6 Thus, for the materials after anion exchange, the new component (around 536.3 eV) could be attributed to the introduction of coordinated oxygen within the resonating structures of chelated β-diketone ligands.
![F 1s and O 1s core-level XPS spectra of (1) the material before anion exchange (IL-modified GaN) and (2) the material after anion exchange (GaN-IM+-[Eu(TTA)4]−).](/image/article/2012/RA/c2ra20582c/c2ra20582c-f3.gif) | ||
| Fig. 3 F 1s and O 1s core-level XPS spectra of (1) the material before anion exchange (IL-modified GaN) and (2) the material after anion exchange (GaN-IM+-[Eu(TTA)4]−). | ||
The thermogravimetric (TG) and the corresponding derivative weight loss (DTG) analyses have been performed for the IL-modified GaN and GaN-IM+-[Eu(TTA)4]−. As shown in Fig. 4A, the IL-modified GaN shows a typical two-step weight loss approach over 225 °C according to the DTG curve, which is coincidental with the weight loss phenomenon observed in previously reported analogous IL-modified materials.16 Similarly, based on the DTG curve presented in Fig. 4B, we have found that the GaN-IM+-[Eu(TTA)4]− shows an obvious three-step weight loss procedure beyond 225 °C. Apparently, the second procedure of weight loss between 300 and 450 °C could be associated with the decomposition of europium tetrakis(β-diketonate) attaching onto the GaN matrices through electrostatic interactions,17 which also can be seen as complementary evidence for the success of anion exchange. In addition, the residual weight of GaN-IM+Cl− and GaN-IM+-[Eu(TTA)4]− is essentially contributed by the same composition. After normalization to the residual weight of GaN-IM+-[Eu(TTA)4]−, reasonable loading percentages for the IL (5.40%) and europium complex (5.17%) are deduced. The loading percentage deviation (0.33%) of IL between IL-modified GaN and GaN-IM+-[Eu(TTA)4]− is acceptable.
![TG and DTG curves for IL-modified GaN (A) and GaN-IM+-[Eu(TTA)4]− (B).](/image/article/2012/RA/c2ra20582c/c2ra20582c-f4.gif) | ||
| Fig. 4 TG and DTG curves for IL-modified GaN (A) and GaN-IM+-[Eu(TTA)4]− (B). | ||
The X-ray diffraction (XRD) patterns (Fig. S1, ESI†) of the pure GaN and GaN-IM+-[Eu(TTA)4]− are determined at room temperature within the 2θ range of 10–70°. Both of the spectra show similar sharp peaks that originate from the crystal-line-natured GaN, which means the crystal structure of GaN matrices has been well preserved after the surface functionalization although the peak intensities in the XRD pattern of GaN-IM+-[Eu(TTA)4]− show a slight decrease. According to the XRD patterns, both of the samples display a hexagonal structure which is in good agreement with the JCPDS file (JCPDS 65-3410 Hexagonal-type, space group P63mc). In addition, the scanning electron micrograph (SEM, Fig. S2, ESI†) for GaN-IM+-[Eu(TTA)4]− shows a homogeneous and regular microstructure. Based on the SEM and XRD patterns, we conclude that the immobilization of IL and anion exchange have little influence on the microstructure of the GaN matrices. The ultraviolet–visible diffuse reflection absorption spectra of GaN-IM+-[Eu(TTA)4]− is performed on powdered samples and presented in Fig. S3, ESI.† It can be observed from the figure that a broad absorption band is located in the range 200–600 nm. Moreover, the spectra of GaN-IM+-[Eu(TTA)4]− peaks at about 360 nm, which is coincident with the dominant peaks of the excitation spectra for GaN-IM+-[Eu(TTA)4]− shown in Fig. 5. In addition, we can observe an obvious inverse peak at about 614 nm, the characteristic transition of a europium ion under excitation during the measurement.
