Coordination chemistry of Ag(I) with bridging ligands based on pyrazolyl–pyridine termini: polymers, helicates and a bow-tie
Andrew
Stephenson
and
Michael D.
Ward
*
Department of Chemistry, University of Sheffield, S3 7HF, UK. E-mail: m.d.ward@sheffield.ac.uk
First published on 11th September 2012
Abstract
A series of Ag(I) complexes has been prepared containing bridging ligands based on two pyrazolyl–pyridine ligands connected by a flexible spacer. Crystallographic investigations reveal a remarkable range of structural types from simple mononuclear complexes to dinuclear double helicates, a distorted ‘bow-tie’ metallamacrocycle, and one-dimensional chains including a triple helical chain based on double helical molecular units linked by Ag⋯Ag contacts. The different structures arise as a result of the different interactions dominating in each complex, including aromatic π-stacking, Ag⋯Ag interactions, exocyclic lone pair interactions and inter-ligand π-stacking.
Introduction
Our recent investigations into the self-assembly of polyhedral coordination cages has led to a large family of bis-bidentate bridging ligands which contain two pyrazolyl–pyridine termini connected via methylene bridges to a central aromatic core.1–3 These ligands have generally been reacted with labile octahedral metal ions to form a range of polyhedral coordination cages based on a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 metal-to-ligand ratio, including examples ranging from M4L6 tetrahedra2 to M16L24 capped truncated tetrahedra.3
3 metal-to-ligand ratio, including examples ranging from M4L6 tetrahedra2 to M16L24 capped truncated tetrahedra.3
An alternative to using octahedral metal ions with these ligands is to use Ag(I) ions which are generally four coordinate with ligands of this type.4 Assembly with our bis-bidentate bridging ligands would afford (in the absence of any other ligands) assemblies with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal
1 metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand ratio, rather than a 2
ligand ratio, rather than a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio as occurs with octahedral metal ions.1–3 Such 1
3 ratio as occurs with octahedral metal ions.1–3 Such 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes might include dinuclear double helicates [M2L2] and infinite coordination polymers [M1L1]∞.4 Ag(I) has the additional benefit that as a d10 ion with no strong stereoelectronic preferences it is particularly adaptable to the differing geometric requirements of different ligands; and the lack of redox activity [in contrast to Cu(I)] removes a potentially complicating factor from the study of its complexes. In addition the possibility of Ag⋯Ag interactions adds an extra dimension to the use of Ag(I) ions in coordination and metallosupramolecular chemistry.5 Partly for these reasons, Ag(I) complexes have been studied extensively and have been previously shown to have useful properties in the areas of host–guest chemistry,6 sensing,7 catalysis8 and conductivity.9
1 complexes might include dinuclear double helicates [M2L2] and infinite coordination polymers [M1L1]∞.4 Ag(I) has the additional benefit that as a d10 ion with no strong stereoelectronic preferences it is particularly adaptable to the differing geometric requirements of different ligands; and the lack of redox activity [in contrast to Cu(I)] removes a potentially complicating factor from the study of its complexes. In addition the possibility of Ag⋯Ag interactions adds an extra dimension to the use of Ag(I) ions in coordination and metallosupramolecular chemistry.5 Partly for these reasons, Ag(I) complexes have been studied extensively and have been previously shown to have useful properties in the areas of host–guest chemistry,6 sensing,7 catalysis8 and conductivity.9
The importance of weak supramolecular interactions in dictating structures of metal complexes with these ligands is illustrated by the family of polyhedral cages in which the aromatic π-stacking between ligands within a complex plays an important role in stabilising particular structural types;1 stacking interactions with ligands of this type can also play a decisive role in other self-assembled systems.10 In Ag(I) complexes, argentophilic Ag⋯Ag interactions may also contribute to the self assembly process, as has been reported recently by us11 and others.5 In this paper we describe a range of Ag(I) complexes with six different ligands all containing two pyrazolyl–pyridine termini; the complexes have been characterised both in the solid state and in solution. A remarkable range of structural types has emerged based in part on different supramolecular interactions.
Results and discussion
The ligands used in this paper are shown in Scheme 1. They are all bis-bidentate and only differ in the ‘spacer’ between the two pyrazolyl–pyridine units. Of these, the ligands Lfur and Lth,12 L14nap,13 and Lbz (ref. 11) have been reported previously. Lazo and LOMe were synthesised in the usual way by the reaction of 3-(2-pyridyl)pyrazole with the respective bis(bromomethyl) substituted aromatic core fragments under basic conditions; full details are given in the experimental section.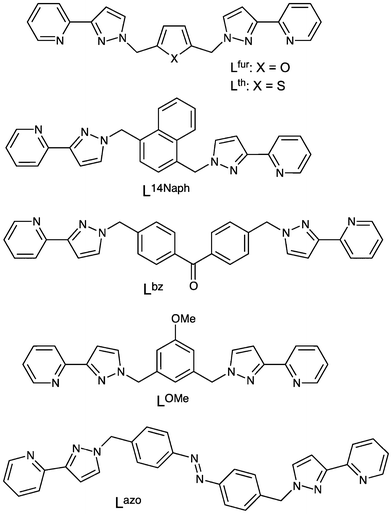 | ||
| Scheme 1 The ligands used in this paper. | ||
A mononuclear Ag(I)–Lfur complex
The complex formed between Lfur and AgClO4 when they are combined in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio is a mononuclear complex with the ligand wrapping around a single Ag(I) ion. This is similar to the previously-reported Cd(II) complex which showed how Lfur could coordinate as a pentadentate chelate with the participation of the furan as a weak O-donor group.12
1 ratio is a mononuclear complex with the ligand wrapping around a single Ag(I) ion. This is similar to the previously-reported Cd(II) complex which showed how Lfur could coordinate as a pentadentate chelate with the participation of the furan as a weak O-donor group.12
Rather unusually the structure of [Ag(Lfur)]ClO4 contains three crystallographically independent complex molecules in the asymmetric unit; however all are structurally quite similar with the two pyrazolyl–pyridine termini of Lfur providing irregular tetradentate chelating coordination (Ag–N distances in the range 2.23–2.56 Å), and the central furan unit providing a long, weak Ag⋯O contact (Fig. 1). Two of the complex cations, containing Ag(1) and Ag(2), form a closely-associated pair (Fig. 2) associated by an Ag(1)⋯Ag(2) interaction of 3.293 Å, which is long for an argentophilic interaction but still less than the sum of the van der Waals' radii for two Ag(I) ions (3.44 Å), and therefore likely significant in terms of an attractive interaction.5f We note that an unbridged Ag⋯Ag contact as long as 3.49 Å has been observed in one case.14 This Ag⋯Ag contact means that the two complex cations are mutually oriented in an ‘overlapping’ manner so as to afford two π-stacking interactions between parallel pairs of pyrazolyl–pyridine fragments, such that this pair of complex cations has two distinct π–π interactions and the Ag⋯Ag interaction between them. The third independent complex unit in contrast, containing Ag(3), forms π-stacking interactions with additional symmetry-equivalent molecules in the unit cell, forming an alternating offset arrangement of molecules which contrasts with the face-to-face ‘eclipsed’ pair of Ag(1) and Ag(2) (Fig. 3). Thus there can be no Ag⋯Ag interactions involving Ag(3) and this may explain why its Ag⋯O contact is shorter (2.853 Å) than in the other two complex units (2.980 and 3.028 Å). Overall, all six independent chelating pyrazolyl–pyridine units associated with the three complex molecules are involved in stacking interactions.
