DOI:
10.1039/C2RA20662E
(Communication)
RSC Adv., 2012,
2, 7660-7665
Sulfamic acid: an efficient, cost-effective and recyclable catalyst for the synthesis of triazole[1,2-a]indazole-trione derivatives†
Received
25th February 2012
, Accepted 19th June 2012
First published on 1st August 2012
Abstract
Sulfamic acid (SA) is successfully utilized as a green, cost-effective and reusable catalyst for the synthesis of triazole[1,2-a]indazole-trione derivatives involving the one-pot, three-component condensation of urazole, aldehydes and cyclic β-diketones in high yields in water as a sole solvent. The salient features of this new methodology are cheaper process, easy availability of the catalyst, mild conditions, versatility, and the catalyst could be recycled easily without affecting the catalytic activity.
Introduction
Developing a simple, eco-friendly approach for the synthesis of compound libraries of medicinal scaffolds is a lucrative area of research in both academic and pharmaceutical R & D.1 MCR protocols in water will be one of the most suitable strategies which will meet the requirements of green chemistry as well as for developing libraries of compounds of pharmaceutical importance.2 With the emphasis on the adoption of cleaner green chemistry processes and concerns over the environmental impact of using volatile organic solvents (VOCs), the potential of water or non-classical solvents has become highly relevant.3 In addition to its abundance and for economical and safety reasons, water has naturally become a substitute and an environmentally benign solvent in organic synthesis.4 Moreover, since the pioneering studies by Breslow5,6 on Diels–Alder reactions, there has been an increasing recognition that organic reactions could proceed well in aqueous media offering key advantages over organic solvents, such as rate enhancement and insolubility of the final products, which facilitates their isolation. The hydrophobic effect of water generates internal pressure and promotes the association of reactants in the solvent cavity during the activation process and accelerates the reaction. Any factor which increases the hydrophobic effect will increase the reaction rate.7 The use of the aqueous medium as solvent also reduces the harmful effects of organic solvents on the environment. This becomes further sophisticated if these reactions can be performed using inexpensive reagents.
The development of mild, low-cost and high-performance acid catalysts has attracted much interest for green chemistry.8 In this regard, sulfamic acid has emerged as a promising substitute for conventional Bronsted- and Lewis acid catalysts. It is water soluble, relatively stable, non-volatile, non-hygroscopic, and non-corrosive and is a commercially available cheap chemical. This catalyst could be easily recycled and reused due to its very high miscibility in water. It has displayed an excellent activity over a vast array of acid-catalyzed organic transformations as witnessed by numerous reports published in the past.9 Nitrogen-containing heterocyclic molecules are of importance as they are part of many natural products, fine chemicals and biologically active pharmaceuticals that are vital for enhancing the quality of life.10 Among them, heterocycles containing a urazole [1,2,4-triazolidine-3,5-diones] moiety and its derivatives are of interest as they exhibit a wide range of biological and clinical applications.11 Urazole derivatives also possess anticonvulsant12 or fungicidal activity13 as well as catalytic activity in radical polymerization.14 Literature reveals a number of methods for the synthesis of heterocycles containing urazole moiety.15,16 Despite the available methodologies, there still exists a demand for devising a more efficient and environmentally benign procedure which allows the ready synthesis of urazole polycyclic systems.
In continuation of our studies aimed at devising new and greener approaches for synthesis of nitrogen containing heterocyclic compounds,17 we herein report a simple, convenient and eco-friendly protocol for the preparation of triazolo[1,2-a]indazolones by one-pot, three-component condensation of urazole, aldehydes and cyclic β-diketones using sulfamic acid (SA) as a reusable catalyst in water as a sole solvent (Scheme 2).
![Synthesis of library of triazole[1,2-a]indazole-trione derivatives.](/image/article/2012/RA/c2ra20662e/c2ra20662e-s2.gif) |
| | Scheme 2 Synthesis of library of triazole[1,2-a]indazole-trione derivatives. | |
Results and discussion
Our initial efforts were focused on the search for a catalyst for the condensation reaction between urazole, aldehydes and cyclic β-diketones. For this purpose, representative reactions involving urazole 1 (1 mmol), benzaldehyde 2a (1.2 mmol) and cyclohexane-1,3-dione 3a (1 mmol) were carried out in the presence of a variety of Bronsted- and Lewis acid-catalysts at different levels of loading in water as a solvent at 50 °C (Scheme 1). After systematic screening, sulfamic acid was found to be the best possible catalyst for this multi-component reaction. Though the various catalysts progressed the reaction to a good extent, from the view point of performance, price, toxicity and ease of handling, SA was found to be the best potential candidate for carrying out this reaction. The results summarized in Table 1 clearly indicate the essentiality as well as the high catalytic activity of sulfamic acid to yield the desired product 4a in good yields (82%) within a shorter reaction time (70 min). However, the uncatalyzed reaction did not yield any product, even after 15 h (Table 1, entry 1).
![Model reaction for the synthesis of triazole[1,2-a]indazole-triones.](/image/article/2012/RA/c2ra20662e/c2ra20662e-s1.gif) |
| | Scheme 1 Model reaction for the synthesis of triazole[1,2-a]indazole-triones. | |
Table 1 Compared performances of various acid catalysts for the model reactiona
| Entry |
Catalyst (x mol%) |
Time (min) |
Yieldb (%) |
|
Reaction conditions: urazole 1 (1 mmol), benzaldehyde 2a (1.2 mmol), cyclohexane-1,3-dione 3a (1 mmol); catalyst: (x mol%); temp: 50 °C; solvent: water (10 ml).