![Excitation and emission spectra of GaN-IM+-[Eu(TTA)4]−.](/image/article/2012/RA/c2ra20582c/c2ra20582c-f5.gif) | ||
| Fig. 5 Excitation and emission spectra of GaN-IM+-[Eu(TTA)4]−. | ||
The excitation and emission spectra of the obtained GaN-IM+-[Eu(TTA)4]− is shown in Fig. 5. The excitation spectrum is obtained by detecting the characteristic emission of a europium(III) ion at 614 nm and dominated by a broad band centered at about 360 nm in the ultraviolet region. The broad excitation band suggests that the combination of TTA and functionalized GaN matrices can sensitize the transition of the europium ion effectively. The emission spectra of GaN-IM+-[Eu(TTA)4]− is obtained by using the most appropriate wavelength (360 nm) as the excitation wavelength based on its excitation spectra. As shown in the right side of Fig. 5, the emission lines are assigned to the transitions 5D0 → 7FJ (J = 0–4) located at 578, 589, 614, 651, and 699 nm for europium ion. The emission spectrum is dominated by the very intense 5D0 → 7F2 transition at 614 nm, suggesting that an effective energy transfer takes place from the matrices to the europium ion. Besides, since the 5D0 → 7F1 transition of the europium ion is a parity-allowed magnetic dipole transition and relatively independent from its chemical environment, while the 5D0 → 7F2 transition is a typical electric dipole transition and highly sensitive to its environment, we can use the intensity ratios I(5D0 → 7F2)/I(5D0 → 7F1) as an indicator for the local environment of europium ion. Here, the intensity ratio of the red/orange intensities is approximately 16.1 (Table S1, ESI†), indicating that the europium(III) ions are located at an asymmetric environment. In order to further investigate the photoluminescence property, the decay curve of GaN-IM+-[Eu(TTA)4]− is detected and fitted (Fig. 6) into a single exponential function in the form ln(St/S0) = −k1t = −t/τ. From which, the lifetime value is calculated as 316 μs. Furthermore, based on the emission spectra and the lifetime (τ) of GaN-IM+-[Eu(TTA)4]−, the emission quantum efficiency (η) of the 5D0 excited state of europium(III) ion is determined to be 26.3% according to ref. 18 (details for calculation process of luminescence quantum efficiency can be seen in ESI†).
![Luminescent decay curves of GaN-IM+-[Eu(TTA)4]−. (Excitation wavelength = 360 nm; emission wavelength = 614 nm; circles: experimental data; solid line: fitted according to I = I0 + Aexp[−(t − t0)/τ.]](/image/article/2012/RA/c2ra20582c/c2ra20582c-f6.gif) | ||
| Fig. 6 Luminescent decay curves of GaN-IM+-[Eu(TTA)4]−. (Excitation wavelength = 360 nm; emission wavelength = 614 nm; circles: experimental data; solid line: fitted according to I = I0 + Aexp[−(t − t0)/τ.] | ||
In summary, we have successfully functionalized the GaN surface with europium complexes by immobilizing a novel IL to it and then performing an anion exchange. The tetrakis β-diketonate europium(III) complex anion has been chemically bonded to the surface of GaN through an imidazolium salt molecular bridge under the drive of an electrostatic interaction, resulting in a novel GaN-based luminescent material. The physical properties, especially the photophysical properties, have been investigated in detail. The photoluminescent properties reveal that GaN is a favorable matrix for constructing luminescent rare earth hybrid materials. Moreover, this achievement provides a new approach to design novel functional GaN materials.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (20971100, 91122003), Program for New Century Excellent Talents in University (NCET 2008-08-0398).References
- G. Kamler, J. Zachara, S. Podsiadlo, L. Adamowicz and W. Gebicki, J. Cryst. Growth, 2000, 212, 39 CrossRef CAS.
- A. Gautam and F. C. J. M. van Veggel, Chem. Mater., 2011, 23, 4817 CrossRef CAS.
- (a) H. Q. Wu, C. B. Poitras, M. Lipson, M. G. Spencer, J. Hunting and F. J. DiSalvo, Appl. Phys. Lett., 2005, 86, 191918 CrossRef; (b) A. J. Steckl, J. C. Heikenfeld, D. S. Lee, M. J. Garter, C. C. Baker, Y. Q. Wang and R. Jones, IEEE J. Sel. Top. Quantum Electron., 2002, 8, 749 CrossRef CAS.
- B. Baur, G. Steinhoff, J. Hernando, O. Purrucker, M. Tanaka, B. Nickel, M. Stutzmann and M. Eickhoff, Appl. Phys. Lett., 2005, 87, 269301 CrossRef.
- (a) A. Matsumoto, K. Tsutsumi, K. Schumacher and K. K. Unger, Langmuir, 2002, 18, 4014 CrossRef CAS; (b) J. B. Brzoska, I. Benazouz and F. Rondelez, Langmuir, 1994, 10, 4367 CrossRef CAS; (c) S. Mitchell, A. Bonilla and J. Perez-Ramirez, Mater. Chem. Phys., 2011, 127, 278 CrossRef CAS.