·(MeCN)2/3.](/image/article/2012/RA/c2ra21757k/c2ra21757k-f1.gif) | ||
| Fig. 1 Structure of one of the three crystallographically independent complex cations in the crystal structure of [Ag(Lfur)](ClO4)·(MeCN)2/3. | ||
·(MeCN)2/3.](/image/article/2012/RA/c2ra21757k/c2ra21757k-f2.gif) | ||
| Fig. 2 Structure of the stacked pair of complex cations in the crystal structure of [Ag(Lfur)](ClO4)·(MeCN)2/3. | ||
·(MeCN)2/3 showing (i) the disposition of the three independent complex units with respect to each other, and (ii) the stacking interactions between the Ag(3) units (dashed lines).](/image/article/2012/RA/c2ra21757k/c2ra21757k-f3.gif) | ||
| Fig. 3 A view of the structure of [Ag(Lfur)](ClO4)·(MeCN)2/3 showing (i) the disposition of the three independent complex units with respect to each other, and (ii) the stacking interactions between the Ag(3) units (dashed lines). | ||
A dinuclear double helicate with Ag(I) and Lth
Replacement of the central furan unit of Lfur by a thienyl unit in Lth removes the ability of the central heterocyclic ring to coordinate to Ag(I) with the result that the ligand now acts as a bis-bidentate bridging ligand in the dinuclear double helicate [Ag2(Lth)2](ClO4)2, in which the two ligands are wrapped helically around a pair of metal ions and the Ag(I) ions are four coordinate from two bidentate N-donor ligand fragments.The exocyclic lone pair of electrons on each thienyl S atom, whilst not coordinated to the Ag(I) ions, is however directed towards a planar, coordinated and relatively electropositive pyrazolyl–pyridine unit from the alternate ligand as shown in Fig. 4. The separation between the S atom and the mean plane of the coordinated pyrazolyl–pyridine unit at which it is directed are 3.41 and 3.46 Å, marginally less than the sum of the relevant van der Waals radii (3.5 Å) and therefore indicative of a weak attractive interaction of the type we have seen before.12 The complex has no crystallographic symmetry but the two ligands are in similar environments.
2. The dashed lines indicate the contact between the thiophene lone pair from one ligand and a coordinated pyrazolyl–pyridine of the other (see main text).](/image/article/2012/RA/c2ra21757k/c2ra21757k-f4.gif) | ||
| Fig. 4 Structure of the double helical complex cation in the crystal structure of [Ag2(Lth)2](ClO4)2. The dashed lines indicate the contact between the thiophene lone pair from one ligand and a coordinated pyrazolyl–pyridine of the other (see main text). | ||
The complex also displays π-stacking between pyrazolyl–pyridine units from different complexes. This is shown in Fig. 5, in which the central complex unit interacts with four more via such stacking interactions; these interactions dominate the crystal packing with no Ag⋯Ag interactions being present and result in the formation of two-dimensional sheets in the crystal. The Ag–Ag distance within a dinuclear helicate is 8.62 Å, and the shortest Ag–Ag distance between dinuclear helicates is 5.49 Å.
2, showing how each molecule stacks with four others (dashed lines).](/image/article/2012/RA/c2ra21757k/c2ra21757k-f5.gif) | ||
| Fig. 5 Part of the two-dimensional sheet in the crystal structure of [Ag2(Lth)2](ClO4)2, showing how each molecule stacks with four others (dashed lines). | ||
This lack of coordination of the thienyl groups in [Ag2(Lth)2](ClO4)2 compared to the furan groups in [Ag(Lfur)]ClO4 may be partly electronic, because the S atom of thiophene is less basic than the O atom of furan due to the greater aromatic character of thiophene. It could also be partly steric, because the larger size of the S atom results in a geometric change in the central heterocyclic ring which moves the two pendant pyrazolyl–pyridine arms of each ligand further apart in Lth, such that they are less able to chelate to a single metal ion: the separation between the two methylene C atoms of each ligand in the structure of [Ag(Lfur)]ClO4 averages 4.84 Å over the three independent complex units, whereas in [Ag2(Lth)2](ClO4)2 the two methylene C atoms within each ligand are separated by 5.39 Å. A very similar change in the arrangement of the two substituent arms pendant from furan vs. thiophene cores has been exploited by Fujita and coworkers in the assembly of Pd-based ‘nanospheres’ of different sizes with ligands that are apparently almost isostructural.15
ES mass spectrometry confirmed the existence of the dinuclear structure in solution. Significantly, in the 1H NMR spectrum both ligands are equivalent and have two fold symmetry, indicating adoption of the maximum possible molecular symmetry (D2) for the double helix. The methylene protons appeared as a singlet rather than as a coupled pair of diastereotopic protons which are inequivalent due to the chirality of the helicate protons. This implies the presence of a dynamic process resulting in the interconversion of each molecule between two enantiomeric forms that is fast on the NMR timescale, no doubt facilitated by the high kinetic lability of Ag(I).
A dinuclear double helicate with L14naph and Ag(I)
The reaction of AgClO4 and L14naph in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio also affords a simple dinuclear double helicate complex, [Ag2(L14naph)2](ClO4)2. Unlike the previous example however this is a very irregular structure with the two ligands clearly adopting substantially different conformations to allow one of the two naphthyl groups (ligand shown in blue) to lie stacked between the two coordinated pyrazolyl–pyridine units of the alternate ligand; the atoms of the pyridyl rings lying either side of, and parallel to, the central naphthyl group are separated from the naphthyl mean plane by ca. 3.5 Å (Fig. 6). This is precisely the type of alternating stacking (acceptor/donor/acceptor) that underpins the assembly of the polyhedral cages when this family of ligands is combined with octahedrally-coordinating transition-metal dications.1
1 ratio also affords a simple dinuclear double helicate complex, [Ag2(L14naph)2](ClO4)2. Unlike the previous example however this is a very irregular structure with the two ligands clearly adopting substantially different conformations to allow one of the two naphthyl groups (ligand shown in blue) to lie stacked between the two coordinated pyrazolyl–pyridine units of the alternate ligand; the atoms of the pyridyl rings lying either side of, and parallel to, the central naphthyl group are separated from the naphthyl mean plane by ca. 3.5 Å (Fig. 6). This is precisely the type of alternating stacking (acceptor/donor/acceptor) that underpins the assembly of the polyhedral cages when this family of ligands is combined with octahedrally-coordinating transition-metal dications.1
2 showing also the interaction of Ag(1) with one of the (disordered) perchlorate anions.](/image/article/2012/RA/c2ra21757k/c2ra21757k-f6.gif) | ||
| Fig. 6 Structure of the double helical complex cation in the crystal structure of [Ag2(L14naph)2](ClO4)2 showing also the interaction of Ag(1) with one of the (disordered) perchlorate anions. | ||
The coordination geometry about the Ag(I) ions appears to be very flattened with a large gap in the coordination sphere arising from the fact that the N(pyridyl)–Ag–N(pyridyl) angles are very large [169.1° at Ag(1) and 165.2° at Ag(2)]. At Ag(1) this allows a long contact with the O atom of a perchlorate anion [Ag(1)⋯O(23X), 2.812 Å], but at Ag(2) the closest Ag⋯O contact [with O(14X)] is 3.26 Å such that Ag(1) can reasonably be described as five coordinate whereas Ag(2) is four coordinate; however disorder of the perchlorate anions means that these Ag⋯O distances should be regarded as approximate. The Ag⋯Ag distance within the complex is 7.72 Å. We note that a related structure of an Ag(I) complex with the same L14naph ligand has been reported previously.16 Again , ES mass spectrometry indicates the retention of the dinuclear structure in solution, and the 1H NMR spectrum shows (as with the earlier double helicate) that the asymmetry observed in the solid state is lost in solution. In addition we see again that the methylene protons are equivalent rather than diastereotopic, implying a dynamic process that results in each molecule changing its chirality fast on the NMR timescale.