Isolated yields.
|
| 1 |
Uncatalyzed |
900 |
Nil |
| 2 |
L -proline (10) |
530 |
Trace |
| 3 |
H2NSO3H (10) |
70 |
82 |
| 4 |
Amberlyst-15 (10) |
180 |
76 |
| 5 |
PMA–SiO2 (2) |
300 |
65 |
| 6 |
PMA–SiO2 (5) |
280 |
70 |
| 7 |
PMA–SiO2 (10) |
250 |
78 |
| 8 |
HClO4–SiO2 (2) |
200 |
62 |
| 9 |
HClO4–SiO2 (5) |
180 |
68 |
| 10 |
HClO4–SiO2 (10) |
140 |
78 |
| 11 |
pTSA (5) |
220 |
58 |
| 12 |
pTSA (10) |
180 |
66 |
| 13 |
pTSA (20) |
150 |
72 |
| 14 |
pTSA (30) |
90 |
80 |
| 15 |
Montmorillonite K-10 (10) |
320 |
55 |
| 16 |
I2 (10) |
650 |
48 |
| 17 |
InCl3 (30) |
220 |
56 |
With the best catalyst in hand, we then studied the influence of different amounts of catalyst (Table 2) and temperature on the reaction time and yield (Fig. 1) for the model reaction. The best catalytic activity of sulfamic acid was optimized to be at 20 mol% at 50 °C and any excess of the catalyst, beyond this proportion did not have any significant effect on the conversion and yield of product. Further prolongation of the reaction time and increasing the temperature seemed ineffective for improvement of the product yield. Thus, using the model reaction (Scheme 1) when carried out in the presence of 20 mol% of SA in water at 50 °C, the corresponding product 4a was obtained after 40 min in 92% yield (Table 2, Entry 6). The present method is better in terms of yield as well as mildness of the reaction conditions as compared to the methods reported earlier by Bazgir et al. and Hamidian et al.16 These methods are inferior in terms of lower yield (90%) and harshness of reaction conditions employed.
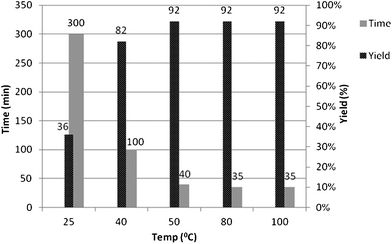 |
| | Fig. 1 Effect of temperature on the model reaction.a | |
Table 2 Catalytic activity evaluation of SA for the synthesis of triazolo[1,2-a]indazole-trionesa
The efficacy of our protocol was well evaluated using a wide range of aldehydes (Scheme 2). As indicated in Table 3, it seemed that there was no remarkable electronic effect from the substituents on aldehyde moiety, since the aryl aldehydes with both electron-donating and electron-withdrawing groups could be applied as efficient candidates for the synthesis of corresponding triazolo[1,2-a]indazolone derivatives in good yields. However, the aliphatic aldehydes reacted slowly as compared to the aryl aldehydes and gave low yields of the products (Table 3, entries 18–21). The present reaction was further investigated using ketones like acetophenone, cyclohexanone etc. as the carbonyl source in place of aldehyde but they were ineffective and the reaction did not proceed under the optimized reaction conditions.
Table 3 Sulfamic acid mediated synthesis of triazole[1,2-a]indazole-trione derivativesa
| S. no. |
R |
R1 |
4
|
Time (min) |
Yieldb (%) |
|
Reaction conditions: urazole 1 (1 mmol), aldehyde 2(a–o) (1.2 mmol), cyclohexane-1,3-dione 3a or dimedone 3b (1 mmol); catalyst: H2NSO3H (20 mol%); temp: 50 °C; solvent: water (10 ml).
Isolated yields.
|
| 1 |
C6H5 (2a) |
H (3a) |
4a
|
40 |
92 |
| 2 |
4-Cl-C6H4 (2b) |
H |
4b
|
45 |
87 |
| 3 |
4-MeO-C6H4 (2c) |
H |
4c
|
40 |
88 |
| 4 |
4-Me-C6H4 (2d) |
H |
4d
|
45 |
92 |
| 5 |
3-HO-C6H4 (2e) |
H |
4e
|
50 |
90 |
| 6 |
3-NO2-C6H4 (2f) |
H |
4f
|
60 |
88 |
| 7 |
4-NO2-C6H4 (2g) |
H |
4g
|
70 |
90 |
| 8 |
Piperonyl (2h) |
H |
4h
|
50 |
86 |
| 9 |
C6H5 |
Me (3b) |
4i
|
40 |
92 |
| 10 |
4-Br-C6H4 (2i) |
Me |
4j
|
50 |
90 |
| 11 |
4-MeO-C6H4 |
Me |
4k
|
45 |
87 |
| 12 |
4-Me-C6H4 |
Me |
4l
|
40 |
91 |
| 13 |
3-HO-C6H4 |
Me |
4m
|
45 |
90 |
| 14 |
3-NO2-C6H4 |
Me |
4n
|
50 |
90 |
| 15 |
4-NO2-C6H4 |
Me |
4o
|
60 |
87 |
| 16 |
2-HO-C6H4 (2j) |
Me |
4p
|
50 |
88 |
| 17 |
2-Thienyl (2k) |
Me |
4q
|
60 |
85 |
| 18 |
Propyl (2l) |
Me |
4r
|
90 |
65 |
| 19 |
Ethyl (2m) |
H |
4s
|
90 |
68 |
| 20 |
Isobutyl (2n) |
H |
4t
|
90 |
65 |
| 21 |
Hexyl (2o) |
H |
4u
|
90 |
62 |
The products 4(a–u) are stable solids and were characterized by using IR, 1H NMR, 13C NMR and ESI-MS spectra and elemental analysis data. An appropriate mechanistic rationale portraying the probable sequence of events is indicated in Scheme 3. Firstly, it involves the protonation of the carbonyl group of the aldehyde 2(a–o) followed by attack of the enolate form of the cyclic β-diketone. The hydrogen ion donated by sulfamic acid helps in the enolization of 1,3-dicarbonyl compounds to form the enolate intermediate. Then, the formation of the heterodiene (5) takes place by Knoevenagel condensation of enolate 3(a–b) and aldehyde 2(a–o) in the presence of sulfamic acid. This step is followed by subsequent Michael addition of urazole (1) to heterodiene (5) followed by concerted cyclization via the condensation of amino and carbonyl group of the intermediate (6) to furnish the corresponding products 4(a–u) along with water as the by-product. In this whole process, sulfamic acid is regenerated and is reused for the next run.