- A. Arranz, C. Palacio, D. Garcia-Fresnadillo, G. Orellana, A. Navarro and E. Munoz, Langmuir, 2008, 24, 8667 CrossRef CAS.
- (a) J. Lopez-Gejo, A. Navarro-Tobar, A. Arranz, C. Palacio, E. Munoz and G. Orellana, ACS Appl. Mater. Interfaces, 2011, 3, 3846 CrossRef CAS; (b) J. Lopez-Gejo, A. Arranz, A. Navarro, C. Palacio, E. Munoz and G. Orellana, J. Am. Chem. Soc., 2010, 132, 1746 CrossRef CAS.
- (a) T. Welton and J. P. Hallett, Chem. Rev., 2011, 111, 3508 CrossRef; (b) A. Monge-Marcet, R. Pleixats, X. Cattoen, M. Wong and C. Man, Catal. Sci. Technol., 2011, 1, 1544 RSC.
- (a) M. A. Neouze, B. Basnar, M. Litschauer, S. Abermann, E. Bertagnolli and G. Strasser, Chem. Commun., 2011, 47, 361 RSC; (b) A. V. Mudring, S. Tang and J. Cybinska, Helv. Chim. Acta, 2009, 92, 2375 CrossRef; (c) K. Lunstroot, K. Driesen, P. Nockemann, L. Viau, P. H. Mutin, A. Vioux and K. Binnemans, Phys. Chem. Chem. Phys., 2010, 12, 1879 RSC.
- M. A. Neouze, J. Mater. Chem., 2010, 20, 9593 RSC.
- (a) M. A. Neouze, M. Litschauer, M. Puchberger and H. Peterlik, J. Mater. Chem., 2010, 20, 1269 RSC; (b) M. Li, P. J. Pham, C. U. Pittman and T. Y. Li, Microporous Mesoporous Mater., 2009, 117, 436 CrossRef CAS.
- (a) L. Maggini, H. Traboulsi, K. Yoosaf, J. Mohanraj, J. Wouters, O. Pietraszkiewicz, M. Pietraszkiewicz, N. Armaroli and D. Bonifazi, Chem. Commun., 2011, 47, 1625 RSC; (b) S. M. Bruno, R. A. S. Ferreira, F. A. A. Paz, L. D. Carlos, M. Pillinger, P. Ribeiro-Claro and I. S. Goncalves, Inorg. Chem., 2009, 48, 4882 CrossRef CAS.
- Y. Zhao and B. Yan, Dalton Trans., 2012, 41, 5334 RSC.
- L. R. Melby, N. J. Rose, E. Abramson and J. C. Caris, J. Am. Chem. Soc., 1964, 86, 5117 CrossRef CAS.
- T. I. Morrow and E. J. Maginn, J. Phys. Chem. B, 2002, 106, 12807 CrossRef CAS.
- (a) M. Litschauer and M. A. Neouze, Monatsh. Chem., 2008, 139, 1151 CrossRef CAS; (b) M. Litschauer and M. A. Neouze, J. Mater. Chem., 2008, 18, 640 RSC.
- S. M. Bruno, R. A. S. Ferreira, F. A. A. Paz, L. D. Carlos, M. Pillinger, P. Ribeiro-Claro and I. S. Goncalves, Inorg. Chem., 2009, 48, 4882 CrossRef CAS.
- (a) O. L. Malta, M. A. C. dosSantos, L. C. Thompson and N. K. Ito, J. Lumin., 1996, 69, 77 CrossRef CAS; (b) R. A. S. Ferreira, L. D. Carlos, R. R. Goncalves, S. J. L. Ribeiro and V. D. Bermudez, Chem. Mater., 2001, 13, 2991 CrossRef; (c) P. C. R. Soares-Santos, H. I. S. Nogueira, V. Felix, M. G. B. Drew, R. A. S. Ferreira, L. D. Carlos and T. Trindade, Chem. Mater., 2003, 15, 100 CrossRef CAS; (d) Y. J. Li, B. Yan and L. Wang, Dalton Trans., 2011, 40, 6722 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra20582c |
| This journal is © The Royal Society of Chemistry 2012 |
