Bone bonding ability—how to measure it?
Wojciech
Chrzanowski
*a,
Weai Jiet
Yeow
a,
Ramin
Rohanizadeh
a and
Fariba
Dehghani
b
aFaculty of Pharmacy, Pharmacy and Bank Building A15, University of Sydney, NSW 2006, Australia
bSchool of Chemical and Biomolecular Engineering, University of Sydney, NSW 2006, Australia
First published on 6th August 2012
Abstract
Bone bonding ability can be examined using in vitro assays. However, conventional approaches use static conditions simulating body fluids without biologically relevant compounds (proteins/growth factors). These features could have a major impact on surface properties, thus we investigated the effect of perfusion and simulated body fluid (SBF) composition of medium on the formation of apatite layer on nickel-titanium alloy (NiTi) with modified surfaces. Apatite layer formation in SBF was investigated under three conditions: static, dynamic (perfusion) and dynamic in serum supplemented SBF (S-SBF). It was found that perfusion and protein supplemented media altered results significantly when compared with standard approaches. Adequate media that contained both organic and inorganic phases provided a more accurate model for bone bonding ability testing. It was also confirmed that both plasma modified and ground to a mirror finish (and cleaned) encourage formation of apatite layers in all test conditions, which suggests the positive interaction of the material in bone tissue environment.
Introduction
The ability of materials to integrate within hard tissues can be assessed by in vitro tests in simulated body fluids (SBF). These tests were originally developed by Kokubo1–3 by using a fluid that mimics the ionic composition of human plasma. Bone bonding ability (i.e., the potential to bind with hard tissue) can be monitored by the formation of apatite layers on the surface of synthetic materials. It is well established that the materials that encourage apatite layer formation are generally integrated with human tissue.4–6 However, there is still debate whether such methodology can be used universally for all types of materials. In particular, in the last decade several revised compositions of SBF have been proposed that mimic the body environment much better than SBF composition used in the original assay by Kokubo.1,7 In fact apatite was not formed on the surface of β-TCP when using SBF for predicting bone bonding ability, however, the results of in vivo tests showed that this material integrated extensively with bone in the body.5,6 Due to relatively complex preparation of media and lack of carbonate content Bohner and Lemaitre7 proposed modified versions of SBF, and demonstrated similar efficacy in predicting bone bonding ability of materials. This new medium also contains only inorganic ions that are prepared by mixing two solutions to prevent premature precipitation prior to experiment, which was its main advantage. Chrzanowski8–10 proposed the feasibility of rapid surface analysis (<5 min) for determining the apatite layer formation using precise chemical and topographical techniques such as XPS and AFM. It was demonstrated that this approach provides a good correlation with cell-based assays. Importantly, Chrzanowski gave a new direction for assessing bone bonding ability by introducing organic compounds such as proteins into SBF media. His study demonstrated that organic content of media interacts immediately with the surface and plays a significant role in surface reactivity.11It is pertinent to note that body fluids contain inorganic ions as well as biologically relevant compounds (e.g., proteins, growth factors). Therefore, it is critical to include the effects of homeostasis and perfusion in SBF test to mimic precisely the physiological environment. Proteins interact with the surface immediately after placing the implant in the body and create a thin film that promotes cell responses.12 It is therefore important using media that contains relevant proteins for predicting bone bonding ability of an implant. Similarly, it is necessary to take into account the impact of dynamic conditions in the body.
The objective of this study was to evaluate the effects of media composition and test conditions on the formation of apatite layer and material/media interactions for assessing bone bonding ability of a material. We selected NiTi as a test material because this alloy possesses a limited activity in the body. However, it has been demonstrated that modified surfaces (polished, plasma treated) are osteoinductive—capable to stimulate osteoblastic cell differentiation and unregulated expression of osteogenic genes.
Experimental
Superelastic NiTi alloy (Johnson Matthey Inc. UK) was used in this study. Samples were cut into square shapes of 8 × 8 mm and the surface was ground to a mirror finish using SiC paper (grit 500–2400). Prior to the surface modification by plasma sputtering, the samples were precisely cleaned using ultrasonication in propan-2-ol, washing with deionised water (18 MΩ cm−1) each step for 5 min, immersing in nitric acid (63%) for 10 min, and finally ultrasonicating in deionised water for 5 min. Samples were dried with pressurised air and kept in a closed container to avoid contamination.13 These cleaned NiTi samples (coded as NT) were also used as control.Surface modification
Surface characterisation
In static conditions the samples were immersed in individual vials containing 5 mL of SBF (without any organic component) and incubated at 37 °C with 90% relative humidity. Three samples of each group were removed from the vials at day 1, 3, 7 and day 14. Samples were washed with 1 mL of deionised water three times and air dried.