A one-dimensional coordination polymer with LOMe and Ag(I)
The reaction of AgBF4 with LOMe in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio affords a one-dimensional coordination polymer. Each bridging ligand spans two metal ions, presenting a bidentate N-donor site to each—as in the double helicate described above—but the ligands are not in register with one another but alternate along the spine of the Ag(I) ions to give an …Ag–L–Ag–L… infinite sequence. All the Ag(I) ions are again four coordinate, from the pyrazolyl–pyridine chelates of two different ligands (Fig. 7). The chain has a ‘zigzag’ structure which is emphasised in Fig. 8, with alternating ligands coloured in red and blue.
1 ratio affords a one-dimensional coordination polymer. Each bridging ligand spans two metal ions, presenting a bidentate N-donor site to each—as in the double helicate described above—but the ligands are not in register with one another but alternate along the spine of the Ag(I) ions to give an …Ag–L–Ag–L… infinite sequence. All the Ag(I) ions are again four coordinate, from the pyrazolyl–pyridine chelates of two different ligands (Fig. 7). The chain has a ‘zigzag’ structure which is emphasised in Fig. 8, with alternating ligands coloured in red and blue.
2·(C7H8)}∞ showing the coordination environment around the Ag(1) centre, and emphasising the stacking between the methoxyphenyl unit from one ligand (blue) and the coordinated pyrazolyl–pyridine unit of another (red).](/image/article/2012/RA/c2ra21757k/c2ra21757k-f7.gif) | ||
| Fig. 7 Part of the 1-D chain structure of {[Ag2(LOMe)2](BF4)2·(C7H8)}∞ showing the coordination environment around the Ag(1) centre, and emphasising the stacking between the methoxyphenyl unit from one ligand (blue) and the coordinated pyrazolyl–pyridine unit of another (red). | ||
2·(C7H8)}∞, illustrating the stacking interactions between the chains (dashed lines).](/image/article/2012/RA/c2ra21757k/c2ra21757k-f8.gif) | ||
| Fig. 8 A view of an adjacent pair of 1-D chains in the crystal structure of {[Ag2(LOMe)2](BF4)2·(C7H8)}∞, illustrating the stacking interactions between the chains (dashed lines). | ||
The main non-covalent interaction to supplement the metal–ligand coordinate bonds within each chain is a π-stacking interaction between the central phenyl ring of one ligand and the pyrazole ring of a pyrazolyl–pyridine unit of a neighbouring ligand; this is emphasised in Fig. 7 with the methoxyphenyl spacer (blue) stacking with a coordinated pyrazolyl–pyridine ligand of the next ligand (in red). Every phenyl ring is stacked with an adjacent pyrazolyl–pyridine unit in this way, with a separation between the parallel, overlapping aromatic fragments of 3.3 Å. As this stacking is partly charge-transfer in nature it will be facilitated by the methoxy substituent, which will make the phenyl ring more electron rich and therefore strengthen the interaction with the pyrazolyl–pyridine unit which is electron deficient by virtue of coordination to a metal cation. Within the chains we also see that the CH3 group of the methoxy group is pointing directly at the face of a pyridyl ring of a neighbouring ligand with the C(methyl)⋯π(pyridyl) separation being 3.18 Å, indicative of a CH⋯π interaction. There is also π-stacking between the pyrazolyl–pyridine units of adjacent chains which results in the one-dimensional chains being associated into 2-D sheets (shown by dashed lines in Fig. 8).
A distorted ‘bow-tie’ metallamacrocycle [Ag4(Lazo)4](BF4)4
The reaction of AgBF4 with Lazo yielded a complex with an unprecedented structure in this series of {AgL}n complexes with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ag
1 Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratio—a cyclic tetramer [Ag4(Lazo)4](BF4)4. Unlike such rings which can often have a near-planar cyclic helical structure,17 this one is based on a distorted near-tetrahedral array of Ag(I) ions with four of the six edges occupied by bridging ligands. Effectively the assembly has a ‘bow-tie’-like appearance (Fig. 9) based on the disposition of metal ions, with all Ag(I) ions being four coordinate from the pyrazolyl–pyridine termini of two different ligands.
L ratio—a cyclic tetramer [Ag4(Lazo)4](BF4)4. Unlike such rings which can often have a near-planar cyclic helical structure,17 this one is based on a distorted near-tetrahedral array of Ag(I) ions with four of the six edges occupied by bridging ligands. Effectively the assembly has a ‘bow-tie’-like appearance (Fig. 9) based on the disposition of metal ions, with all Ag(I) ions being four coordinate from the pyrazolyl–pyridine termini of two different ligands.
4·2(C7H8) showing the positions of all four metal ions and two of the four ligands. The Ag ions that are connected by a bridging ligand are shown as being connected by a gold-coloured bond to clarify the structure.](/image/article/2012/RA/c2ra21757k/c2ra21757k-f9.gif) | ||
| Fig. 9 A partial view of the complex cation of [Ag4(Lazo)4](BF4)4·2(C7H8) showing the positions of all four metal ions and two of the four ligands. The Ag ions that are connected by a bridging ligand are shown as being connected by a gold-coloured bond to clarify the structure. | ||
The complex cation lies on a C2 axis and therefore contains two independent Ag(I) ions. Around the set of four ions in the cyclic array, the successive Ag⋯Ag separations are 9.71 Å [Ag(1)⋯Ag(2)], 14.01 Å [Ag(2)⋯Ag(2′)], 9.71 Å again [Ag(2′)⋯Ag(1A)] and 15.39 Å [Ag(1′)⋯Ag(1)]. The two edges of the approximate Ag4 tetrahedron that are not connected by bridging ligands, i.e. Ag(1)⋯Ag(2′) and Ag(1′)⋯Ag(2), are both 13.93 Å in length.