![Suitable mechanism for the formation of triazole[1,2-a]indazole-triones.](/image/article/2012/RA/c2ra20662e/c2ra20662e-s3.gif) |
| | Scheme 3 Suitable mechanism for the formation of triazole[1,2-a]indazole-triones. | |
The recycling potential of the sulfamic acid catalyst was investigated for the model reaction (Scheme 1) at 50 °C in water as the solvent and the results obtained are summarized in Table 4. As sulfamic acid is insoluble in most of the common organic solvents, upon completion of the reaction, ethyl acetate was added to extract the product formed and the water extract containing the catalyst was reused for consecutive reactions with fresh substrates. Furthermore, to rule out the possibility of catalyst leaching, we also tried the following procedure for the model reaction (Scheme 1) up to the fourth run. After completion of the reaction, the water extract that was left was subjected to evaporation under reduced pressure and the solid catalyst that was recovered was weighed, which came out to be approximately 0.1950 g, nearly 20 mol% catalyst. The experimental results revealed that SA could be reused four times with only a slight decrease in the yield. Loss of the weight of SA is attributed to handling.
Experimental
General
Chemicals were purchased from Sigma-Aldrich and Sisco Research Laboratories and were used without further purification. All reactions and purity of triazole[1,2-a]indazole-trione derivatives were monitored by thin-layer chromatography (TLC) using aluminium plates coated with silica gel F254 plates (Merck) using 30% ethyl acetate and 70% hexane as an eluent. The spots were detected either under ultraviolet (UV) light or by placing them in an iodine chamber. Melting points were determined in open capillary tubes using Thomas Hoover melting point apparatus and are uncorrected. Infrared (IR) spectra were recorded on a Perkin-Elmer FTIR-1710 spectrophotometer using nujol film. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a JEOL JNM-ECX 400P FT NMR spectrometer using tetramethylsilane (TMS) as an internal standard and the value of chemical shift values are recorded on the δ scale and coupling constants (J) values are in hertz (Hz). Mass spectra were recorded on a Waters LCT Micromass spectrometer. Elemental analysis was performed on a Hereaus CHN rapid analyzer. The temperature of the reaction mixture was measured through a non-contact infrared mini gun thermometer (AZ minigun type, model 8868).
General procedure for the synthesis of triazole[1,2-a]indazole-trione derivatives
A mixture of urazole 1 (1 mmol), aldehyde RCHO 2(a–o) (1.2 mmol), cyclic β-diketone 3a (C6H8O2) or 3b (C8H12O2) (1 mmol) and sulfamic acid (20 mol%) were stirred in water (10 ml) at 50 °C until the TLC indicated the completion of the reaction. After the completion of the reaction, the reaction mixture was cooled to room temperature and ethyl acetate (5 ml × 3) was added to the reaction mixture to extract the product. The combined organic layers were washed with water, dried over anhydrous sodium sulphate and concentrated under reduced pressure to obtain the neat product. Products thus obtained were subjected to purification either by recrystallization from absolute ethanol or column chromatography on silica gel (100–200 mesh size) using hexane–ethyl acetate in varying proportions as eluent, which afforded the respective triazole[1,2-a]indazole-trione derivatives, 4(a–u). All the synthesized products were stable solids and their authenticity was established on the basis of their spectral analysis (IR, 1H NMR, 13C NMR, ESI-MS) and elemental analysis data. The spectral data for synthesized compounds are listed below.
Recycling and reusability of sulfamic acid
One of the unique features of sulfamic acid is its immiscibility with common organic solvents. Thus, upon completion of the reaction, the product was extracted using ethyl acetate, dried over anhydrous sodium sulphate and evaporated under reduced pressure to obtain the product. The water extract that remained contained the sulfamic acid catalyst which was used as such for the consecutive runs without any appreciable loss in its catalytic activity, for up to four runs.
Spectral data for the synthesized derivatives 4(a–u)
5,6,7,9-Tetrahydro-9-(phenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4a).
M.Pt.: 246–250 °C; IR (film, νmax cm−1): 3250, 2956, 1782, 1731, 1655; 1H NMR (CDCl3, 400 MHz): δ 1.93–2.04 (m, 2H, CH2), 2.25–2.38 (m, 2H, CH2), 2.47–2.66 (m, 2H, CH2), 6.08 (s, 1H, CH-Ar), 7.06–7.39 (m, 5H, Ar-H), 10.10 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 19.6, 27.4, 34.4, 52.7, 116.8, 126.6, 127.4, 127.7, 128.7, 136.4, 139.3, 149.8, 153.6, 192.8; ESI-MS: 282.98 (M+); Anal calcd. for C15H13N3O3: C, 63.60; H, 4.63; N, 14.83; Found: C, 63.28; H, 4.49; N, 14.66.