In a dynamic condition, a custom-built perfusion reactor was used in the bioactivity test (Fig. 1). SBF was perfused at a rate of 1 mL min−1 in a closed loop. The reactor and reservoir were kept in the incubator at 37 °C and 90% relative humidity. The SBF in the reservoir was replaced every three days.
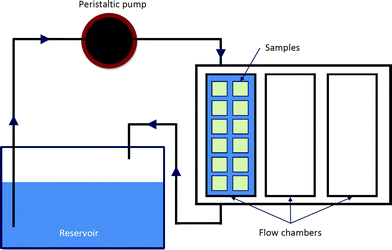 | ||
| Fig. 1 Schematic diagram of dynamic system. | ||
Tests were conducted without controlling CO2 content because our tests do not involve cells and control of CO2 was not critical. Similarly other bioactivity tests are proposed to be run without CO2 control.1,7
Zeiss EVO 50 SEM/EDX spectrometer was used to analyse the samples. EDX maps were recorded at different magnification and at least four different locations at the sample. Ca/P ratio was calculated as an average from all the locations.
TF-XRD Shimadzu XRD-6000: S6000 with Cu-Kα radiation (λ = 1.54 Å) at a generator voltage of 45 kV and a current of 40 mA was used. The data collection was performed by the 2θ scan method with 1° as incident beam angle and a scan speed of 2° min−1 at 0.02° step angle.
3. Results and discussion
3.1. Surface characterization
| Sample | Ni (at.%) | Ti (at.%) | O (at.%) | N (at.%) | C (at.%) | Ca (at.%) |
|---|---|---|---|---|---|---|
| NT | 2.84 ± 0.12 | 18.63 ± 0.18 | 48.89 ± 0.33 | 0.70 ± 0.09 | 27.22 ± 0.42 | 1.73 ± 0.10 |
| PC | 1.53 ± 0.10 | 19.38 ± 0.19 | 53.92 ± 0.39 | 2.06 ± 0.17 | 23.15 ± 0.50 | n/a |
Chemical analysis evidenced a small quantity of calcium on NT samples. The presence of calcium was not expected, calcium is regarded as a typical laboratory contaminant and it is believed that it appeared during polishing and washing steps. Calcium contaminations were previously reported and associated with laboratory contaminations.
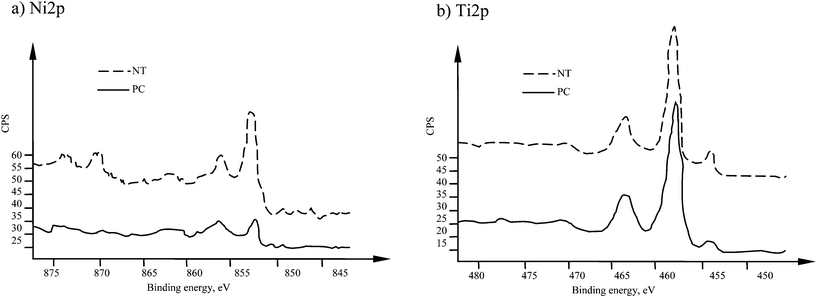
The analysis of high resolutions spectra in Fig. 2 show that Ni2p line had two main peaks at binding energies of 852.7 and 856.2 eV. These peaks correspond to metallic nickel and nickel oxides (Ni(OH)2 or Ni2O3).10,12 Titanium spectra for NT sample (Fig. 2b) have main peaks at 454.4 eV, and the second double-peak at 458.9 eV and 464.7 eV. These energies correspond to metallic titanium and titanium dioxide.
Surface roughness analysis in Fig. 3a shows that in general the surfaces are smooth and the estimated mean roughness is 4 nm. Plasma treatment exhibited negligible impact on the surface roughness. As depicted in Fig. 3b, grinding process resulted in creating some scratch marks (e.g., depth <10 nm) on the surface of both types of samples.
