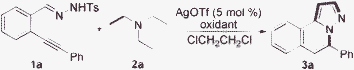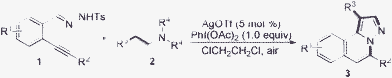Reaction of N′-(2-alkynylbenzylidene)hydrazide with tertiary amine: a concise synthesis of H-pyrazolo[5,1-a]isoquinolines†
Chao
Ye
ac,
Xingxin
Yu
a,
Guanyinsheng
Qiu
a and
Jie
Wu
*ab
aDepartment of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433, China. E-mail: jie_wu@fudan.edu.cn; Fax: +86 21 6564 1740; Tel: +86 21 6510 2412
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Road, Shanghai 200032, China
cKey Laboratory of Functional Small Organic Molecules, Ministry of Education and College of Chemistry & Chemical Engineering, Jiangxi Normal University, Nanchang, Jiangxi 330022, China
First published on 11th May 2012
Abstract
The reaction of N′-(2-alkynylbenzylidene)hydrazide with tertiary amine via C–H bond activation in the presence of cooperative catalysis was reported, which generates H-pyrazolo[5,1-a]isoquinolines in good yields under mild conditions.
Currently, the pursuit of practical and efficient approaches for the rapid generation of small molecules is of utmost urgency and importance in the studies of chemical genetics.1 Recently, we have been involved in the development of methodologies for the construction of natural product-like compounds2 for different biological evaluations. During the studies, we identified some hits of H-pyrazolo[5,1-a]isoquinolines as CDC25B, TC-PTP, and PTP1B inhibitors.3 Therefore, efficient approaches for the generation of diverse H-pyrazolo[5,1-a]isoquinolines are highly desirable. Recently, Li and co-workers reported an iron-catalyzed oxidation of tertiary amines in the presence of tert-butyl hydroperoxide (TBHP) for the synthesis of β-1,3-dicarbonyl aldehydes.4 During the reaction process, α,β-unsaturated aldehydes generated by tertiary amine oxidation in situ acted as key intermediates. Prompted by this result and our efforts in the tandem reactions5 for the synthesis of N-heterocycles, we conceived that the iron-catalyzed oxidation of tertiary amines could be applied in the reaction of N′-(2-alkynylbenzylidene)hydrazides as well for the generation of H-pyrazolo[5,1-a]isoquinolines. We envisioned that the isoquinolinium-2-yl amide6 and α,β-unsaturated aldehyde produced in situ would act as the intermediate in the transformation. The reaction would proceed through 6-endo cyclization, [3 + 2] cycloaddition, and aromatization to afford the desired product A. To our surprise, compound A was not obtained when a model reaction of N′-(2-alkynylbenzylidene)hydrazide 1a with triethylamine was studied in the presence of silver triflate and an iron catalyst with TBHP (Scheme 1). Instead, compound 3a was isolated in 8% yield, unexpectedly. No reaction occurred in the absence of iron catalyst or silver triflate. The presence of silver triflate has been demonstrated to promote the 6-endo cyclization of N′-(2-alkynylbenzylidene)hydrazide to form isoquinolinium-2-yl amide.6 With this interesting result in hand, we were curious to know the possible route for the formation of compound 3a.
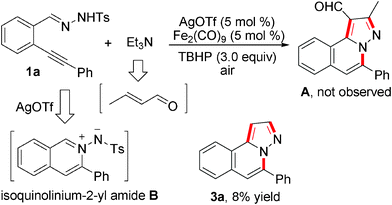 | ||
| Scheme 1 An unexpected result from an iron-catalyzed reaction of N′-(2-alkynylbenzylidene)hydrazide 1a with triethylamine. | ||
It seems that the transformation is related to C–H activation7–9 and C–N bond cleavage during the process. Catalytic dehydrogenative oxidation of the C–H bond adjacent to a nitrogen atom have been reported.7,8 For instance, Loh and co-workers described a dioxygen–copper catalytic system for the production of α-amino acetals via a rearrangement of a tertiary amine through the oxidation of the aliphatic C–H bond.8c We conceived that the result in Scheme 1 might proceed through a similar route in the presence of an iron catalyst. We reasoned that in the presence of an iron catalyst with TBHP, the tertiary amine would be transferred to an enamine through the oxidation of an aliphatic C–H bond of the tertiary amine, which then acted as a nucleophile to attack the in situ generated isoquinolinium-2-ylamide. The subsequent release of a tosyl group and aromatization would produce the H-pyrazolo[5,1-a]isoquinoline 3a. Based on these considerations, we started to explore the optimal conditions of this conversion.