5,6,7,9-Tetrahydro-9-(4-chlorophenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4b).
M.Pt.: 182–186 °C; IR (film, νmax cm−1): 3199, 2954, 1782, 1738, 1664; 1H NMR (CDCl3, 400 MHz): δ 2.01–2.30 (m, 2H, CH2), 2.41–2.63 (m, 2H, CH2), 2.77–2.92 (m, 2H, CH2), 4.70 (br s, 1H, NH), 6.00 (s, 1H, CH-Ar), 7.30–7.94 (m, 4H, Ar-H); 13C NMR (CDCl3, 100 MHz): δ 19.6, 28.3, 34.3, 52.8, 119.6, 126.5, 127.3, 129.4, 137.1, 139.4, 148.8, 150.6, 191.7; ESI-MS: 316.97 (M+); Anal calcd. for C15H12ClN3O3: C, 56.70; H, 3.81; N, 13.23; Found: C, 56.54; H, 3.65; N, 13.08.
5,6,7,9-Tetrahydro-9-(4-methoxyphenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4c).
M.Pt.: 176–180 °C; IR (film, νmax cm−1): 3299, 2956, 2365, 1858, 1740, 1665; 1H NMR (CDCl3, 400 MHz): δ 1.95–2.07 (m, 2H, CH2), 2.26–2.41 (m, 2H, CH2), 2.55–2.68 (m, 2H, CH2), 3.73 (s, 3H, OCH3), 4.75 (br s, 1H, NH), 6.03 (s, 1H, CH-Ar), 6.73–7.22 (m, 4H, Ar-H); 13C NMR (CDCl3, 100 MHz): δ 20.3, 28.2, 37.7, 52.9, 55.8, 115.6, 118.8, 127.8, 133.6, 140.7, 149.3, 154.6, 157.9, 194.5; ESI-MS: 313.05 (M+); Anal calcd. for C16H15N3O4: C, 61.34; H, 4.83; N, 13.41; Found: C, 61.20; H, 4.68; N, 13.25.
5,6,7,9-Tetrahydro-9-(4-methylphenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4d).
M.Pt.: 220–224 °C; IR (film, νmax cm−1): 3303, 2953, 1782, 1735, 1670; 1H NMR (CDCl3, 400 MHz): δ 1.93–2.02 (m, 2H, CH2), 2.23 (s, 3H, CH3), 2.29–2.42 (m, 2H, CH2), 2.53–2.64 (m, 2H, CH2), 6.28 (s, 1H, CH-Ar), 6.99–7.77 (m, 4H, Ar-H), 9.94 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 20.2, 21.0, 36.9, 37.1, 116.9, 128.6, 128.8, 135.9, 141.4, 143.8, 149.8, 163.8, 196.6; ESI-MS: 297.04 (M+); Anal calcd. for C16H15N3O3: C, 64.64; H, 5.09; N, 14.13; Found: C, 64.50; H, 4.94; N, 13.91.
5,6,7,9-Tetrahydro-9-(3-hydroxyphenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4e).
M.Pt.: 238–240 °C; IR (film, νmax cm−1): 3327, 2917, 1768, 1719, 1685; 1H NMR (CDCl3, 400 MHz): δ 1.89–2.05 (m, 2H, CH2), 2.29–2.41 (m, 2H, CH2), 2.50–2.68 (m, 2H, CH2), 4.75 (br s, 1H, NH), 6.04 (s, 1H, CH-Ar), 7.11–7.42 (m, 4H, Ar-H), 9.91 (br s, 1H, OH); 13C NMR (CDCl3, 100 MHz): δ 19.7, 27.6, 38.8, 54.5, 114.8, 116.6, 118.3, 121.2, 127.5, 137.7, 141.8, 148.6, 153.4, 155.7, 193.6; ESI-MS: 299.0 (M+); Anal calcd. for C15H13N3O4: C, 60.20; H, 4.38; N, 14.04; Found: C, 60.00; H, 4.16; N, 13.90.
5,6,7,9-Tetrahydro-9-(3-nitrophenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4f).
M.Pt.: 146–150 °C; IR (film, νmax cm−1): 3089, 2933, 1784, 1735, 1654; 1H NMR (CDCl3, 400 MHz): δ 1.88–1.94 (m, 2H, CH2), 2.25–2.31 (m, 2H, CH2), 2.34–2.38 (m, 2H, CH2), 6.14 (s, 1H, CH-Ar), 7.30–8.19 (m, 4H, Ar-H), 10.10 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 19.5, 26.8, 33.4, 53.8, 117.7, 120.8, 121.3, 128.6, 130.5, 136.7, 138.9, 145.4, 150.8, 154.6, 196.7; ESI-MS: 328.03 (M+); Anal calcd. for C15H12N4O5: C 54.88; H 3.68; N 17.07; Found: C, 54.64; H, 3.53; N, 16.92.
5,6,7,9-Tetrahydro-9-(4-nitrophenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4g).
M.Pt.: 152–156 °C; IR (film, νmax cm−1): 3402, 3082, 2924, 1762, 1711, 1605; 1H NMR (CDCl3, 400 MHz): δ 1.88–2.16 (m, 2H, CH2), 2.27–2.55 (m, 2H, CH2), 2.60–2.94 (m, 2H, CH2), 4.77 (br s, 1H, NH), 6.12 (s, 1H, CH-Ar), 7.55–8.33 (m, 4H, Ar-H); 13C NMR (CDCl3, 100 MHz): δ 20.2, 28.1, 37.6, 55.4, 118.7, 122.8, 127.7, 139.2, 139.8, 144.6, 148.3, 153.7, 195.2; ESI-MS: 328.02 (M+); Anal calcd. for C15H12N4O5: C, 54.88; H, 3.68; N, 17.07; Found: C, 54.65; H, 3.44; N, 16.87.