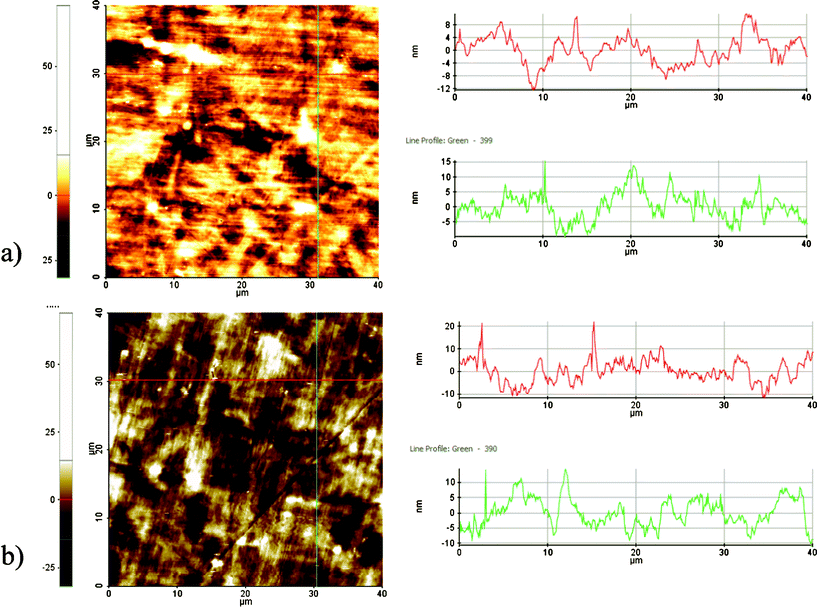 | ||
| Fig. 3 AFM images of the (a) cleaned-NT and (b) plasma cleaned- PC. | ||
| SFE (mN m−1) | γd | γp | γt | FP |
|---|---|---|---|---|
| NT | 29.80 ± 0.33 | 14.68 ± 0.19 | 44.48 ± 0.50 | 0.33 ± 0.005 |
| PC | 15.64 ± 0.24 | 42.14 ± 0.32 | 57.78 ± 0.56 | 0.73 ± 0.007 |
3.2 Bioactivity test using SBF
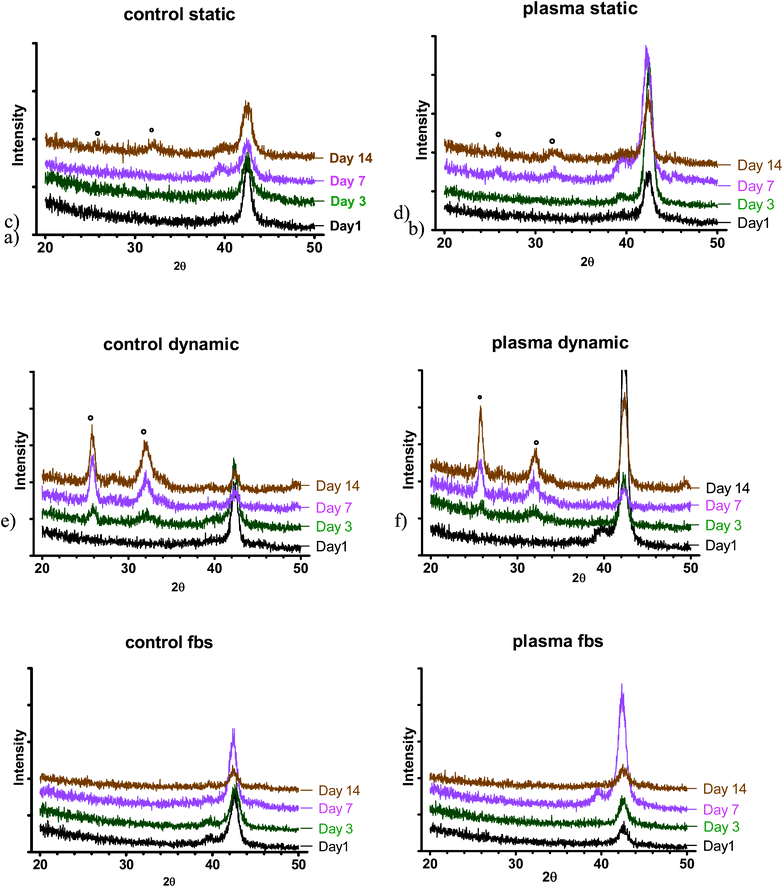 | ||
| Fig. 4 XRD spectra at various time points: a) control (NT) b) PC in static SBF; c) control (NT), d) PC in dynamic SBF; e) control (NT), f) PC in dynamic S-SBF. * indicates position of apatite peaks. | ||
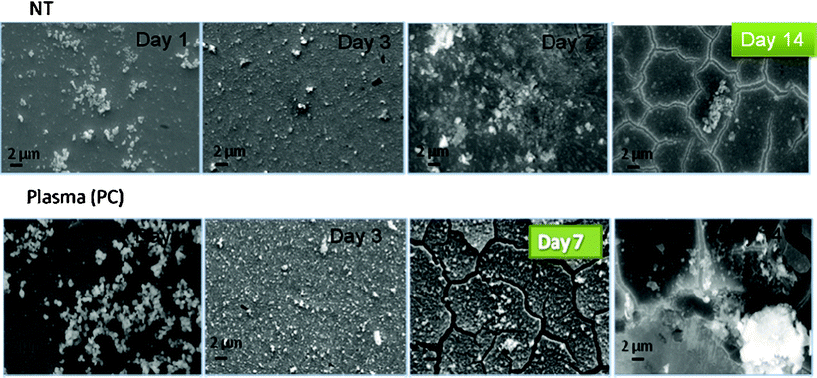 | ||
| Fig. 5 SEM images for NT and PC samples after incubation in SBF under static conditions. | ||
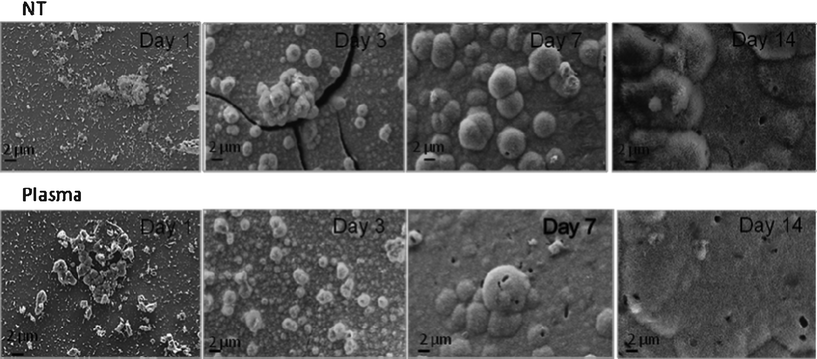 | ||
| Fig. 6 SEM images for NT and PC samples after incubation in SBF under dynamic conditions. | ||
Under dynamic conditions, shown in Fig. 7, the precipitation of apatite layer was substantially accelerated and for both types of samples a thick layer of apatite was observed at day three. The precipitation layers were prone to cracking under vacuum and even delaminated after the third day of incubation due to their substantial thickness.
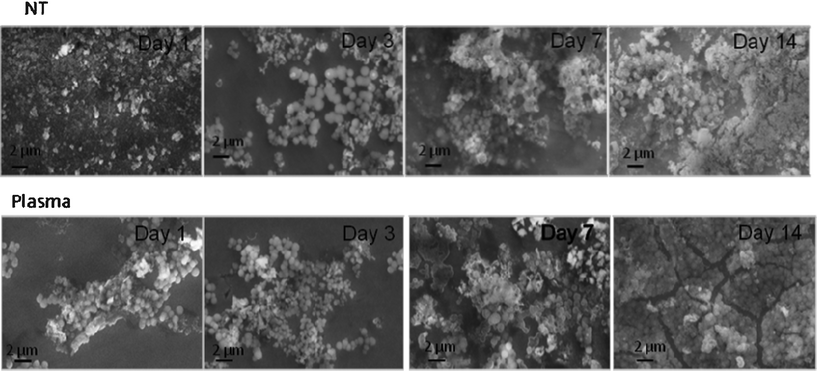 | ||
| Fig. 7 SEM images for NT and PC samples after incubation in FBS supplemented SBF under dynamic conditions. | ||