The main non-covalent interaction stabilising the structure is a set of two three-layer π-stacks in which an azobenzene unit from one ligand lies sandwiched between two phenyl rings of azobenzene groups from two different ligands. In Fig. 10 the (crystallographically equivalent) pair of ligands coloured red and blue have their azobenzene units sandwiched in this way, with the orange and green ligands providing the outer phenyl rings to complete the stacks. Notably, the coordinated pyrazolyl–pyridine units are not involved in any stacking interactions within the complex molecule. In addition, there are two (crystallographically equivalent) [BF4]− anions located at the periphery of the complex which participate in an array of CH⋯F hydrogen-bonding interactions with the methylene protons of the blue and red ligands (Fig. 11). Such interactions are shown by the relatively short C⋯F contacts in the range 2.7–2.9 Å with C(126) and C(146) (Fig. 11). Such H-bonding interactions involving these methylene protons have been shown in previous examples of polyhedral cage complexes to play an important role in the recognition and binding of H-bond accepting guest molecules18 and these interactions clearly help to stabilise this unusual bow-tie structure also.
4·2(C7H8) with all ligands coloured separately for clarity. The dashed lines illustrate the stacking between the central azobenzene groups of the red and blue ligands with phenyl rings from the green and yellow ligands.](/image/article/2012/RA/c2ra21757k/c2ra21757k-f10.gif) | ||
| Fig. 10 A view of the complex cation of [Ag4(Lazo)4](BF4)4·2(C7H8) with all ligands coloured separately for clarity. The dashed lines illustrate the stacking between the central azobenzene groups of the red and blue ligands with phenyl rings from the green and yellow ligands. | ||
4·2(C7H8) showing how the methylene groups of two of the ligands form CH⋯F interactions with tetrafluoroborate anions (dashed lines; see main text).](/image/article/2012/RA/c2ra21757k/c2ra21757k-f11.gif) | ||
| Fig. 11 An alternative view of the complex cation of [Ag4(Lazo)4](BF4)4·2(C7H8) showing how the methylene groups of two of the ligands form CH⋯F interactions with tetrafluoroborate anions (dashed lines; see main text). | ||
The [Ag4(Lazo)4]4+ complex cations are associated into 1-D chains via CH⋯π interactions between the coordinated pyrazolyl–pyridine units and phenyl protons of an adjacent complex; these chains associate further into 2-D sheets via stacking interactions between the pyrazolyl–pyridine units of adjacent complexes. The ES mass spectrum shows only signals consistent with an Ag2(Lazo)2 dinuclear species, possibly a double helicate: there is no trace of any signals arising from the intact tetramer. The 1H NMR spectrum indicates the formation of a symmetric species in which the ligands are all equivalent and have twofold symmetry, in agreement with the mass spectrometry data.
A triple helix of double helicates: the complex of Lbz and Ag(I)
The reaction of AgPF6 with Lbz in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio gives rise to a remarkable polymeric structure which may be described as a ‘triple helix of double helicates’. The first such example was reported briefly in a recent preliminary communication11 and additional examples are presented here. The structure is based on conventional dinuclear double helical [Ag2(Lbz)2]2+ units which assemble via Ag⋯Ag interactions into infinite one-dimensional chains that have a shallow helical twist; then, in an additional level of organisation, three of these 1-D chains are wrapped around one another to afford an infinite triple helical braid. The twisted conformation of the benzophenone spacer group lends itself naturally to the formation of helical architectures.19
1 ratio gives rise to a remarkable polymeric structure which may be described as a ‘triple helix of double helicates’. The first such example was reported briefly in a recent preliminary communication11 and additional examples are presented here. The structure is based on conventional dinuclear double helical [Ag2(Lbz)2]2+ units which assemble via Ag⋯Ag interactions into infinite one-dimensional chains that have a shallow helical twist; then, in an additional level of organisation, three of these 1-D chains are wrapped around one another to afford an infinite triple helical braid. The twisted conformation of the benzophenone spacer group lends itself naturally to the formation of helical architectures.19
The dinuclear double helical unit [Ag2(Lbz)2]2+ is shown in Fig. 12 and is in itself quite conventional. The Ag–N distances lie in the range 2.31–2.47 Å and the Ag–Ag distance is 11.64 Å. The double helical complex unit lies on a twofold axis such that only one metal ion and one ligand are in the asymmetric unit. The flattened coordination geometry around each Ag(I) ion provides space for short Ag⋯Ag contacts between units, whose distance (3.05 Å) constitutes a significant argentophilic interaction.5f In addition this short Ag⋯Ag contact results in π-stacking between the pyrazolyl–pyridine units of adjacent complex molecules, in a manner similar to that seen earlier in the complex with Lfur (Fig. 2). The resulting chain of double helical [Ag2(Lbz)2]2+ units (Fig. 13) has itself a shallow helical twist, with six [Ag2(Lbz)2]2+ units constituting a complete turn and a pitch length of 78.07 Å (equivalent to three times the length of the crystallographic c axis). Surprisingly the sense of twist of this 1-D helical chain is opposite to that of the double helical molecular units from which it is composed.
2}∞.](/image/article/2012/RA/c2ra21757k/c2ra21757k-f12.gif) | ||
| Fig. 12 Structure of the dinuclear double helical complex cation unit of {[Ag2(Lbz)2](PF6)2}∞. | ||
2}∞ showing how the double helical complex cations associate into one-dimensional chains via Ag⋯Ag interactions (orange bonds).](/image/article/2012/RA/c2ra21757k/c2ra21757k-f13.gif) | ||
| Fig. 13 A view of {[Ag2(Lbz)2](PF6)2}∞ showing how the double helical complex cations associate into one-dimensional chains via Ag⋯Ag interactions (orange bonds). | ||
The final level of organisation in this structure is that three such one-dimensional helical strands (crystallographically equivalent) are wrapped around each other to give an infinite triple helical array (Fig. 14). A space-filling view shows that this triple helix effectively forms a cylinder with a narrow channel down the central axis which is occupied by the hexafluorophosphate anions, and short CH⋯F contacts between the F atoms and internally-directed protons from the ligands clearly stabilise the structure. The distance between the successive P atoms of anions within the central channel is 6.50 Å. These anions have CH⋯F hydrogen-bonding interactions with internally-directed H atoms from the ligands, the closest H⋯F distances being 2.31 Å to a pyrazolyl proton, 2.42 Å to a phenyl proton and 2.52 Å to a methylene proton.