5,6,7,9-Tetrahydro-9-(benzo[1,3]-dioxo-5-yl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4h).
M.Pt.: 262–266 °C; IR (film, νmax cm−1): 3401, 2953, 2127, 1788, 1739, 1647; 1H NMR (CDCl3, 400 MHz): δ 1.60–1.75 (m, 2H, CH2), 1.94–2.04 (m, 2H, CH2), 2.18–2.38 (m, 2H, CH2), 5.58 (s, 1H, CH-Ar), 5.80 (s, 2H, CH2-piperonyl), 6.26–6.75 (m, 3H, Ar-H), 8.56 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 19.3, 27.6, 36.8, 53.8, 100.2, 116.5, 118.8, 120.4, 121.3, 137.8, 140.8, 145.6, 149.8, 151.7, 156.5, 194.7; ESI-MS: 327.03 (M+); Anal calcd. for C16H13N3O5: C, 58.72; H, 4.00; N, 12.84; Found: C, 58.60; H, 3.84; N, 12.71.
5,6,7,9-Tetrahydro-6,6-dimethyl-9-(phenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4i).
M.Pt.: 150–154 °C; IR (film, νmax cm−1): 3200, 2958, 1781, 1735, 1596; 1H NMR (CDCl3, 400 MHz): δ 1.05 (s, 3H, CH3), 1.09 (s, 3H, CH3), 2.36 (s, 2H, CH2), 2.81 (2H, AB system 2JHH = 16.9 Hz, CH2), 6.41 (s, 1H, CH-Ar), 7.14–7.65 (m, 5H, Ar-H), 10.00 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 28.4, 28.7, 39.6, 53.4, 59.7, 118.7, 126.5, 127.9, 136.8, 148.7, 150.6, 154.6, 194.2; ESI-MS: 311.07 (M+); Anal calcd. for C17H17N3O3: C, 65.58; H, 5.50; N, 13.50; Found: C, 65.43; H, 5.35; N, 13.37.
5,6,7,9-Tetrahydro-6,6-dimethyl-9-(4-bromophenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4j).
M.Pt.: 192–196 °C; IR (film, νmax cm−1): 3016, 2962, 1765, 1701, 1648; 1H NMR (CDCl3, 400 MHz): δ 0.94 (s, 6H, 2 × CH3), 2.08 (s, 2H, CH2), 2.70 (2H, AB system 2JHH = 19.1 Hz, CH2), 5.93 (s, 1H, CH-Ar), 6.82–7.61 (m, 4H, Ar-H), 9.84 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 27.2, 28.4, 38.7, 52.4, 60.7, 120.7, 125.2, 127.6, 128.7, 143.7, 146.1, 152.4, 156.2, 192.7; ESI-MS: 388.97 (M+); Anal calcd. for C17H16BrN3O3: C, 52.32; H, 4.13; N, 10.77; Found: C, 52.18; H, 3.97; N, 10.62.
5,6,7,9-Tetrahydro-6,6-dimethyl-9-(4-methoxyphenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4k).
M.Pt.: 176–180 °C; IR (film, νmax cm−1): 3015, 2931, 1895, 1697, 1599; 1H NMR (CDCl3, 400 MHz): δ 1.05 (s, 6H, 2 × CH3), 2.41 (s, 2H, CH2), 2.86 (2H, AB system 2JHH = 21.2 Hz, CH2), 3.72 (s, 3H, OCH3), 5.45 (s, 1H, CH-Ar), 6.70–6.96 (m, 4H, Ar-H), 9.83 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 26.4, 27.3, 38.4, 53.5, 58.9, 60.3, 117.7, 119.2, 127.4, 138.1, 141.7, 148.5, 151.6, 155.4, 194.7; ESI-MS: 341.05 (M+); Anal calcd. for C18H19N3O4: C, 63.33; H, 5.61; N, 12.31; Found: C, 63.20; H, 5.48; N, 12.17.
5,6,7,9-Tetrahydro-6,6-dimethyl-9-(4-methylphenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4l).
M.Pt.: 170–174 °C; IR (film, νmax cm−1): 3019, 2929, 1897, 1666, 1598; 1H NMR (CDCl3, 400 MHz): δ 0.99 (s, 3H, CH3), 1.12 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.36 (s, 2H, CH2), 2.96 (2H, AB system 2JHH = 19.0 Hz, CH2), 5.41 (s, 1H, CH-Ar), 6.88–7.22 (m, 4H, Ar-H), 9.84 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 20.6, 27.8, 28.4, 36.2, 52.8, 57.3, 118.6, 126.8, 128.2, 135.9, 140.7, 143.2, 147.5, 156.7, 196.7; ESI-MS: 325.10 (M+); Anal calcd. for C18H19N3O3 : C, 66.45; H, 5.89; N, 12.91; Found : C, 66.31; H, 5.72; N, 12.76.
5,6,7,9-Tetrahydro-6,6-dimethyl-9-(3-hydroxyphenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4m).