The profile of apatite layer formation was influenced by the type of medium. The rate of apatite layer formation was decreased when SBF was supplemented with FBS-S-SBF. The total surface coverage of precipitated apatite was only observed for PC samples after 14 days as depicted in Fig. 7. XRD did not detect apatite even after 14 days but SEM images showed the presence of apatite crystals predominantly in globular form. The results of AFM analysis in Fig. 8 are in agreement with the data acquired from SEM and XRD; the apatetic globules were larger in size on NT samples than those on PC samples.
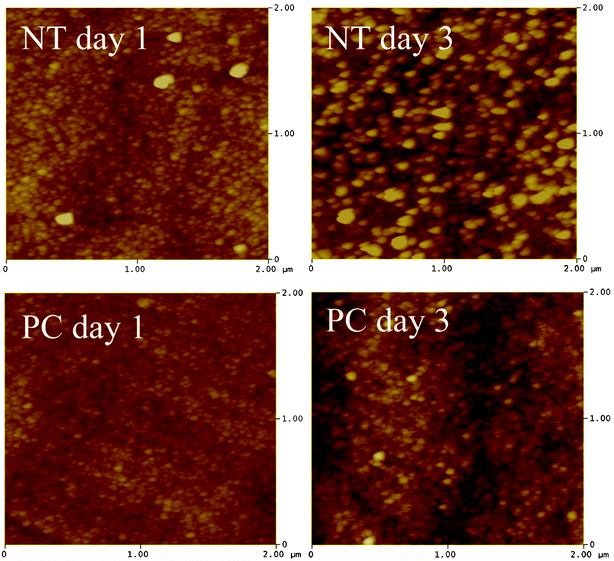 | ||
| Fig. 8 AFM image (2 × 2 μm) of NT and PC samples incubated in S-SBF. | ||
Mean Ca/P ratio in Table 3 is higher than that of stoichiometric hydroxyapatite (1.67) likely attributed to the type of CaP formed on the surface of treated NiTi alloy. Results show a range of Ca/P ratio from 1.37–2.26. There was no significant difference in Ca/P ratio between NiTi and PC for all three incubation conditions; static, dynamic and dynamic S-SBF (p > 0.05). However, by comparing the ratios across incubation conditions, Ca/P ratios for dynamic condition in SBF were significantly higher than both static conditions in SBF and dynamic conditions in S-SBF groups (p < 0.05).