The reaction of N′-(2-alkynylbenzylidene)hydrazide 1a with triethylamine 2a in 1,2-dichloroethane was investigated in the presence of silver triflate and an oxidant (Table 1). Only a trace amount of product 3a was detected when the iron catalyst was changed to FeCl3, FeCl2, or Fe(OTf)3 (Table 1, entries 1–3). To our delight, the product was isolated in 76% yield by switching the catalyst to Fe(acac)3 (Table 1, entry 4). In order to verify that the reaction proceeded through the isoquinolinium-2-yl amide intermediate B, the reaction of isoquinolinium-2-yl amide B with triethylamine 2a was performed under the Fe(acac)3-catalysis conditions. As expected, H-pyrazolo[5,1-a]isoquinoline 3a was produced in 81% yield. The same outcome was generated when 5.0 equiv. of triethylamine was used. Reducing the amount of triethylamine to 2 equiv. decreased the yield. No difference was observed when 10 equiv. of triethylamine was employed (Table 1, entry 6). Lower yields were afforded when the reaction took place in other solvents (Table 1, entries 7–11). Other oxidants were screened. It was found that PhI(OAc)2 was also effective in the conversion (Table 1, entry 12). The yield was higher when the reaction was exposed to air. Further surveys revealed that the reaction worked most efficiently in 1,2-dichloroethane in the presence of 5.0 equiv. of triethylamine.
| Entry | Oxidant | Et3N | Solvent | Yield (%)a |
|---|---|---|---|---|
| a Isolated yield based on N′-(2-alkynylbenzylidene)hydrazide 1a. | ||||
| 1 | FeCl3/TBHP | 1 mL | ClCH2CH2Cl | trace |
| 2 | FeCl2/TBHP | 1 mL | ClCH2CH2Cl | trace |
| 3 | Fe(OTf)3/TBHP | 1 mL | ClCH2CH2Cl | trace |
| 4 | Fe(acac)3/TBHP | 1 mL | ClCH2CH2Cla | 76 |
| 5 | Fe(acac)3/TBHP | 5.0 equiv. | ClCH2CH2Cl | 76 |
| 6 | Fe(acac)3/TBHP | 10.0 equiv. | ClCH2CH2Cl | 77 |
| 7 | Fe(acac)3/TBHP | 5.0 equiv. | MeCN | 58 |
| 8 | Fe(acac)3/TBHP | 5.0 equiv. | EtOH | 52 |
| 9 | Fe(acac)3/TBHP | 5.0 equiv. | THF | 63 |
| 10 | Fe(acac)3/TBHP | 5.0 equiv. | toluene | 57 |
| 11 | Fe(acac)3/TBHP | 5.0 equiv. | 1,4-dioxane | 66 |
| 12 | PhI(OAc)2 | 1 mL | ClCH2CH2Cl | 60 |
| 13 | PhI(OAc)2/air | 1 mL | ClCH2CH2Cl | 80 |
| 14 | PhI(OAc)2/air | 1.0 equiv. | ClCH2CH2Cl | 56 |
| 15 | PhI(OAc)2/air | 2.0 equiv. | ClCH2CH2Cl | 60 |
| 16 | PhI(OAc)2/air | 5.0 equiv. | ClCH2CH2Cl | 75 |
| 17 | PhI(OAc)2/air | 5.0 equiv. | MeCN | 65 |
| 18 | PhI(OAc)2/air | 5.0 equiv. | EtOH | 28 |
| 19 | PhI(OAc)2/air | 5.0 equiv. | THF | 44 |
| 20 | PhI(OAc)2/air | 5.0 equiv. | toluene | 60 |
| 21 | PhI(OAc)2/air | 5.0 equiv. | 1,4-dioxane | 56 |
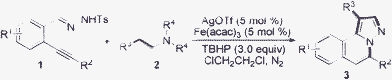