M.Pt.: 122–126 °C; IR (film, νmax cm−1): 3413, 2961, 1778, 1734, 1654; 1H NMR (CDCl3, 400 MHz): δ 0.97 (s, 3H, CH3), 1.00 (s, 3H, CH3), 2.29 (s, 2H, CH2), 2.63 (2H, AB system 2JHH = 16.2 Hz, CH2), 5.80 (s, 1H, CH-Ar), 6.78–6.98 (m, 4H, Ar-H), 8.12 (br s, 1H, NH), 9.80 (br s, 1H, OH); 13C NMR (CDCl3, 100 MHz): δ 26.5, 27.4, 34.2, 50.7, 54.7, 116.2, 117.3, 120.3, 121.5, 128.6, 139.4, 141.6, 149.7, 152.2, 158.3, 195.8; ESI-MS: 327.06 (M+); Anal calcd. for C17H17N3O4: C, 62.38; H, 5.23; N, 12.84; Found: C, 62.15; H, 5.10; N, 12.67.
5,6,7,9-Tetrahydro-6,6-dimethyl-9-(3-nitrophenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4n).
M.Pt.: 134–138 °C; IR (film, νmax cm−1): 3210, 2927, 1762, 1732, 1668; 1H NMR (CDCl3, 400 MHz): δ 1.09 (s, 3H, CH3), 1.25 (s, 3H, CH3), 2.42 (s, 2H, CH2), 2.83 (2H, AB system 2JHH = 17.6 Hz, CH2), 5.51 (s, 1H, CH-Ar), 7.37–8.22 (m, 4H, Ar-H), 10.10 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 29.2, 29.7, 36.4, 53.8, 58.4, 120.8, 122.8, 124.7, 129.6, 133.8, 137.4, 142.7, 149.5, 149.8, 159.7, 194.2; ESI-MS: 356.04 (M+); Anal calcd. for C17H16N4O5: C, 57.30; H, 4.53; N, 15.72; Found: C, 57.10; H, 4.36; N, 15.54.
5,6,7,9-Tetrahydro-6,6-dimethyl-9-(4-nitrophenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4o).
M.Pt.: 224–228 °C; IR (film, νmax cm−1): 3110, 2960, 1775, 1708, 1654; 1H NMR (CDCl3, 400 MHz): δ 0.97 (s, 6H, 2 × CH3), 2.18 (s, 2H, CH2), 2.76 (2H, AB system 2JHH = 17.6 Hz, CH2), 6.10 (s, 1H, CH-Ar), 7.53–8.15 (m, 4H, Ar-H), 10.10 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 28.7, 29.3, 35.2, 52.4, 57.4, 118.8, 122.6, 129.3, 139.7, 146.7, 147.4, 150.8, 154.8, 191.6; ESI-MS: 356.05 (M+); Anal calcd. for C17H16N4O5: C, 57.30; H, 4.53; N, 15.72; Found: C, 57.08; H, 4.37; N, 15.58.
5,6,7,9-Tetrahydro-6,6-dimethyl-9-(2-hydroxyphenyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4p).
M.Pt.: 110–114 °C; IR (film, νmax cm−1): 3064, 2958, 1764, 1712, 1643; 1H NMR (CDCl3, 400 MHz): δ 1.00 (s, 3H, CH3), 1.10 (s, 3H, CH3), 2.30 (s, 2H, CH2), 2.51 (2H, AB system 2JHH = 17.6 Hz, CH2), 4.64 (br s, 1H, NH), 6.02 (s, 1H, CH-Ar), 6.89–7.54 (m, 4H, Ar-H), 10.46 (br s, 1H, OH); 13C NMR (CDCl3, 100 MHz): δ 27.4, 28.2, 37.9, 51.8, 53.3, 116.7, 117.4, 121.8, 126.4, 126.8, 128.7, 129.2, 138.4, 149.9, 153.6, 192.7; ESI-MS: 327.05 (M+); Anal calcd. for C17H17N3O4: C, 62.38; H, 5.23; N, 12.84; Found: C, 62.21; H, 5.10; N, 12.70.
5,6,7,9-Tetrahydro-6,6-dimethyl-9-(2-thienyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4q).
M.Pt.: 136–140 °C; IR (film, νmax cm−1): 3086, 2959, 1786, 1732, 1668; 1H NMR (CDCl3, 400 MHz): δ 0.95 (s, 6H, 2 × CH3), 2.35 (s, 2H, CH2), 2.92 (2H, AB system 2JHH = 16.9 Hz, CH2), 6.26 (s, 1H, CH-Ar), 6.68–7.11 (m, 3H, Ar-H), 9.81 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 26.5, 27.3, 35.7, 49.2, 54.8, 118.6, 120.2, 126.7, 127.2, 137.4, 140.8, 147.8, 153.6, 193.8; ESI-MS: 316.98 (M+); Anal calcd. for C15H15N3O3S: C, 56.77; H, 4.76; N, 13.24; Found: C, 56.58; H, 4.62; N, 13.06.
5,6,7,9-Tetrahydro-6,6-dimethyl-9-(propyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4r).
M.Pt.: 184–186 °C; IR (film, νmax cm−1): 3196, 2959, 2734, 1725, 1601; 1H NMR (CDCl3, 400 MHz): δ 0.86 (t, 3H, *CH3CH2), 0.99 (s, 6H, 2 × CH3), 1.29 (m, 2H, *CH2CH3), 2.16 (m, 2H, CH*CH2CH2), 2.46 (s, 2H, CH2), 2.94 (2H, AB system 2JHH = 17.8 Hz, CH2), 5.28 (m, 1H, CHN), 8.20 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 15.3, 17.8, 26.8, 27.4, 31.2, 36.4, 39.4, 52.7, 121.8, 140.8, 151.7, 156.4, 195.8; ESI-MS: 277.08 (M+); Anal calcd. for C14H19N3O3: C, 60.63; H, 6.91; N, 15.15; Found: C, 60.46; H, 6.73; N, 14.98.