| Ca/P ratio | Static | Dynamic | Dynamic S-SBF | |||
|---|---|---|---|---|---|---|
| NT | PC | NT | PC | NT | PC | |
| Day 1 | 1.56 | 1.64 | 1.44 | 1.37 | 1.37 | 1.47 |
| Day 3 | 1.66 | 1.68 | 1.73 | 1.81 | 1.56 | 1.42 |
| Day 7 | 1.59 | 1.72 | 2.26 | 2.21 | 1.43 | 1.48 |
| Day 14 | 1.91 | 1.92 | 2.24 | 2.26 | 1.49 | 1.68 |
Stoichiometric hydroxyapatite has Ca/P ratio of 1.67. Hydroxyapatite is the most stable form of CaP and a favourable bioactive material for bone replacement due to its similar chemistry to the bone mineral phase. Several samples possessed Ca/P ratio of 1.67, however, their XRD profiles were not comparable with hydroxyapatite. For example samples with the greatest ratio of Ca and P had very clear apatite peaks (PC dynamic 7 and 14 days), while the samples with ratios close to 1.67 did not present any apatite peak in XRD spectra (PC dynamic S-SBF). Generally, peaks similar to that of hydroxyapatite were detected in XRD profile of samples having Ca/P ratio above 1.67. The higher Ca/P observed in this study could be due to the substitution of carbonate for the phosphate group in the precipitated apatite.14
Significant morphological differences of the precipitates were observed for all 3 test conditions (Fig. 9). Precipitates under static SBF conditions had the form of dense, small and uniform globules. Standard SBF and dynamic perfusion conditions resulted in the formation of a more developed structure; many elongated, flake-like crystals were formed, while the incubation in S-SBF resulted in the formation of CaP precipitates that were smooth and more rounded.
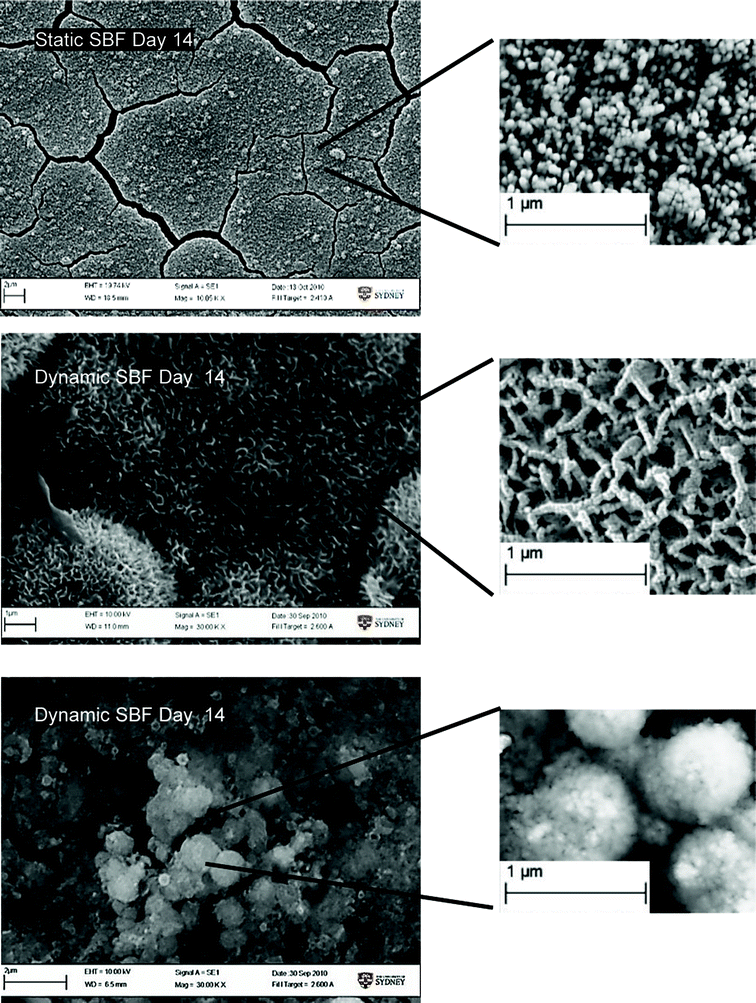 | ||
| Fig. 9 SEM image showing morphological difference in Ca/P growth on samples. | ||
4. Discussion
Plasma modification improves cell responses for various materials.15,16 This strategy has been broadly used as a standard method for the surface modification of some biomaterials. The results achieved so far are promising for enhancing cell-material interactions and suggest that this approach can be beneficial for less active materials such as nickel-titanium alloys.Nickel-titanium alloy is one of the materials used in biomedical applications, which has limited bioactivity due to the high nickel content. Several techniques were used to improve biological relevance of the material and recent studies have demonstrated that cell responses to the alloy were significantly improved by surface plasma modifications.10 These modifications addressed one of the outstanding problems of nickel-titanium alloy, which is its low bone bonding ability.