Having demonstrated the viability of this conversion, we next investigated the scope of the transformation using an AgOTf/PhI(OAc)2 system. To assess the impact of the structural and functional motifs on the reaction, we tested a range of N′-(2-alkynylbenzylidene)hydrazides 1 (Table 2). From the result, it seems that N′-(2-alkynylbenzylidene)hydrazides with an aliphatic group or an aryl group attached to the triple bond (R2) were all good partners in the reactions. Better results were obtained when N′-(2-alkynylbenzylidene)hydrazides 1 with an electron-withdrawing group attached on the aromatic ring were employed. For example, the reaction failed when N′-(2-alkynylbenzylidene)hydrazide 1m was utilized in the reaction with triethylamine 2a (Table 2, entry 23). In extending this useful transformation, reactions of N′-(2-alkynylbenzylidene)hydrazides 1 with tertiary amines 2 in the presence of silver triflate and iron catalyst were investigated in the meantime (Table 3). All reactions worked well to produce the expected H-pyrazolo[5,1-a]isoquinolines in good yields.
| Entry | R1, R2 | R3, R4 | Yield (%)a |
|---|---|---|---|
| a Isolated yield based on N′-(2-alkynylbenzylidene)hydrazide 1. | |||
| 1 | H, Ph (1a) | H, Et (2a) | 75 (3a) |
| 2 | H, Ph (1a) | H, iPr (2b) | 73 (3a) |
| 3 | H, Ph (1a) | Me, nPr (2c) | 57 (3b) |
| 4 | H, Ph (1a) | Et, nBu (2d) | 73 (3c) |
| 5 | H, 4-MeC6H4 (1b) | H, Et (2a) | 77 (3d) |
| 6 | H, 4-MeOC6H4 (1c) | H, Et (2a) | 57 (3e) |
| 7 | H, 4-MeOC6H4 (1c) | Me, nPr (2c) | 45 (3f) |
| 8 | H, 4-ClC6H4 (1d) | H, Et (2a) | 67 (3g) |
| 9 | H, 4-ClC6H4 (1d) | Me, nPr (2c) | 55 (3h) |
| 10 | H, cyclopropyl (1e) | H, Et (2a) | 78 (3i) |
| 11 | 5-Cl, cyclopropyl (1f) | H, Et (2a) | 67 (3j) |
| 12 | 4-F, C6H5 (1g) | H, Et (2a) | 84 (3k) |
| 13 | 4-F, 4-MeC6H4 (1h) | H, Et (2a) | 78 (3l) |
| 14 | 4-F, n-Bu (1i) | H, Et (2a) | 73 (3m) |
| 15 | 5-F, C6H5 (1j) | H, Et (2a) | 73 (3n) |
| 16 | 5-F, C6H5 (1j) | Me, nPr (2c) | 49 (3o) |
| 17 | 5-Me, C6H5 (1k) | H, Et (2a) | 71 (3p) |
| 18 | 5-Me, C6H5 (1k) | Me, nPr (2c) | 55 (3q) |
| 19 | 5-Me, C6H5 (1k) | Et, nBu (2d) | 64 (3r) |
| 20 | 4-OMe, C6H5 (1l) | H, Et (2a) | 41 (3s) |
| 21 | 4-OMe, C6H5 (1l) | Me, nPr (2c) | 52 (3t) |
| 22 | 4-OMe, C6H5 (1l) | Et, nBu (2d) | 45 (3u) |
| 23 | 4,5-(OMe)2, 4-MeOC6H4 (1m) | H, Et (2a) | trace |
| Entry | R1, R2 | R3, R4 | Yield (%)a |
|---|---|---|---|
| a Isolated yield based on N′-(2-alkynylbenzylidene)hydrazide 1. | |||
| 1 | H, Ph (1a) | H, Et (2a) | 76 (3a) |
| 2 | H, Ph (1a) | Me, nPr (2c) | 68 (3b) |
| 3 | H, Ph (1a) | Et, nBu (2d) | 72 (3c) |
| 4 | H, 4-MeC6H4 (1b) | H, Et (2a) | 83 (3d) |
| 5 | H, 4-MeOC6H4 (1c) | H, Et (2a) | 61 (3e) |
| 6 | H, 4-ClC6H4 (1d) | H, Et (2a) | 68 (3g) |
| 7 | H, cyclopropyl (1e) | H, Et (2a) | 67 (3i) |
| 8 | 5-Cl, cyclopropyl (1f) | H, Et (2a) | 72 (3j) |