2}∞. In each strand, one double helical subunit is coloured in a darker shade to emphasise the relationship between the molecular double helical units and the infinite chain structure.](/image/article/2012/RA/c2ra21757k/c2ra21757k-f14.gif) | ||
| Fig. 14 The triple helical infinite chain formed by the intertwining of three helical chains in the structure of {[Ag2(Lbz)2](PF6)2}∞. In each strand, one double helical subunit is coloured in a darker shade to emphasise the relationship between the molecular double helical units and the infinite chain structure. | ||
Additional PF6− anions are located on the external surface of each triple helical cylinder, and also in the channels between the helical chains between the helicates as shown in Fig. 15, an end-on view of the cylindrical triple helicates which illustrates the hexagonal close packing of these cylinders in the extended structure. Accounting for the anions is somewhat complex. Within an asymmetric unit [containing one Ag(I) ion and one ligand] there are two anions with site occupancies of 1/6 which end up in the central channel of the triple helical cylinders. Another anion with 1/6 occupancy lies in the channel between triple helical cylinders and interacts equally with three such cylinders, forming CH⋯F interactions (H⋯F 2.55 Å) with an externally-directed pyridine H atom. The remaining anions, totalling 0.5 occupancy in the asymmetric unit, are associated with the external surface of a particular triple-helical cylinder and form CH⋯F interactions with externally-directed methylene protons (H⋯F, 2.63 Å)
2}∞ perpendicular to that in Fig. 14, showing (i) the arrangement of cylindrical chains, and (ii) the disposition of anions, both along the central cavities of the cylinders and in the channels between the cylinders.](/image/article/2012/RA/c2ra21757k/c2ra21757k-f15.gif) | ||
| Fig. 15 A view of the structure of {[Ag2(Lbz)2](PF6)2}∞ perpendicular to that in Fig. 14, showing (i) the arrangement of cylindrical chains, and (ii) the disposition of anions, both along the central cavities of the cylinders and in the channels between the cylinders. | ||
The fact that essentially identical structures were observed earlier with perchlorate and tetrafluoroborate anions11 as we also observe here with hexafluorophosphate anions implies that, although the anions are involved in weak H-bonding interactions with the complex chain, the size/shape of the anion does not significantly affect this arrangement of helical chains in the crystal. To investigate this further we investigated the crystal structures of the [Ag2(Lbz)2]2+ complexes with nitrate and triflate as the anions. These gave poorer quality crystals which scattered X-rays more weakly, and the structures are hence not reported, but it was clear from these partial determinations that the gross structures are identical in every case, confirming that anion size/shape does not significantly affect the formation of these crystal structures.
The hierarchy of different levels of organisation in these structures is particularly interesting with three distinct levels of supramolecular organisation being evident. The first of these is formation of the dinuclear double helicates from metal ions and ligands. This is followed by the Ag⋯Ag interactions and π-stacking which result in the association of the molecular helicate units into infinite one-dimensional chains. Finally we have the wrapping of three such chains around one another, aided by H-bonding to a central spine of anions, to afford the infinite triple helix which is composed of dinuclear double helical components. This structure illustrates perfectly the opportunities that are available for using self-assembly to generate very elaborate structures from simple components—and the severe difficulties involved in trying to predict and control such a disparate set of supramolecular interactions that operate over such different length scales.
Conclusions
In conclusion, we have shown how six ligands all based on bis-bidentate pyrazolyl–pyridine units but with different spacer groups have yielded a series of Ag(I) complexes in which a wide range of supramolecular interactions control the formation of the structures and their packing in crystals. All complexes are based on a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M
1 M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratio but the series as a whole displays increasing complexity: from a relatively simple mononuclear complex [Ag(Lfur)](ClO4), to dinuclear double helicates [Ag2L2](ClO4)2 with Lth and L14naph, the cyclic tetrameric ‘bow-tie’ complex [Ag4(Lazo)4](BF4)4 , and finally infinite coordination chains based on a simple 1-D coordination polymer {[AgLOMe](BF4)}∞ and a remarkably elaborate ‘triple helix of double helicates’ {[Ag2(Lbz)2](PF6)2}∞.
L ratio but the series as a whole displays increasing complexity: from a relatively simple mononuclear complex [Ag(Lfur)](ClO4), to dinuclear double helicates [Ag2L2](ClO4)2 with Lth and L14naph, the cyclic tetrameric ‘bow-tie’ complex [Ag4(Lazo)4](BF4)4 , and finally infinite coordination chains based on a simple 1-D coordination polymer {[AgLOMe](BF4)}∞ and a remarkably elaborate ‘triple helix of double helicates’ {[Ag2(Lbz)2](PF6)2}∞.
Experimental
The following ligands were prepared using previously published methods: Lfur,12 Lth,12 L14naph,13 and Lbz.11 The following compounds were also prepared according to literature methods: 3-(2-pyridyl)pyrazole,20 3,5-bis(bromomethyl)anisole21 and 4,4′-bis(bromomethyl)azobenzene.22 The instrumentation used for the routine spectroscopic analysis has been described in a recent paper.2 The metal salts and organic reagents used to make the complexes were purchased from Sigma-Aldrich and used as received. The syntheses of the new ligands are given below.New ligand syntheses
The syntheses and purifications of LOMe and Lazo were carried out exactly according to the standard method for this type of ligand, viz reaction of 2.1 equivalents of 3-(2-pyridyl)pyrazole with one equivalent of 3,5-bis(bromomethyl)anisole or 4,4′-bis(bromomethyl)azobenzene, respectively, under basic conditions and then followed by chromatographic purification. Characterisation data are as follows.Data for LOMe. 1H NMR (400 MHz, CDCl3): δ 8.62 (2H, ddd; pyridyl H6), 7.92 (2H, dt; pyridyl H3), 7.69 (2H, td; pyridyl H4), 7.41 (2H, d; pyrazolyl H5), 7.18 (2H, m; pyridyl H5), 6.90 (2H, d; pyrazolyl H4), 6.72 (1H, s; phenyl), 6.70 (2H, s; phenyl), 5.32 (4H, s; CH2), 3.70 (3H, s; CH3). ESMS: m/z 423.48 [M + H]+, 445.48 [M + Na]+. Anal. Calcd for C25H22N6O: C, 71.1; H, 5.2; N, 19.9%. Found: C, 70.9; H, 5.0; N, 19.5%.
Data for Lazo. 1H NMR (400 MHz, CDCl3): δ 8.63 (2H, ddd; pyridyl H6), 7.95 (2H, dt; pyridyl H3), 7.87 (4H, d; phenyl), 7.69 (2H, td; pyridyl H4), 7.45 (2H, d; pyrazolyl H5), 7.35 (4H, d; phenyl), 7.18 (2H, m; pyridyl H5), 6.94 (2H, d; pyrazolyl H4), 5.44 (4H, s; CH2). ESMS: m/z 497.58 [M + H]+, 519.53 [M + Na]+. Anal. Calcd for C30H24N8: C, 72.6; H, 4.9; N, 22.6%. Found: C, 72.2; H, 4.7; N, 22.3%.
Complex syntheses
All complexes were prepared using the same general method: the synthesis of [Ag(Lfur)](ClO4) is given as an illustrative example.To a solution of AgClO4 (0.029 g, 0.13 mmol) in MeOH (7 cm3) was added a solution of Lfur (0.050 g, 0.13 mmol) in CH2Cl2 (7 cm3). The mixture was stirred at room temperature for 24 h, and the resultant precipitate was filtered off, washed with both MeOH and CH2Cl2, and dried in vacuo to give [Ag(Lfur)](ClO4) as a grey powder in 70% yield. X-Ray quality crystals were grown by the slow diffusion of isopropyl ether into a solution of the complex in acetonitrile. ESMS: m/z 490.3, {[Ag(Lfur)]}+. Anal. Calcd for AgC22H18N6ClO5: C, 44.8; H, 3.1; N, 14.3%. Found: C, 44.6; H, 2.9; N, 14.2%. 1H NMR (400 MHz, CD3CN): δ 8.49 (2H, ddd; pyridyl H6), 7.96 (2H, dt; pyridyl H3), 7.96 (2H, td; pyridyl H4), 7.81 (2H, d; pyrazolyl H5), 7.43 (2H, m; pyridyl H5), 6.95 (2H, d; pyrazolyl H4), 6.39 (2H, s; furan), 5.18 (4H, s; CH2).