5,6,7,9-Tetrahydro-9-(ethyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4s).
M.Pt.: 132–134 °C; IR (film, νmax cm−1): 3206, 2962, 2737, 1714, 1667; 1H NMR (CDCl3, 400 MHz): δ 0.56 (t, 3H, *CH3CH2), 1.39–1.45 (m, 2H, *CH2CH3), 1.92–2.00 (m, 2H, CH2), 2.23–2.30 (m, 2H, CH2), 2.37–2.48 (m, 2H, CH2), 4.76 (m, 1H, CHN), 6.12 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 11.2, 20.8, 22.3, 33.4, 38.4, 46.7, 11.6, 139.8, 147.2, 152.2, 193.6; ESI-MS: 235.03 (M+); Anal calcd. for C11H13N3O3: C, 56.16; H, 5.57; N, 17.86; Found: C, 56.03; H, 5.42; N, 17.70.
5,6,7,9-Tetrahydro-9-(isobutyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4t).
M.Pt.: 120–124 °C; IR (film, νmax cm−1): 3276, 3192, 2952, 1734, 1622; 1H NMR (CDCl3, 400 MHz): δ 0.85 (d, 6H, 2 × CH3), 1.22 (m, 2H, CH-CH2), 1.65 (m, 1H, CH(CH3)2), 1.94–2.02 (m, 2H, CH2), 2.42–2.46 (m, 2H, CH2), 2.48–2.52 (m, 2H, CH2), 4.08 (m, 1H, CHN), 7.78 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 18.6, 22.7, 24.2, 31.8, 40.6, 44.5, 49.3, 119.6, 138.6, 148.6, 152.4, 192.7; ESI-MS: 263.06 (M+); Anal calcd. for C13H17N3O3: C, 59.30; H, 6.51; N, 15.96; Found: C, 59.13; H, 6.36; N, 15.80.
5,6,7,9-Tetrahydro-9-(hexyl)-[1,2,4]-triazolo[1,2-a]indazole-1,3,8(2H,5H,9H)-trione (4u).
M.Pt.: 166–168 °C; IR (film, νmax cm−1): 3086, 2959, 1786, 1732, 1668; 1H NMR (CDCl3, 400 MHz): δ 0.76 (t, 3H, *CH3CH2), 1.36 (m, 2H, *CH2CH3), 1.68 (m, 2H, CH-*CH2–CH2), 1.84 (m, 6H, 3 × CH2), 1.92–1.98 (m, 2H, CH2), 2.23–2.30 (m, 2H, CH2), 2.37–2.44 (m, 2H, CH2), 6.38 (m, 1H, CHN), 8.33 (br s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 14.8, 19.2, 24.7, 25.2, 28.7, 29.6, 31.8, 33.2, 38.4, 49.6, 119.7, 137.8, 146.6, 151.8, 192.7; ESI-MS: 291.10 (M+); Anal calcd. for C15H21N3O3: C, 61.84; H, 7.27; N, 14.42; Found: C, 61.52; H, 7.12; N, 14.28.
Conclusion
In summary, we have developed a green and efficient multi-component reaction protocol for the synthesis of triazolo[1,2-a]indazolones using sulfamic acid as a recyclable and cost-effective catalyst. Simple work up procedure, general applicability, ambient conditions and use of water as a reaction medium makes this protocol eco-friendly and distinctly superior to many other methods reported earlier. Moreover, it is noteworthy to mention that the catalyst could be reused for the four successive runs without any significant change in its activity.
Acknowledgements
Author (R. Chauhan) thanks UGC (University Grants Commission) for providing a Junior Research Fellowship and also to the Director of University Science and Instrumentation Centre, University of Delhi, Delhi for providing the instrumentation facilities.
References
-
(a)
Organic Reactions in Water. Principles, Strategies and Applications, ed. U. M. Lindstrom, Blackwell Publishing, Oxford, UK, 2007 Search PubMed;
(b) L. Weber, Drug Discov. Today, 2002, 7, 143 CAS;
(c) C. Hulme and V. Gore, Curr. Med. Chem., 2003, 10, 51 CrossRef CAS;
(d) A. Domling, Chem. Rev., 2006, 106, 17 CrossRef;
(e) I. Kanizsai, S. Gyonfalvi, Z. Szakonyi, R. Sillanpaa and F. Fulop, Green Chem., 2007, 9, 357 RSC.
-
(a)
Multicomponent Reactions, ed. J. Zhu and H. Bienayme, Wiley-VCH Verlag GmbH & Co.; KGaA, Weinheim, 2005 Search PubMed;
(b) K. Kumaravel and G. Vasuki, Curr. Org. Chem., 2009, 13, 1820 CrossRef CAS;
(c) A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 CrossRef CAS;
(d) D. Tejedor and F. Garcia-Tellado, Chem. Soc. Rev., 2007, 36, 484 RSC.
- R. A. Sheldon, Green Chem., 2005, 7, 267 RSC.
-
(a)
P. A. Grieco, Organic Synthesis in Water, Blackie Academic and Professional, London, 1998 Search PubMed;
(b)
C. J. Li and T. H. Han, Organic Reactions in Aqueous Media, John Wiley and Sons, New York, 1997, p. 159 Search PubMed.
- R. Breslow, Acc. Chem. Res., 1991, 24, 159 CrossRef CAS.
- R. Breslow, Acc. Chem. Res., 2004, 37, 471 CrossRef CAS.