This characteristic is particularly critical for orthopaedics (i.e., spinal applications) of the alloy (e.g., spinal cages/discs). Chrzanowski has demonstrated that positive cell responses and upregulation of osteocalcin and osteopontin expressions was achieved by the plasma modifications.9,10 The surfaces showed osteogenic character and the results are in good agreement with the apatite layer formation in SBF,8 which is attributed to bone bonding ability of materials. However, these tests were conducted using a standard protocol:8 static conditions in simulated body fluid developed by Kokubo.1,2 Such a model replicates only the inorganic components of body media while it has been already shown that surfaces react with proteins within a few minutes forming a layer that can totally change interactions (precipitations) with ions from the body fluid.9,17 Therefore, to mimic the natural body environment it is critical to introduce dynamic (perfusion) conditions and supplement traditional simulated body fluids with organic components such as proteins. Both dynamic and modified media make the test more biologically relevant and result in a new standard method in bone bonding ability testing.
In this study we investigated the effects of bone bonding ability testing conditions, i.e., perfusion and body media composition on formation of apatite layer to validate a new standard in these tests. Secondly, the study was conducted to demonstrate the advantages of plasma modifications on apatite layer formation. Two groups of samples were used: (i) ground to a mirror finish and, (ii) plasma modified nickel-titanium. It has been previously shown that both surfaces have osteogenic character and demonstrate similar high bioactivity.18
The formation of apatite layers and its compositions in different conditions (static vs. dynamic) has demonstrated that perfusion condition accelerated the precipitation of calcium phosphates. The flow of the medium allowed for increasing the deposition and mineralisation of precipitates on the surface. In dynamic conditions, the precipitates had higher Ca/P ratio and apatite peaks were detected even at the third day of incubation regardless of the type of substrate. However, in standard-static-conditions they were observed at day 7 for plasma treated samples and at day 14 for untreated samples. It is believed that the continuous exchange of media, thus delivery of ions present in dynamic conditions, contributed to the accelerated formation of apatite layer. Most likely the physical interactions of fluid and ‘pushing’ the media to the surface could have an impact on the apatite layer formation. We demonstrated that perfusion is a key factor in the assessment of apatite layer formation and provides data in conditions that represent more closely the body environment. It is anticipated that both nickel-titanium samples used in the study exhibit similar levels of activity. These results support our hypothesis: perfusion of the medium in the bone bonding ability test has an impact on the results. The combination of perfusion and correct medium composition provides a model for the bioactivity testing that is closer to the real scenario.
Further analyses were conducted under dynamic conditions using protein supplemented medium. The formation of apatite layers was comparable for both types of samples. However, the results of SEM analysis show that the rate of formation of this layer was decreased when compared with both static and dynamic conditions using standard SBF. Hence, the adsorption of proteins present in the serum had a negative impact on initially formed CaP, by reducing the surface availability and affinity to precipitate bonelike apatite.19 The main composition of serum is albumin which is negatively charged at physiological pH.20,21 Thus Ca2+ ions bind to the albumin22 and decrease the rate of CaP precipitation. This phenomena may also be attributed to the rapid absorption of protein in serum on the surface of NiTi23,24 that impede Ca2+ and PO42− ion binding sites on the surface of the samples, hence nucleation of apatite crystals.20 Importantly, no apatite peaks were detected in XRD spectra, which indicates that apatite was not formed on the surface. It can be anticipated that the Ca/P layer was predominately amorphous. The results are not in full agreement with our previous studies8–10,25 because osteogenic character was not confirmed here. However, precipitates were clearly observed for both types of the samples at similar levels and it confirmed our hypothesis: organic components of medium used in the bone bonding ability tests change drastically the test results. It is still postulated that these results are more relevant to predict material behaviour in vivo but care must be taken because no apatite was observed in these conditions but samples were reported to trigger osteoblastic differentiation and mineralization.10,12,18 This effect could indicate that such tests could produce false negative results and further measurement such as protein adsorption and cell responses are still required.