| 9 | 4-F, C6H5 (1g) | H, Et (2a) | 70 (3k) |
| 10 | 5-F, C6H5 (1j) | Me, nPr (2c) | 56 (3o) |
| 11 | 5-Me, C6H5 (1j) | H, Et (2a) | 72 (3p) |
| 12 | 5-Me, C6H5 (1j) | Me, nPr (2c) | 67 (3q) |
| 13 | 4-OMe, C6H5 (1k) | H, Et (2a) | 46 (3s) |
| 14 | 4-OMe, C6H5 (1k) | Me, nPr (2c) | 67 (3t) |
In conclusion, we have reported an unexpected reaction of N′-(2-alkynylbenzylidene)hydrazide with a tertiary amine via C–H bond activation in the presence of cooperative catalysis. H-Pyrazolo[5,1-a]isoquinolines are generated in good yields under mild conditions. During the reaction process, three bonds are formed with the concurrent cleavage of a C–N bond of the tertiary amine. Currently, studies of catalytic dehydrogenative oxidation of the C–H bond adjacent to a nitrogen atom for the synthesis of other N-heterocycles is under investigation in our laboratory.
Acknowledgements
Financial support from the National Natural Science Foundation of China (21032007) and Jiangxi Normal University is gratefully acknowledged.References
- (a) D. P. Walsh and Y.-T. Chang, Chem. Rev., 2006, 106, 2476 CrossRef CAS; (b) P. Arya, D. T. H. Chou and M.-G. Baek, Angew. Chem., Int. Ed., 2001, 40, 339 CrossRef CAS; (c) S. L. Schreiber, Science, 2000, 287, 1964 CrossRef CAS.
- (a) For recent selected examples, see: G. Qiu, Y. He and J. Wu, Chem. Commun., 2012, 48, 3836 RSC; (b) G. Qiu, G. Liu, S. Pu and J. Wu, Chem. Commun., 2012, 48, 2903 RSC; (c) S. Ye, G. Liu, S. Pu and J. Wu, Org. Lett., 2012, 14, 70 CrossRef CAS; (d) Z. Chen, L. Gao, S. Ye, Q. Ding and J. Wu, Chem. Commun., 2012, 48, 3975 RSC.
- Z. Chen and J. Wu, Org. Lett., 2010, 12, 4856 CrossRef CAS.
- W. Liu, J. Liu, D. Ogawa, Y. Nishihara, X. Guo and Z. Li, Org. Lett., 2011, 13, 6272 CrossRef CAS and references cited therein.
- (a) For reviews, see: J. Montgomery, Angew. Chem., Int. Ed., 2004, 43, 3890 CrossRef CAS; (b) E. Negishi, C. Coperet, S. Ma, S. Y. Liou and F. Liu, Chem. Rev., 1996, 96, 365 CrossRef CAS; (c) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS; (d) R. Grigg and V. Sridharan, J. Organomet. Chem., 1999, 576, 65 CrossRef CAS; (e) T. Miura and M. Murakami, Chem. Commun., 2007, 217 RSC; (f) M. Malacria, Chem. Rev., 1996, 96, 289 CrossRef CAS; (g) K. C. Nicolaou, T. Montagnon and S. A. Snyder, Chem. Commun., 2003, 551 RSC; (h) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134 CrossRef CAS; (i) D. Enders, C. Grondal and M. R. M. Hüttl, Angew. Chem., Int. Ed., 2007, 46, 1570 CrossRef CAS; (j) L. F. Tietze, G. Brasche and K. Gericke, Domino Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006 Search PubMed; (k) L. F. Tietze, M. A. Düfert, T. Hungerland, K. Oum and T. Lenzer, Chem.–Eur. J., 2011, 17, 8452 CrossRef CAS.
- (a) S. Li, Y. Luo and J. Wu, Org. Lett., 2011, 13, 4312 CrossRef CAS; (b) S. Li and J. Wu, Org. Lett., 2011, 13, 712 CrossRef CAS; (c) X. Yu, Q. Yang, H. Lou, Y. Peng and J. Wu, Org. Biomol. Chem., 2011, 9, 7033 RSC; (d) X. Yu, X. Pan and J. Wu, Tetrahedron, 2011, 67, 1145 CrossRef CAS; (e) Z. Chen, X. Pan and J. Wu, Synlett, 2011, 964 CAS; (f) S. Ye, X. Yang and J. Wu, Chem. Commun., 2010, 46, 5238 RSC; (g) X. Yu, S. Ye and J. Wu, Adv. Synth. Catal., 2010, 352, 2050 CrossRef CAS; (h) X. Yu, Z. Chen, X. Yang and J. Wu, J. Comb. Chem., 2010, 12, 374 CrossRef CAS; (i) H. Ren, S. Ye, F. Liu and J. Wu, Tetrahedron, 2010, 66, 8242 CrossRef CAS; (j) Z. Chen, X. Yang and J. Wu, Chem. Commun., 2009, 3469 RSC; (k) Z. Chen, Q. Ding, X. Yu and J. Wu, Adv. Synth. Catal., 2009, 351, 1692 CrossRef CAS; (l) Z. Chen, M. Su, X. Yu and J. Wu, Org. Biomol. Chem., 2009, 7, 4641 RSC.
- (a) For representative reviews, see: K. R. Campos, Chem. Soc. Rev., 2007, 36, 1069 RSC; (b) K. Godula and D. Sames, Science, 2006, 312, 67 CrossRef CAS; (c) M. Tobisu and N. Chatani, Angew. Chem., Int. Ed., 2006, 45, 1683 CrossRef CAS; (d) G. E. Dobereiner and R. H. Crabtree, Chem. Rev., 2010, 110, 681 CrossRef CAS; (e) D. Dyker, Ed. Handbook of C-H Transformations,Wiley-VCH, Weinheim, 2005 Search PubMed; (f) Special issue on selective functionalization of C-H bonds, Chem. Rev., 2010, 110, 575–1211 Search PubMed.
- (a) R. G. Bergman, Nature, 2007, 446, 391 CrossRef CAS; (b) K. Godula and D. Sames, Science, 2006, 312, 67 CrossRef CAS; (c) J.-S. Tian and T.-P. Loh, Angew. Chem., Int. Ed., 2010, 49, 8417 CrossRef CAS; (d) S. Li and J. Wu, Org. Lett., 2011, 13, 712 CrossRef CAS.
- (a) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS; (b) R. Giri, B. F. Shi, K. M. Engle, N. Maugel and J.-Q. Yu, Chem. Soc. Rev., 2009, 38, 3242 RSC; (c) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS; (d) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef CAS; (e) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS; (f) J. L. Bras and J. Muzart, Chem. Rev., 2011, 111, 1170 CrossRef; (g) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS; (h) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS; (i) L. Ackermann, Chem. Rev., 2011, 111, 1315 CrossRef CAS; (j) B. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A.-M. Resmerita, N. K. Garg and V. Percec, Chem. Rev., 2011, 111, 1346 CrossRef CAS; (k) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS; (l) Y. Kuninobu and K. Takai, Chem. Rev., 2011, 111, 1938 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: experimental procedures, characterization data, 1H and 13C NMR spectra of compounds 3. See DOI: 10.1039/c2ra20899g |
| This journal is © The Royal Society of Chemistry 2012 |