X-Ray crystallography
Details of the crystal, data collection and refinement parameters are summarised in Table 1; the selected structural parameters are in Table 2. Data were corrected for absorption using empirical methods (SADABS)23 based upon symmetry-equivalent reflections combined with measurements at different azimuthal angles. The structures were solved by direct methods and refined by full-matrix least squares on weighted F2 values for all reflections using the SHELX suite of programs.24 Non-hydrogen atoms were refined anisotropically. Hydrogen atoms were placed in calculated positions, refined using idealized geometries (riding model) and were assigned fixed isotropic displacement parameters.| Compound | [Ag(Lfur)](ClO4)·(MeCN)2/3 | [Ag2(Lth)2](ClO4)2 | {[Ag2(LOMe)2](BF4)2·(C7H8)}∞ |
|---|---|---|---|
| Formula | AgC46.67H20ClN6.67O5 | Ag2C44H36Cl2N12O8S2 | Ag2C57H52B2F8N12O2 |
| Molecular weight | 617.11 | 1211.61 | 1326.47 |
| T/K | 120(2) | 150(2) | 100(2) |
| Crystal system | Monoclinic | Orthorhombic | Monoclinic |
| Space group | C2/c | Pca21 | P21/n |
| a/Å | 38.9323(11) | 18.5223(9) | 10.4096(4) |
| b/Å | 15.4688(4) | 11.5704(6) | 11.3764(5) |
| c/Å | 25.4156(6) | 21.6258(11) | 23.0044(11) |
| α/° | 90 | 90 | 90 |
| β/° | 109.234(2) | 90 | 92.754(3) |
| γ/° | 90 | 90 | 90 |
| V/Å3 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 451.8(7) 451.8(7) |
4634.6(4) | 2721.1(2) |
| Z | 24 | 4 | 2 |
| ρ/g cm−3 | 1.702 | 1.736 | 1.619 |
| μ/mm−1 | 0.998 | 1.119 | 0.804 |
| Data, restraints, parameters, Rint | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 509/297/1058/0.0487 509/297/1058/0.0487 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 768/1/616/0.0579 768/1/616/0.0579 |
3888/430/363/0.0694 |
| Final R1, wR2a | 0.0301, 0.0824 | 0.0387, 0.0927 | 0.0611, 0.1484 |
| Compound | [Ag4(Lazo)4](BF4)4·2(C7H8) | [Ag2(L14naph)2](ClO4)2 | {[Ag2(Lbz)2](PF6)2}∞ |
|---|---|---|---|
| Formula | Ag4C134H112B4F16N32 | Ag2C56H44Cl2N12O8 | Ag2C62H48F12N12O2P2 |
| Molecular weight | 2949.28 | 1299.67 | 1498.80 |
| T/K | 100(2) | 150(2) | 100(2) |
| Crystal system | Monoclinic | Monoclinic | Hexagonal |
| Space group | C2/c | P21/c | P6(3)22 |
| a/Å | 34.111(6) | 15.6463(10) | 22.275(13) |
| b/Å | 11.236(2) | 24.0292(15) | 22.275(13) |
| c/Å | 40.686(8) | 14.4368(9) | 26.024(15) |
| α/° | 90 | 90 | 90 |
| β/° | 111.635(2) | 105.090(3) | 90 |
| γ/° | 90 | 90 | 120 |
| V/Å3 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 495(5) 495(5) |
5240.6(6) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 183(11) 183(11) |
| Z | 4 | 4 | 6 |
| ρ/g cm−3 | 1.351 | 1.647 | 1.335 |
| μ/mm−1 | 0.611 | 0.919 | 0.644 |
| Data, restraints, parameters, Rint | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 197/836/645/0.1137 197/836/645/0.1137 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480/301/795/0.0489 480/301/795/0.0489 |
3922/589/394/0.0963 |
| Final R1, wR2a | 0.1167, 0.3099 | 0.0395, 0.0988 | 0.0884, 0.2250 |
| a The value of R1 is based on ‘observed’ data with I > 2σ(I); the value of wR2 is based on all data. | |||
| [Ag(Lfur)](ClO4)·(MeCN)2/3 | |||
|---|---|---|---|
| a N(31) and N(31B) are two disorder components of the same pyridine ring. | |||
| Ag(1)–N(42A) | 2.231(2) | Ag(2)–N(42B) | 2.438(2) |
| Ag(1)–N(11A) | 2.236(2) | Ag(3)–N(42C) | 2.263(3) |
| Ag(1)–N(31A) | 2.479(2) | Ag(3)–N(11C) | 2.319(3) |
| Ag(1)–N(22A) | 2.575(2) | Ag(3)–N(22C) | 2.402(2) |
| Ag(2)–N(22B) | 2.343(2) | Ag(3)–N(31C) | 2.512(3) |
| Ag(2)–N(31B) | 2.344(2) | Ag(1)–Ag(2) | 3.2932(3) |
| Ag(2)–N(11B) | 2.369(2) | ||
| N(42A)–Ag(1)–N(11A) | 160.89(8) | N(22B)–Ag(2)–N(42B) | 133.39(8) |
| N(42A)–Ag(1)–N(31A) | 70.87(8) | N(31B)–Ag(2)–N(42B) | 70.39(8) |
| N(11A)–Ag(1)–N(31A) | 97.33(8) | N(11B)–Ag(2)–N(42B) | 136.45(8) |
| N(42A)–Ag(1)–N(22A) | 128.98(8) | N(42C)–Ag(3)–N(11C) | 150.96(9) |
| N(11A)–Ag(1)–N(22A) | 70.05(8) | N(42C)–Ag(3)–N(22C) | 137.00(9) |
| N(31A)–Ag(1)–N(22A) | 125.14(8) | N(11C)–Ag(3)–N(22C) | 71.51(8) |
| N(22B)–Ag(2)–N(31B) | 152.46(8) | N(42C)–Ag(3)–N(31C) | 70.61(9) |
| N(22B)–Ag(2)–N(11B) | 71.00(8) | N(11C)–Ag(3)–N(31C) | 94.53(9) |
| N(31B)–Ag(2)–N(11B) | 101.71(8) | N(22C)–Ag(3)–N(31C) | 131.43(8) |
| [Ag2(Lth)2](ClO4)2 | |||
|---|---|---|---|
| Ag(1)–N(11A) | 2.223(4) | Ag(2)–N(31B) | 2.306(4) |
| Ag(1)–N(11B) | 2.265(4) | Ag(2)–N(31A) | 2.338(4) |
| Ag(1)–N(22B) | 2.389(4) | Ag(2)–N(42A) | 2.355(4) |
| Ag(1)–N(22A) | 2.463(4) | Ag(2)–N(42B) | 2.380(4) |
| N(11A)–Ag(1)–N(11B) | 168.60(13) | N(31B)–Ag(2)–N(31A) | 164.32(12) |
| N(11A)–Ag(1)–N(22B) | 118.40(12) | N(31B)–Ag(2)–N(42A) | 120.61(14) |