-
(a) C. M. Kleiner and P. R. Schreiner, Chem. Commun., 2006, 4315 RSC;
(b) M. C. Pirrung and K. D. Sarma, J. Am. Chem. Soc., 2004, 126, 444 CrossRef CAS;
(c) A. Lubineau, J. Org. Chem., 1986, 51, 2142 CrossRef CAS;
(d) P. H. Von Hippel and T. Schleich, Acc. Chem. Res., 1969, 2, 257 CrossRef CAS.
- G. Centi, P. Ciambelli, S. Perathoner and P. Russo, Catal. Today, 2002, 75, 3 CrossRef CAS.
-
(a) T.-S. Jin, G. Sun, Y.-W. Li and T.-S. Li, Green Chem., 2002, 4, 255 RSC;
(b) R. Nagarajan, C. J. Magesh and P. T. Perumal, Synthesis, 2004, 69 CAS;
(c) M. Xia and Y.-D. Lu, J. Fluorine Chem., 2006, 127, 1119 CrossRef CAS;
(d) S. D. Mitragotri, D. M. Pore, U. V. Desai and P. P. Wadgaonkar, Catal. Commun., 2008, 9, 1822 CrossRef CAS;
(e) K. Niknam and D. Saberi, Tetrahedron Lett., 2009, 50, 5210 CrossRef CAS;
(f) L. Wu, S. Ma, F. Yan and C. Yang, Monatsh. Chem., 2010, 141, 565 CrossRef CAS.
-
(a)
P. N. Craig, in Comprehensive Medicinal Chemistry, ed. C. J. Drayton, Pergamon Press, New York, 1991, vol. 8 Search PubMed;
(b) F. M. Awadallah, F. Müller, A. H. Lehmann and A. H. Abadi, Bioorg. Med. Chem., 2007, 15, 5811 CrossRef CAS;
(c) M. C. Bagley, T. Davis, M. C. Dix, M. J. Rokicki and D. Kipling, Bioorg. Med. Chem. Lett., 2007, 17, 5107 CrossRef CAS;
(d) E. Conchon, F. Anizon, B. Aboab and M. Prudhomme, J. Med. Chem., 2007, 50, 4669 CrossRef CAS.
-
(a) X. Lei, N. Zaarur, M. Y. Sherman and J. A. Porco Jr., J. Org. Chem., 2005, 70, 6474 CrossRef CAS;
(b) A. Kiriazis, T. Ruffer, S. Jantti, H. Lang and J. Yli-Kauhaluoma, J. Comb. Chem., 2007, 9, 263 CrossRef CAS;
(c) P. D. Boatman, J. Urban, M. Nguyen, M. Qabar and M. Kahn, Bioorg. Med. Chem. Lett., 2003, 13, 1445 CrossRef CAS;
(d) V. M. Kolb, J. P. Dworkin and S. L. Miller, J. Mol. Evol., 1994, 38, 549 CrossRef CAS.
-
C. R. Jacobson, A. D’Adamo and C. E. Cosgrove, US Pat., 3663564, 1972, Chem. Abstr., 77, 62007a Search PubMed.
-
T. Shigematsu, M. Tomita, T. Shibahara, M. Nakazawa and S. Munakata, Jpn. Pat., 52083562, 1977, Chem. Abstr., 87, 6891f Search PubMed.
-
E. Baumgartner, U. Blumenstein, R. Bueschl and N. Reieber, Ep. Pat., 390026, 1990, Chem. Abstr., 114, 103011f Search PubMed.
-
(a) S. Tanaka, K. Seguchi, K. Itoh and A. Sera, J. Chem. Soc., Perkin Trans. 1, 1994, 2335 RSC;
(b) S. Meehan and R. D. Little, J. Org. Chem., 1997, 62, 3779 CrossRef CAS;
(c) P. Y. F. Deghati, M. J. Wanner and G.-J. Koomen, Tetrahedron Lett., 1998, 39, 4561 CrossRef CAS;
(d) Y. Arroyo, J. F. Rodriguez, M. Santos, M. A. Sanz Tejedor, I. Vaca and J. L. Garcia Ruano, Tetrahedron: Asymm., 2004, 15, 1059 CrossRef CAS.
-
(a) A. Bazgir, M. Seyyedhamzeh, Z. Yasaei and P. Mirzaei, Tetrahedron Lett., 2007, 48, 8790 CrossRef CAS;
(b) H. Hamidian, S. Fozooni, A. Hassankhani and S. Z. Mohammadi, Molecules, 2011, 16, 9041 CrossRef CAS.
-
(a) M. Kidwai, D. Bhatnagar and R. Chauhan, J. Heterocycl. Chem., 2012 DOI:10.1002/jhet.1037;
(b) M. Kidwai, R. Chauhan and D. Bhatnagar, J. Sulf. Chem., 2011, 32, 37 CrossRef CAS;
(c) M. Kidwai, A. Jahan, R. Chauhan and N. K. Mishra, Tetrahedron Lett., 2012, 53, 1728 CrossRef CAS;
(d) M. Kidwai, R. Chauhan, D. Bhatnagar, A. K. Singh, B. Mishra and S. Dey, Monatsh. Chem., 2012 DOI:10.1007/s00706-012-0742-4.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra20662e |
| ‡ Presently working as Vice-chancellor (President), Jiwaji University, Gwalior (M. P.), India. |
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. ![Synthesis of library of triazole[1,2-a]indazole-trione derivatives.](/image/article/2012/RA/c2ra20662e/c2ra20662e-s2.gif)
![Model reaction for the synthesis of triazole[1,2-a]indazole-triones.](/image/article/2012/RA/c2ra20662e/c2ra20662e-s1.gif)

![Suitable mechanism for the formation of triazole[1,2-a]indazole-triones.](/image/article/2012/RA/c2ra20662e/c2ra20662e-s3.gif)
