Visible light-driven organic form-stable phase change materials for solar energy storage†
Yunming
Wang
,
Bingtao
Tang
* and
Shufen
Zhang
State Key Laboratory of Fine Chemicals, Dalian University of Technology, P.O. Box 89, West Campus, 2# Linggong Rd, Dalian 116024, China. E-mail: tangbt@dlut.edu.cn; Fax: 086-411-84986264; Tel: 086-411-84986267
First published on 11th May 2012
Abstract
A novel visible light-driven organic form-stable phase change material (VLDOPCM) exhibited excellent performances of light-harvesting, light-thermal conversion, thermal energy storage and form-stable effect, which is promoted by the dye as an effective “photon capture and molecular heater” for direct and efficient use of solar radiation.
Solar-energy conversion and storage materials are arguably some of the most promising options for supplying renewable, carbon-neutral energy on a global scale,1–9 especially solar-thermal energy, a direct and efficient application of solar radiation.10 However, overcoming the gaps between time and space solar irradiation is difficult. Moreover, visible light, which accounts for approximately 44%11 of solar radiation, can not be directly or effectively applied due to low thermal efficiency.10 Therefore, efficient harvest and thermal conversion of visible light and thermal energy storage are essential for the improvement of solar energy utilization.
In the present paper, a new strategy to improve the efficiency of solar energy utilization by introducing a dye, 1,4-bis((2-hydroxyethyl)amino)anthracene-9,10-dione, into a form-stable phase change material (PCM) is described (ESI,† Scheme S1). The novel materials (Scheme 1) are able to harvest visible light and convert it to thermal energy more effectively compared with traditional organic PCMs for latent heat thermal energy storage.12–17 As a photon antenna, the dye is used to capture sunlight and convert light to thermal energy around the visible region due to its strong absorbance and excellent light-thermal conversion capability (η = 0.937). Thermal energy is effectively stored and released during the phase transition process of the form-stable PCM, which is shape-stable during the phase change process. Therefore, the visible light-driven organic form-stable PCM (VLDOPCM) containing the dye is capable of rapid light-harvesting, light-thermal conversion, and thermal energy storage (Scheme 1). Moreover, VLDOPCM exhibits many desirable characteristics, e.g., direct use without additional encapsulation, shape stability, and easy preparation. Compared with common solar thermal water tanks with sensible heat storage (SHS),2,18 the new materials have some smart features, such as latent heat storage (LHS) with high storage density, shape stability, and adjustable phase transition temperature. Therefore, the novel VLDOPCM bridges the time gap between solar energy requirement and supply. Of crucial importance is the realization of low-cost dye drivers in which photons are efficiently harvested due to the absorption of visible sunlight (Fig. 1a and ESI,† Fig. S4). Then, the visible light is converted to thermal energy as a crucial consequence of the nonradiative process of excited dye by visible irradiation. In our research, VLDOPCM shows a reversible (more than 200 cycles) phase transition (crystalline state change) via on–off switching of visible light irradiation, of which no notable deterioration is observed. Our current study is the first report that describes organic light-to-heat conversion materials via a visible light-driven reversible phase transition based on a dye and form-stable PCM. The obtained novel material has potential applications in renewable and clean energy sources.
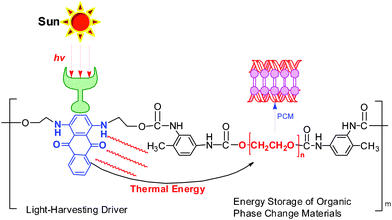 | ||
| Scheme 1 The light-driven transition diagram of the novel VLDOPCM. | ||
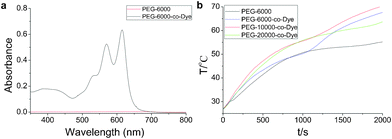 | ||
| Fig. 1 (a) UV-visible absorption spectra of PEG-6000 and VLDOPCM (λ max1 = 591 nm and λ max2 = 637 nm, THF). (b) The light-driven temperature-dependence spectrum of VLDOPCM and PEG-6000 (controlled sample) for a heating process (control sample = 4.0 g, P = 0.30 W, ambient temperature = 26.7 °C). | ||
The UV-visible absorption spectra of VLDOPCM and pure PEG are shown in Fig. 1a. VLDOPCM has a perfect and broad absorption between 500 and 650 nm (black line in Fig. 1a), whereas pure PEG-6000 has no absorption in the visible region (colored line in Fig. 1a). The visible light that accounts for approximately 44% of solar radiation extends across the 400 < λ < 700 nm interval and has its peak at 555 nm.2 Clearly, VLDOPCM can absorb a large part of the solar radiation. In our current research, visible irradiation experiments of the conversion efficiency (η) were carried out for VLDOPCM, as described in detail in the experimental section (ESI,† Fig. S1 and S2). Using the measured irradiated area of 4.90 cm2 and the visible light (λ max = 600 nm) power of 0.33 W, the transduction efficiency of converting absorbed visible light energy into heat is approximately 0.937. The calculated η is remarkably close to 1, as described in detail in the ESI† (Fig. S3 and eqn (1)). Under sunlight irradiation, the dyes were successfully used as light-harvesting antennae for the novel VLDOPCM and showed high light-harvesting efficiency.