| N(11B)–Ag(1)–N(22B) | 72.85(12) | N(31A)–Ag(2)–N(42A) | 71.84(14) |
| N(11A)–Ag(1)–N(22A) | 73.58(13) | N(31B)–Ag(2)–N(42B) | 71.80(14) |
| N(11B)–Ag(1)–N(22A) | 102.59(12) | N(31A)–Ag(2)–(42B) | 113.76(14) |
| N(22B)–Ag(1)–N(22A) | 104.94(11) | N(42A)–Ag(2)–N(42B) | 112.89(11) |
| {[Ag2(LOMe)2](BF4)2·(C7H8)}∞ | |||
|---|---|---|---|
| Ag(1)–N(211) | 2.237(5) | Ag(1)–N(111) | 2.392(4) |
| Ag(1)–N(122) | 2.249(4) | Ag(1)–N(222) | 2.408(4) |
| N(211)–Ag(1)–N(122) | 157.2(2) | N(211)–Ag(1)–N(222) | 72.27(19) |
| N(211)–Ag(1)–N(111) | 119.8(2) | N(122)–Ag(1)–N(222) | 121.29(18) |
| N(122)–Ag(1)–N(111) | 70.90(17) | N(111)–Ag(1)–N(222) | 121.83(19) |
| [Ag2(L14naph)2](ClO4)2 | |||
|---|---|---|---|
| Ag(1)–N(11B) | 2.326(2) | Ag(2)–N(31B) | 2.276(3) |
| Ag(1)–N(11A) | 2.345(3) | Ag(2)–N(31A) | 2.279(3) |
| Ag(1)–N(22B) | 2.389(2) | Ag(2)–N(42B) | 2.410(3) |
| Ag(1)–N(22A) | 2.393(2) | Ag(2)–N(42A) | 2.425(3) |
| N(11B)–Ag(1)–N(11A) | 169.07(9) | N(31B)–Ag(2)–(31A) | 165.22(10) |
| N(11B)–Ag(1)–N(22B) | 71.02(9) | N(31B)–Ag(2)–N(42B) | 72.49(9) |
| N(11A)–Ag(1)–N(22B) | 118.37(9) | N(31A)–Ag(2)–N(42B) | 114.89(9) |
| N(11B)–Ag(1)–N(22A) | 110.71(8) | N(31B)–Ag(2)–N(42A) | 113.57(10) |
| N(11A)–Ag(1)–N(22A) | 70.45(9) | N(31A)–Ag(2)–N(42A) | 71.83(9) |
| N(22B)–Ag(1)–N(22A) | 119.80(8) | N(42B)–Ag(2)–N(42A) | 132.16(9) |
| [Ag4(Lazo)4](BF4)4·2(C7H8) | |||
|---|---|---|---|
| Ag(1)–N(111) | 2.272(4) | Ag(2)–N(131) | 2.273(4) |
| Ag(1)–N(222) | 2.280(4) | Ag(2)–N(322) | 2.319(4) |
| Ag(1)–N(211) | 2.334(4) | Ag(2)–N(311) | 2.320(4) |
| Ag(1)–N(122) | 2.375(5) | Ag(2)–N(142) | 2.364(4) |
| N(111)–Ag(1)–N(222) | 129.7(2) | N(131)–Ag(2)–N(322) | 129.2(2) |
| N(111)–Ag(1)–N(211) | 152.15(17) | N(131)–Ag(2)–N(311) | 153.5(2) |
| N(222)–Ag(1)–N(211) | 72.98(16) | N(322)–Ag(2)–N(311) | 72.19(18) |
| N(111)–Ag(1)–N(122) | 72.44(19) | N(131)–Ag(2)–N(142) | 71.95(18) |
| N(222)–Ag(1)–N(122) | 125.1(2) | N(322)–Ag(2)–N(142) | 126.6(2) |
| N(211)–Ag(1)–N(122) | 109.93(16) | N(311)–Ag(2)–N(142) | 109.87(19) |
| {[Ag2(Lbz)2](PF6)2}∞ | |||
|---|---|---|---|
| Ag(1)–N(11) | 2.318(6) | Ag(1)–N(31B)a | 2.371(9) |
| Ag(1)–N(42) | 2.338(7) | Ag(1)–N(22) | 2.395(6) |
| Ag(1)–N(31)a | 2.476(9) | Ag(1)–Ag(1)#1 | 3.052(3) |
| N(11)–Ag(1)–N(42) | 119.4(3) | N(42)–Ag(1)–N(22) | 133.2(3) |
| N(11)–Ag(1)–N(31B)a | 161.9(4) | N(31B)–Ag(1)–N(22) | 110.0(3) |
| N(42)–Ag(1)–N(31B)a | 72.6(3) | N(11)–Ag(1)–N(31)a | 164.2(3) |
| N(11)–Ag(1)–N(22) | 72.7(3) | N(42)–Ag(1)–N(31)a | 70.8(3) |
The dataset for [Ag2(Lbz)2](PF6)2 was collected at the National Crystallography Service at the University of Southampton.25 The SQUEEZE function in PLATON was used to eliminate regions of diffuse electron density in solvent-accessible voids in the structure; full details are in the CIF. All other datasets were collected at the University of Sheffield on a Bruker APEX-2 or SMART CCD diffractometer equipped with graphite-monochromated Mo-Kα radiation from a sealed-tube source.
All of the structures suffered from disorder to some extent. For [Ag(Lfur)](ClO4)·(MeCN)2/3, [Ag2(Lth)2](ClO4)2 and [Ag2(L14naph)2](ClO4)2 this is simple disorder of perchlorate anions. For {[Ag2(LOMe)2](BF4)2·(C7H8)}∞, as well as anion disorder, the toluene solvent molecule is disordered over a symmetry element. This crystal scattered relatively weakly and geometric restraints were applied to all 6- and 5-membered rings; global displacement restraints were also applied. [Ag4(Lazo)4](BF4)4·2(C7H8) also displayed disorder of both the anions and the toluene solvent molecule. In addition the azo linkage of one of the ligands had its N atoms disordered over two positions, which could be split into N(171)/N(172) for one arrangement and N(173)/N(174) for the other (both with a trans conformation). Geometric restraints were applied to these fragments, and to all six- and five-membered aromatic rings, to keep their geometries sensible. Diffuse electron density that could not be successfully modelled was removed using the SQUEEZE function in PLATON. Finally, {[Ag2(Lbz)2](PF6)2}∞ scattered very weakly and required the use of geometric restraints on six- and five-membered rings to keep the refinement stable. One pyridine ring [containing N(31)] was disordered over two closely-spaced sites. Regions of diffuse electron density corresponding to severely disordered solvent molecules were removed using the SQUEEZE function in PLATON.
Full details of all of these issues and how they were resolved are in the individual CIFs, CCDC deposition numbers 895598 – 895603.