The type of CaP formed on the surface can be identified by conducting the morphology analysis. The flaky and porous structure of CaP was formed under dynamic SBF conditions and it is similar to the morphology of octacalcium phosphate (OCP). However, the smooth and globular CaP as observed in both static and dynamic S-SBF incubated conditions tended to be more needle-shape apatite-like crystals.26 Based on EDX results, apatite formed in dynamic conditions using supplemented SBF showed lower Ca/P ratios because the Ca2+ ions could bind to proteins present in the media.21 It can be speculated that due to lower Ca2+ ions available for precipitation, calcium deficient hydroxyapatites was formed. The chemical and structural analysis of precipitates revealed that apatite diffraction peaks are detected only when the ratio of Ca/P was above 1.67, which refers to stoichiometric hydroxyapatite. This finding suggests that carbonate groups are substituted for phosphate groups during apatite formation. The higher Ca/P ratio of precipitated apatite has been reported in previous studies.14,27
Bone bonding ability tests serve as in vitro analysis to reduce the number of animals for in vivo tests.1,2 Differences in CaP film formation observed in the standard method for both tested materials were not observed in the modified methods, which is in agreement with our previous in vitro studies. The addition of serum and presence of shear force mimics bodily conditions, thus apatite forming ability in such conditions can be considered as a new approach for predicting bone bonding ability. However, osseointegration, bone tissue growth at bone-implant interface without the formation of fibrous tissue,28,29 is a complex process and this proposed test is an alternative strategy that acts as a screening method prior to cell/tissue/animal based tests to minimise the required in vitro and in vivo tests. We demonstrated that various factors affect bioactivity and a new method developed for testing bone bonding ability mimics the human body environment and provide insights for materials integration with bone prior to in vivo studies. We found that plasma treated samples have been found to improve apatite film formation when a standard approach7 was used. However, when dynamic conditions and FBS supplemented media were used, negligible effects of treatment on the ability to form CaP layer were noted. These tests demonstrated that the apatite layers were readily formed on both types of samples and it is anticipated that both materials integrate within surrounding hard tissues. This finding is in full agreement with previous results, where insignificant differences between both types of samples were reported and expressions of osteogenic gens were at the same level.30 Hence, tests with the use of dynamic conditions and serum supplemented media correlate well with cell-based experiments.
This study demonstrates that lower nickel content and more favourable surface free energy improved the apatite layer formation only for standard method (SBF without organic components in static condition). It could be stated that changes in surface energy and small differences in chemistry did not play a significant role in the layer formation in modified techniques due to the extensive interactions of surface with proteins that alter apatite layer formation. This data was in agreement with previous work.
5. Conclusions
The Perfusion of simulated body fluid is an important factor in the assessment of apatite layer formation. Typically in the dynamic condition, interactions between medium components and surfaces were enhanced, which resulted in accelerating the deposition of apatite film. Furthermore, it was found that organic components of medium used in bone bonding ability tests change drastically the results due to the protein/drug/peptides interactions with the surface of biomaterials. The media that contains both organic and inorganic phases provides a more accurate model for bioactivity testing. It was also confirmed that both plasma modified and ground to a mirror finish (and cleaned) encouraged formation of apatite layers in all test conditions, which allows to predict the positive integration of material with bone.Despite the high rate of the layer precipitations on the control ground nickel-titanium and plasma treated when using perfusion and supplement medium no apatite peak was detected by XRD. The apatite might have formed but its level was below detection of XRD. It was postulated that perfusion and media that contain organic compounds are more relevant to predict material behaviour in vivo because they capture early protein/peptides interactions with surfaces, which are key in cell-surface communication. The lack of apatite formation suggests that such tests could produce false negative results and further aspects such as protein adsorption and cell responses are still required.
References
- T. Kokubo and H. Takadama, Biomaterials, How useful is SBF in predicting in vivo bone bioactivity?, 2006, 27(15), 2907–2915 CAS.
- T. Kokubo, Acta Materialia, Apatite formation on surfaces of ceramics, metals and polymers in body environment, 1998, 46(7), 2519–2527 CAS.
- T. Kokubo, Materials Science and Engineering: C, Design of bioactive bone substitutes based on biomineralization process, 2005, 25(2), 97–104 Search PubMed.
- K. Nishio, Journal of Biomedical Materials Research, et al., The effect of alkali- and heat-treated titanium and apatite-formed titanium on osteoblastic differentiation of bone marrow cells, 2000, 52(4), 652–661 Search PubMed.
- J. Isaac, Journal of Biomedical Materials Research Part A, et al., : Bone-like tissue formation on a biomimetic titanium surface in an explant model of osteoconduction, 2009, 89A(3), 585–593 Search PubMed.
- A. Fukuda, Acta Biomaterialia, et al., Bone bonding bioactivity of Ti metal and Ti–Zr–Nb–Ta alloys with Ca ions incorporated on their surfaces by simple chemical and heat treatments, 2011, 7(3), 1379–1386 Search PubMed.
- M. Bohner and J. Lemaitre, Biomaterials, Can bioactivity be tested in vitro with SBF solution?, 2009, 30(12), 2175–2179 Search PubMed.