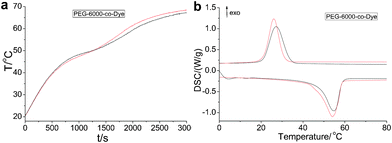
The incident photon-to-heat conversion and energy storage curves of the VLDOPCM and pure PEG-6000 (control sample) are shown in Fig. 1b. The samples were prepared in the weighing bottle, and foam insulation was used during the procedure (Fig. S3†). Upon irradiation with the solar light (0.30 W, ambient temperature = 26.7 °C, sample = 4.0 g), the rate of temperature increase was faster than that of the pure PEG-6000, and the temperature of the VLDOPCMs, working under irradiation of ante meridiem (AM) 34 min sunlight, was as high as 70 °C, which is higher than that of the pure PEG-6000 (55.1 °C) and environment (26.7 °C). This phenomenon was ascribed to the VLDOPCMs which can absorb visible sunlight well and effectively covert light to thermal energy. Under sunlight irradiation, the control sample (PEG-6000) temperature also gradually increased because of the absorption of the near-infrared (NIR) sunlight by PEG.19 Therefore, under sunlight irradiation, the dye of VLDOPCM acts as an effective “photon capture and molecular heater” to convert visible light into heat energy and store heat in the VLDOPCM by phase transition.
As the irradiation time increased, the growth platform of the temperature for PEG-6000-co-Dye first appeared between 48.5 and 58.7 °C, and then PEG-10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-co-Dye and PEG-20
000-co-Dye and PEG-20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-co-Dye. This finding proved that VLDOPCM undergoes a phase transition via absorbing large amounts of heat energy. With an increase in the average molecular weight of PEG, the beginning temperatures of phase transition became higher, and the platform of time vs. temperature became longer and higher. Compared with the phase transition of the pure PEG from solid to liquid, the shape-stabilized VLDOPCM can retain its original shape and can not flow before and after the phase transition, which is why there is no risk of VLDOPCMs leaking during the repeated melting and crystallization process, even if the temperature is raised to 80 °C or higher (ESI,† Fig. S5). Thus, VLDOPCM’s phase transition can be considered to be a form-stable phase transition, which agrees well with the results described below.
000-co-Dye. This finding proved that VLDOPCM undergoes a phase transition via absorbing large amounts of heat energy. With an increase in the average molecular weight of PEG, the beginning temperatures of phase transition became higher, and the platform of time vs. temperature became longer and higher. Compared with the phase transition of the pure PEG from solid to liquid, the shape-stabilized VLDOPCM can retain its original shape and can not flow before and after the phase transition, which is why there is no risk of VLDOPCMs leaking during the repeated melting and crystallization process, even if the temperature is raised to 80 °C or higher (ESI,† Fig. S5). Thus, VLDOPCM’s phase transition can be considered to be a form-stable phase transition, which agrees well with the results described below.
To further demonstrate the controlled transition enthalpy of the materials, thermal properties of the pure PEG and VLDOPCMs were measured by DSC technique (Fig. 2 and Table 1), including melting and crystallization points and latent heats. The crystallizations of the novel VLDOPCMs and PEG were transformed and broken during the phase change.20–22 The latent energy was then released. Compared with the pure PEG (178.7 J g−1, 197.2 J g−1, 178.5 J g−1), the VLDOPCM (78.7 J g−1, 108.6 J g−1, 142.9 J g−1) showed a greater loss of latent heat (Fig. 2 and Table 1). The reason was that PEG’s soft segment crystallization in VLDOPCMs was confined by the hard dye segment. Notably, the transition enthalpy of the materials has been greatly improved, while the average molecular weight of the PEG monomer increased (Fig. 2b). Conversely, the transition temperature of VLDOPCMs slightly changed. Although pure PEG and VLDOPCM underwent a phase transition with high transition enthalpy, their phase transition states were quite different. Shape stabilized properties of VLDOPCM were characterized by hot stage-digital camera technology. The samples were placed on the hot stage and heated at 5 °C min−1 in the temperature range 30–100 °C. The changes in the samples were observed via tracking photographs by the digital camera. Compared with the phase transition of the pure PEG from solid to liquid, the VLDOPCM’s phase transition remained solid and no liquid was observed in the complete heating process, even if the temperature was raised to 80 °C or higher (ESI,† Fig. S5). Thus, VLDOPCMs have a good energy storage effect and good machinability, which broaden their application areas.
| Samples | Phase transition | ΔH (J g−1) | Tp (°C) | ||
|---|---|---|---|---|---|
| Heating cycle | Cooling cycle | Heating cycle | Cooling cycle | ||
| a Tp = Peak transition temperature of samples; ΔH = phase transition enthalpy of samples. | |||||
| PEG-6000 | Solid–liquid | 178.7 | 178.1 | 65.9 | 34.8 |
| PEG-10 000 | Solid–liquid | 197.2 | 201.1 | 67.0 | 46.7 |
| PEG-20 000 | Solid–liquid | 178.5 | 179.1 | 66.9 | 40.1 |
| PEG-6000-co-Dye | Form–stable | 78.7 | 81.67 | 54.8 | 27.2 |
| PEG-10 000-co-Dye | Form–stable | 108.6 | 109.0 | 55.7 | 36.4 |
| PEG-20 000-co-Dye | Form–stable | 142.9 | 132.6 | 58.2 | 38.8 |
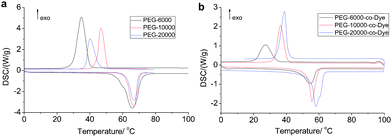 | ||
| Fig. 2 DSC curves of solar thermal conversion materials (5 °C min−1). | ||
Fig. 3 shows the schematic structure and corresponding electron energy band diagram of VLDOPCM. The energy inputs from light often produce an electronic excited state. The dπ–dπ* (or dπ*–dσ*) excited states of dyes are short-lived and dissociative. Rapid transition to the ground state by the ‘thermal’ approach is presented by a, b, and c (Fig. 3 inset).23 The thermal energy was stored by changing the PCM’s crystallinity, so the crystallizations of the novel VLDOPCMs were transformed and broken during phase transitions and the latent energy was released. To further prove the mechanism of solar thermal conversion, fluorescence experiments on VLDOPCM in different solvents, such as toluene, chloroform, and water, were conducted. No fluorescence was issued, and the efficiency of light-to-heat conversion (η) was determined to be remarkably close to 1 (η = 0.937). Therefore, the sunlight radiated VLDOPCM through nonradiative decay from excited states to the ground state, generated heat, and then stored the energy by phase transitions of organic photothermal conversion materials.
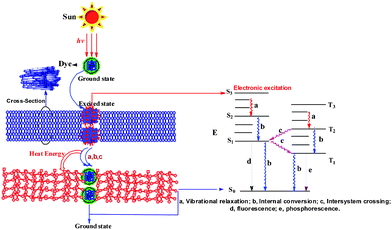 | ||
| Fig. 3 The proposed mechanism of solar thermal conversion and energy storage. | ||
To prove the sunlight irradiation durability of the VLDOPCM, sunlight irradiation cycling tests were carried out, and the result of a 200 cycle test is shown in Fig. 4. The tests were conducted in a sunlight operation placed for 20 min, followed by covering the irradiation for 20 min in a temperature-controlled water bath (10 °C) to ensure the solid–solid cycles. The spectral features obtained before (black line in Fig. 4a) and after (red line in Fig. 4a) the irradiation are virtually identical. Additionally, VLDOPCMs were then checked with DSC for their latent heat storage capacity and melting temperature change (Fig. 4b). Compared with 78.7 and 81.7 J g−1 before the cycles, the latent heats of melting and freezing were 79.5 and 81.8 J g−1 after 200 solid–solid cycles, respectively. Amazingly, no notable deterioration of VLDOPCM was observed even after 200 cycle operations.
 | ||
| Fig. 4 (a) Simulated sunlight irradiation spectra of VLDOPCM before (black line) and after (red line) the 200 cycles under sunlight irradiations. (b) DSC curves of VLDOPCM before (black line) and after (red line) the 200 cycle irradiations. | ||
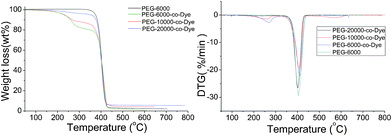
The thermogravimetric (TG) and derivative thermogravimetric (DTG) analysis curves of VLDOPCMs and monomer-PEG are shown in Fig. 5, and the results of the thermal decomposition temperature are summarized in Table 2. From the TG, DTG curves and Table 2, there are two-step degradation processes. The first step of degradation was when the temperature reached 260 °C and the polyurethane chain of VLDOPCMs (PEG-co-Dye) started to degrade.22 The second step of degradation occurred roughly from 400 °C to 420 °C, corresponding to the degradation of the PEG chain.24,25 The onset temperatures of degradation can be calculated from the TG curves by extrapolating from the curve at the peak of degradation back to the initial weight of the PEG and TDI. The weight loss of VLDOPCM was almost equal to the theoretical amount during the first degradation step. Therefore, the TG analysis results were consistent with the expected degradation mechanism.
| Samples | T-5wt% (°C) | Tmax1 (°C) | Tmax2 (°C) | Char yield at 650 °C (wt%) |
|---|---|---|---|---|
| PEG6000 | 373.5 | / | 403.9 | 2.1 |
| PEG6000-co-Dye | 231.6 | 270.4 | 410.8 | 0.1 |
| PEG10 000-co-Dye | 228.4 | 262.7 | 408.6 | 4.3 |
| PEG20 000-co-Dye | 352.1 | 270.1 | 401.6 | 5.4 |
 | ||
| Fig. 5 TG and DTG curves of PEG and PEG-co-Dye. | ||
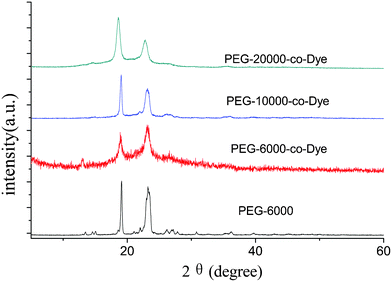
To reveal the crystallization further, X-ray diffraction of pure PEG and VLDOPCM are shown in Fig. 6. Sharp and intense diffraction peaks at 19.12° and 23.24° were observed for VLDOPCM and PEG-6000.21,26,27 Obviously, pure PEG and VLDOPCM showed similar diffraction curves, upon which the diffraction angle and crystal plane distance were nearly the same. Although the VLDOPCM’s diffraction peak height was lower than that of pure PEG, which means its crystal particles become smaller and its crystallinity decreases, they have the same crystal structure and unit cell type. Thus these results are consistent with the DSC results discussed above.
 | ||
| Fig. 6 X-Ray diffraction of PEG and PEGco-Dye polymer. | ||
In conclusion, a novel VLDOPCM was designed and synthesized by condensation polymerization based on dye and PEG monomers. A photon antenna, dye (1,4-bis((2-hydroxyethyl)amino)anthracene-9,10-dione) was used to capture and convert visible sunlight to thermal energy around the visible region. The thermal energy was effectively stored and released during the phase transition process of the phase change material (PCM-PEG). As functional materials with efficient light-to-heat conversion, the VLDOPCMs excellently performed light-harvest and thermal energy storage, and exhibited form-stable properties, which bridged the time gap between solar energy requirement and supply. Therefore, the newly prepared VLDOPCM may open a door to the design and development of driven molecular devices and machines, which would be useful in many areas of energy production. Our concept of the solar energy conversion materials will stimulate further fundamental work on the structures of the dyes as “photon captures and molecular heaters” for the form-stable PCMs. Further study is necessary to better understand and design more materials that enable and promote light-to-heat conversion and energy storage.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (20 804 007), the State Key Program of National Natural Science Foundation of China (20 836 001), the Doctoral Fund of Ministry of Education of China (200 801 411 032), and the National Key Technology R&D Program (2011BAE07B01).References
- A. V. Ruban, M. P. Johnson and C. D. P. Duffy, Energy Environ. Sci., 2011, 4, 1643–1650 CAS.
- C. G. Granqvist, Adv. Mater., 2003, 15, 1789–1803 CrossRef CAS.
- C. Liu, F. Li, L.-P. Ma and H.-M. Cheng, Adv. Mater., 2010, 22, E28–E62 CrossRef CAS.
- Y. Hou, B. L. Abrams, P. C. K. Vesborg, M. E. Björketun, K. Herbst, L. Bech, A. M. Setti, C. D. Damsgaard, T. Pedersen, O. Hansen, J. Rossmeisl, S. Dahl, J. K. Nørskov and I. Chorkendorff, Nat. Mater., 2011, 10, 434–438 CrossRef CAS.
- I. Thomann, B. A. Pinaud, Z. Chen, B. M. Clemens, T. F. Jaramillo and M. L. Brongersma, Nano Lett., 2011, 11, 3440–3446 CrossRef CAS.
- Y. Zeng, J. Yao, B. A. Horri, K. Wang, Y. Wu, D. Li and H. Wang, Energy Environ. Sci., 2011, 4, 4074–4078 CAS.
- M. Roeb, M. Neises, N. Monnerie, C. Sattler and R. Pitz-Paal, Energy Environ. Sci., 2011, 4, 2503–2511 CAS.
- K. Shimura and H. Yoshida, Energy Environ. Sci., 2010, 3, 615–617 CAS.
- R. H. Crabtree, Energy Environ. Sci., 2008, 1, 134–138 CAS.
- N. Armaroli and V. Balzani, Angew. Chem., Int. Ed., 2007, 46, 52–66 CrossRef CAS.
- X.-Z. Guo, Y.-D. Zhang, D. Qin, Y.-H. Luo, D.-M. Li, Y.-T. Pang and Q.-B. Meng, J. Power Sources, 2010, 195, 7684–7690 CrossRef CAS.
- J. P. Wang, X. X. Zhang and X. C. Wang, Renewable Energy, 2011, 36, 2984–2991 CrossRef CAS.
- A. A. Aydin and H. Okutan, Sol. Energy Mater. Sol. Cells, 2011, 95, 2417–2423 CrossRef CAS.
- I. Ceron, J. Neila and M. Khayet, Energy Build., 2011, 43, 1869–1874 CrossRef.
- T. Matsunaga, N. Yamada, R. Kojima, S. Shamoto, M. Sato, H. Tanida, T. Uruga, S. Kohara, M. Takata, P. Zalden, G. Bruns, I. Sergueev, H. C. Wille, R. P. Hermann and M. Wuttig, Adv. Funct. Mater., 2011, 21, 2232–2239 CrossRef CAS.
- D. G. Wood, M. B. Brown, S. A. Jones and D. Murnane, J. Phys. Chem. C, 2011, 115, 8369–8375 CAS.
- X. X. Zhang, P. F. Deng, R. X. Feng and J. A. Song, Sol. Energy Mater. Sol. Cells, 2011, 95, 1213–1218 CrossRef CAS.
- Y. Shen, A. G. Skirtach, T. Seki, S. Yagai, H. Li, H. Moöhwald and T. Nakanishi, J. Am. Chem. Soc., 2010, 132, 8566–8568 CrossRef CAS.
- N. Sarier and E. Onder, Thermochim. Acta, 2007, 454, 90–98 CrossRef CAS.
- W. D. Li and E. Y. Ding, Sol. Energy Mater. Sol. Cells, 2007, 91, 764–768 CrossRef CAS.
- J. C. Su and P. S. Liu, Energy Convers. Manage., 2006, 47, 3185–3191 CrossRef CAS.
- Q. Cao and P. S. Liu, Eur. Polym. J., 2006, 42, 2931–2939 CrossRef CAS.
- J. W. Schwede, I. Bargatin, D. C. Riley, B. E. Hardin, S. J. Rosenthal, Y. Sun, F. Schmitt, P. Pianetta, R. T. Howe, Z. X. Shen and N. A. Melosh, Nat. Mater., 2010, 9, 762–767 CrossRef CAS.
- N. Sarier and E. Onder, Thermochim. Acta, 2010, 510, 113–121 CrossRef CAS.
- Q. H. Meng and J. L. Hu, Sol. Energy Mater. Sol. Cells, 2008, 92, 1245–1252 CrossRef CAS.
- L. Feng, J. Zheng, H. Yang, Y. Guo, W. Li and X. Li, Sol. Energy Mater. Sol. Cells, 2011, 95, 644–650 CrossRef CAS.
- L. Zhang, J. Q. Zhu, W. B. Zhou, J. Wang and Y. Wang, Thermochim. Acta, 2011, 524, 128–134 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra20904g |
| This journal is © The Royal Society of Chemistry 2012 |