Acknowledgements
We thank EPSRC for financial support, the EPSRC National Crystallography Service at the University of Southampton for the crystallographic data collection of {[Ag2(Lbz)2](PF6)2}∞, and Dr Benjamin Hall for assistance with the synthesis of LOMe.References
- M. D. Ward, Chem. Commun., 2009, 4487 RSC.
- B. R. Hall, L. E. Manck, I. S. Tidmarsh, A. Stephenson, B. F. Taylor, E. J. Blaikie, D. A. Vander Griend and M. D. Ward, Dalton Trans., 2011, 40, 12132 RSC.
- A. Stephenson, S. P. Argent, T. Riis-Johannessen, I. S. Tidmarsh and M. D. Ward, J. Am. Chem. Soc., 2011, 133, 858 CrossRef CAS.
- (a) H. Fenton, I. S. Tidmarsh and M. D. Ward, Dalton Trans., 2009, 4199 RSC; (b) S. P. Argent, H. Adams, T. Riis-Johannessen, J. C. Jeffery, L. P. Harding, W. Clegg, R. W. Harrington and M. D. Ward, Dalton Trans., 2006, 2996 Search PubMed; (c) S. P. Argent, H. Adams, L. P. Harding, T. Riis-Johannessen, J. C. Jeffery and M. D. Ward, New J. Chem., 2005, 29, 904 RSC.
- (a) A. M. Stadler, N. Kyritsakas, G. Vaughan and J.-M. Lehn, Chem.–Eur. J., 2007, 13, 59 CrossRef CAS; (b) M. Barboiu, G. Vaughan, N. Kyritsakas and J.-M. Lehn, Chem.–Eur. J., 2003, 9, 763 CrossRef CAS; (c) G. Baum, E. C. Constable, D. Fenske, C. E. Housecroft and T. Kulke, Chem. Commun., 1998, 2659 Search PubMed; (d) W. L. Leong and J. J. Vittal, Chem. Rev., 2011, 111, 688 CrossRef CAS; (e) T. C. W. Mak, X.-L. Zhao, Q.-M. Wang and G.-C. Guo, Coord. Chem. Rev., 2007, 251, 2311 CrossRef CAS; (f) P. Pyykkö, Chem. Rev., 1997, 97, 597 CrossRef.
- G.-G. Hou, Y. Wu, J.-P. Ma and Y.-B. Dong, CrystEngComm, 2011, 13, 6850 RSC.
- N. Gerasimchuk, A.N. Esaulenko, K.N. Dalley and C. Moore, Dalton Trans., 2010, 39, 749 RSC.
- M.-T. Chen, B. Landers and O. Navarro, Org. Biomol. Chem., 2012, 10, 2206 CAS.
- X.-F. Zheng and L.-G. Zhu, CrystEngComm, 2010, 12, 2878 RSC.
- L. Holland, W.-Z. Shen, P. von Grebe, P. J. Sanz Miguel, F. Pichierri, A. Springer, C. A. Schalley and B. Lippert, Dalton Trans., 2011, 40, 5159 RSC.
- A. Stephenson and M. D. Ward, Chem. Commun., 2012, 48, 3605 RSC.
- A. Stephenson and M. D. Ward, Dalton Trans., 2011, 40, 10360 RSC.
- A. Stephenson and M. D. Ward, Dalton Trans., 2011, 40, 7824 RSC.
- G. W. Eastland, M. A. Mazid, D. R. Russell and M. C. R. Symons, J. Chem. Soc., Dalton Trans., 1980, 1682 RSC.
- (a) M. Tominaga, K. Suzuki, M. Kawano, T. Kusukawa, T. Ozeki, S. Sakamoto, K. Yamaguchi and M. Fujita, Angew. Chem., Int. Ed., 2004, 43, 5621 CrossRef CAS; (b) Q.-F. Sun, J. Iwasa, D. Ogawa, Y. Ishido, S. Sato, T. Ozeki, Y. Sei, K. Yamaguchi and M. Fujita, Science, 2010, 328, 1144 CrossRef CAS.
- J.-L. Du, T.-L. Hu, S.-M. Zhang, Y.-F. Zeng and X.-H. Bu, CrystEngComm, 2008, 10, 1866 RSC.
- (a) S. P. Argent, H. Adams, T. Riis-Johannessen, J. C. Jeffery, L. P. Harding, O. Mamula and M. D. Ward, Inorg. Chem., 2006, 45, 3905 CrossRef CAS; (b) N. K. Al-Rasbi, H. Adams, L. P. Harding and M. D. Ward, Eur. J. Inorg. Chem., 2007, 4770 CrossRef CAS; (c) B. Hasenknopf, J.-M. Lehn, B. O. Kneisel, G. Baum and D. Fenske, Angew. Chem., Int. Ed. Engl., 1996, 35, 1838 CrossRef CAS; (d) B. Hasenknopf, J.-M. Lehn, N. Boumediene, A. Dupont-Gervais, A. van Dorsselaer, B. Kneisel and D. Fenske, J. Am. Chem. Soc., 1997, 119, 10956 CrossRef CAS; (e) G. Baum, E. C. Constable, D. Fenske, C. E. Housecroft and T. Kulke, Chem. Commun., 1999, 195 Search PubMed; (f) F. Tuna, J. Hamblin, A. Jackson, G. Clarkson, N. W. Alcock and M. J. Hannon, Dalton Trans., 2003, 2141 RSC; (g) P. L. Jones, K. J. Byrom, J. C. Jeffery, J. A. McCleverty and M. D. Ward, Chem. Commun., 1997, 1361 RSC; (h) O. Mamula, A. von Zelewsky, P. Brodard, C. W. Schlapfer, G. Bernardinelli and H. Stoeckli-Evans, Chem.–Eur. J., 2005, 11, 3049 CrossRef CAS.
- (a) S. Turega, M. Whitehead, B. R. Hall, M. F. Haddow, C. A. Hunter and M. D. Ward, Chem. Commun., 2012, 48, 2752 RSC; (b) R. L. Paul, Z. R. Bell, J. C. Jeffery, J. A. McCleverty and M. D. Ward, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4883 CrossRef CAS.
- X.-D. Chen and T. C. W. Mak, Dalton Trans., 2005, 3646 RSC.
- (a) A. J. Amoroso, A. M. W. Cargill Thompson, J. C. Jeffery, P. L. Jones, J. A. McCleverty and M. D. Ward, J. Chem. Soc., Chem. Commun., 1994, 2751 RSC; (b) H. Brunner and T. Scheck, Chem. Ber., 1992, 125, 701 CrossRef CAS; (c) Y. Lin and S. A. Lang, J. Heterocycl. Chem., 1977, 14, 345 CrossRef CAS.
- S. In, S. J. Cho, K. H. Lee and J. Kang, Org. Lett., 2005, 7, 3993 CrossRef CAS.
- F. Bonardi, G. London, N. Nouwen, B. L. Feringa and A. J. M. Driessen, Angew. Chem., Int. Ed., 2010, 49, 7234 CrossRef CAS.
- G. M. Sheldrick, SADABS: A program for absorption correction with the Siemens SMART system, University of Göttingen, Germany, 1996 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr. Sect. A, 2008, 64, 112 CrossRef.
- S. J. Coles and P. A. Gale, Chem. Sci., 2012, 3, 683 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