- W. Chrzanowski, Acta Biomaterialia, et al., : Effect of surface treatment on the bioactivity of nickel–titanium, 2008, 4(6), 1969–1984 Search PubMed.
- W. Chrzanowski, Journal of Biomedical Materials Research Part A, et al., In vitro studies on the influence of surface modification of Ni–Ti alloy on human bone cells, 2010, 93A(4), 1596–1608 Search PubMed.
- W. Chrzanowski, Journal of Biomaterials Applications, et al., : Biocompatible, Smooth, Plasma-Treated Nickel–Titanium Surface – An Adequate Platform for Cell Growth, 2012, 26(6), 707–731 Search PubMed.
- W. Chrzanowski, Journal of Biomedical Materials Research Part A, et al., In vitro studies on the influence of surface modification of Ni–Ti alloy on human bone cells., 2010, 93(4), 1596–1608 Search PubMed.
- W. Chrzanowski, Acta Biomaterialia, et al., : Effect of surface treatment on the bioactivity of nickel-titanium, 2008, 4(6), 1969–1984 Search PubMed.
- W. Chrzanowski, Materials Science and Engineering: C, et al., Impaired bacterial attachment to light activated Ni–Ti alloy, 2010, 30(2), 225–234 Search PubMed.
- R. Rohanizadeh, Journal of Biomedical Materials Research, et al., : Apatite precipitation after incubation of biphasic calcium-phosphate ceramic in various solutions: Influence of seed species and proteins, 1998, 42(4), 530–539 Search PubMed.
- P. K. Chu, Surface and Coatings Technology, Enhancement of surface properties of biomaterials using plasma-based technologies, 2007, 201(19–20), 8076–8082 Search PubMed.
- L. Tan, R. A. Dodd and W. C. Crone, Biomaterials, Corrosion and wear-corrosion behavior of NiTi modified by plasma source ion implantation, 2003, 24(22), 3931–3939 Search PubMed.
- W. Chrzanowski, Archives of Materials Science and Engineering, et al., Study on bioactivity of NiTinol after surface treatment, 2008, 21(1), 3 Search PubMed.
- W. Chrzanowski, Engineering surface properties of nickel-titanium alloy for improved osteointegration. 1 ed. Vol. 1., 2012Gliwice: Printing House of the Silesian University of Technology. 161 Search PubMed.
- S. Areva, Journal of Biomedical Materials Research Part A, et al., Use of sol–gel-derived titania coating for direct soft tissue attachment, 2004, 70A(2), 169–178 Search PubMed.
- Y. Wang, Materials Science and Engineering: C, et al.,In vitro behavior of fluoridated hydroxyapatite coatings in organic-containing simulated body fluid, 2007, 27(2), 244–250 Search PubMed.
- F. Sussman and H. Weinstein, Proceedings of the National Academy of Sciences, On the ion selectivity in Ca-binding proteins: the cyclo(-L-Pro-Gly-)3 peptide as a model, 1989, 86(20), 7880–7884 Search PubMed.
- H. A. Saroff and M. S. Lewis, The Journal of Physical Chemistry, THE BINDING OF CALCIUM IONS TO SERUM ALBUMIN, 1963, 67(6), 1211–1216 Search PubMed.
- A. S. Svetlana, Bio-Medical Materials and Engineering, Surface, corrosion and biocompatibility aspects of Nitinol as an implant material, 2002, 12(1), 69–109 Search PubMed.
- S. Shabalovskaya, J. Anderegg and J. Van Humbeeck, Acta Biomaterialia, Critical overview of Nitinol surfaces and their modifications for medical applications, 2008, 4(3), 447–467 Search PubMed.
- K. Page, Journal of Materials Science, et al., Study of the adhesion of <i>Staphylococcus aureus</i> to coated glass substrates, 2011, 46(19), 6355–6363 Search PubMed.
- C. Gobel, Journal of Materials Chemistry, et al., Phase formation and morphology of calcium phosphate-gelatine-composites grown by double diffusion technique: the influence of fluoride, 2004, 14(14), 2225–2230 Search PubMed.
- R. Rohanizadeh, M. Trécant-Viana and G. Daculsi, Calcified Tissue International, Ultrastructural Study of Apatite Precipitation in Implanted Calcium Phosphate Ceramic: Influence of the Implantation Site, 1999, 64(5), 430–436 Search PubMed.
- D. A. Puleo and A. Nanci, Biomaterials, Understanding and controlling the bone-implant interface, 1999, 20(23-24), 2311–2321 Search PubMed.
- J. Davies, Journal of Dental Education, Understanding peri-implant endosseous healing, 2003, 67(8), 932–949 Search PubMed.
- W. Chrzanowski, Journal of Biomaterials Applications, et al., Biocompatible, smooth, plasma-treated nickel–titanium surface – an adequate platform for cell growth, 2011 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |