Operando 57Fe Mössbauer and XRD investigation of LixMnyFe1-yPO4/C composites (y = 0.50; 0.75)†
Alexis
Perea
*a,
Moulay T.
Sougrati
a,
Costana M.
Ionica-Bousquet
a,
Bernard
Fraisse
a,
Cécile
Tessier
b,
Laurent
Aldon
a and
Jean-Claude
Jumas
a
aICGM/AIME (CNRS UMR 5253), Université Montpellier II, CC 15-02, Place E. Bataillon, 34095 Montpellier Cedex 5, France. E-mail: alexis.perea@univ-montp2.fr
bSaft Recherche Division, 111-113 Bld, Alfred Daney, 33074 Bordeaux, France
First published on 7th August 2012
Abstract
LiMn0.50Fe0.50PO4 and LiMn0.75Fe0.25PO4 powders have been synthesized by ceramic route. A comparative investigation of their electrochemical behaviour using operando57Fe Mössbauer and X-ray diffraction is reported. The complementarity of operando techniques used in this study allows the monitoring of changes of the local electronic environment and the lattice modifications that are directly linked to the redox reaction mechanisms. During the charge, LiMn0.50Fe0.50PO4 has been found to undergo three well defined and reversible reactions via an intermediate phase containing simultaneously FeII and FeIII cations. LiMn0.75Fe0.25PO4 undergoes a two reaction mechanism. FeIII Mössbauer signature has been found to be sensitive to the oxidation of MnII since this oxidation is accompanied with a significant increase of the iron quadrupole splitting. This study summarizes the different mechanisms observed for compositions with different manganese content.
1. Introduction
Since the work on the Fe3+/Fe2+ redox couple in phospho olivines was first reported in 1997, LiFePO4 has been intensively studied as a promising cathode material1–4 for the next generation of lithium-ion batteries because of its low cost, safety and environmental compatibility. Numerous studies have attempted to improve the electrochemical performances of the material and to get deeper insight in the lithium electrochemical mechanism.5–9 On the other hand, attempts have been made to enhance the potential of olivine cathode materials by using manganese phosphate instead of iron phosphate.3,10 Despite the recent advances in cycling insulating materials, thanks to nano-coating techniques and new preparation routes11,12 and even though some authors have reported promising results,11–13 LiMnPO4 is not used as active material in lithium battery. This is due to the fact that it shows much lower effective energy density than the lithium iron phosphate owing to the low practical capacity evidenced. In addition, LiMnPO4 requires very slow charge and discharge rates.2,14–16 The main reason is the large kinetic barrier at the mismatched interface of MnPO4/LiMnPO4.4 It is also worth noticing that during the charge, LiMnPO4 exhibits higher volume change than LiFePO4.2,15,17A good compromise between LiFePO4 and LiMnPO4 is the partially substituted phases LiMnyFe1−yPO4,3,15,18 that would introduce the advantage of combining good electrochemical performances of LiFePO4 with a higher potential for manganese (∼4.1 V compared with ∼3.4 V vs. Li+/Li for iron) in order to enhance the energy density.
It is however not yet clear which composition has to be preferred for practical application. Yamada et al. suggested that LiMn0.6Fe0.4PO4 composition exhibits the best electrochemical performances.15 LiMn0.4Fe0.6PO4 has also been reported to give good specific capacity after 100 cycles.19 Chang et al. have reported that LiMn0.3Fe0.7PO4 shows the excellent cycling performances,20 whereas Xu et al. found that LiMn0.1Fe0.9PO4 shows the best cycling performances.21 Despite increasing interest in the Mn substituted phases, their electrochemical mechanisms are not yet well understood. It is, for example, not clear why the Fe3+/Fe2+ and Mn3+/Mn2+ redox potentials are shifted (vs. Mn content) and why the polarizations related to these two couples show an opposite behaviour.22
Operando techniques are increasingly employed for the investigation of the batteries electrochemical behaviour. Few analyses of LiMnyFe1−yPO4 have been reported using XRD and X-ray absorption spectroscopy (XAS).23–25
We have recently reported an operando study of LixMnyFe1−yPO4/C composites (y = 0; 0.25).1,26 In order to establish a global mechanism, we have prepared LiMn0.50Fe0.50PO4/C and LiMn0.25Fe0.75PO4/C using solid-state reaction. Operando XRD and 57Fe Mössbauer Spectroscopy analyses have been used to investigate the evolution of iron local environment, oxidation state and lattice structure during the electrochemical redox process.
2. Experimental section
2.1 Synthesis procedure
LiMnyFe1−yPO4/C (y = 0.5; 0.75) composites were obtained by solid-state reaction from Li2CO3, FeC2O4·2H2O, Mn(COOCH3)2·4H2O, NH4H2PO4 and a source of carbon (all chemicals of 99% purity from Aldrich) taken in stoichiometric quantities. The precursors were first ball milled during 90 min and then thermally treated in a furnace under argon flow at 600 °C.2.2 Characterization of materials
After preparation, the compounds were crushed in an agate mortar and powder XRD (PHILIPS X′Pert MPD θ–2θ diffractometer equipped with the X′celerator detector) patterns were recorded using Cu-Kα radiation (λ = 1.5418 Å) and a nickel filter.The residual carbon content was evaluated by a Flash EA 1112 analyser based on the Dynamic Flash Combustion which produced complete combustion of the sample followed by an accurate and precise determination of the elemental gases produced. 57Fe Mössbauer spectra were recorded in the constant acceleration mode and in transmission geometry on a standard Mössbauer spectrometer composed of electronic devices from Ortec and Wissel. A 57Co(Rh) source with a nominal activity of 370 MBq was used. The source and the absorber were always kept at RT. All the isomer shifts are given relative to α-Fe at RT.
2.3 Electrochemical behaviour
Electrodes containing 80 wt.% sample and 20 wt.% carbon black were prepared for cycling tests. Standard Swagelok cells Li|1 M LiPF6 (propylenecarbonate (PC): ethylene carbonate (EC): dimethyl carbonate (DMC) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v)| LiMnyFe1−yPO4 were assembled in an argon-filled glove box and tested using a VMP system.
3, v/v)| LiMnyFe1−yPO4 were assembled in an argon-filled glove box and tested using a VMP system.
To identify the electrochemical reaction mechanisms, we carried out operando XRD and 57Fe Mössbauer spectroscopy measurements for both LiMnyFe1−yPO4 (y = 0.50; 0.75) electrodes using an electrochemical cell designed especially for this aim.27
To preserve beryllium window from the oxidation due to high working voltage, it was covered by a 2 μm thick foil of pure aluminium (Goodfellow). Here, the cells were charged and discharged using a C/40 rate, at room temperature in the voltage ranges 2.75–4.6 V. Each operando XRD scan was recorded during 1 h in which Li extraction/insertion was performed and the results correspond to a change of composition of Δx = 0.025 Li (where x is in LixMnyFe1−yPO4/C (y = 0.50; 0.75)). A 2 h acquisition time was used for the operando Mössbauer measurement. Each spectrum corresponds to Δx = 0.05 extracted/inserted Li. For y = 0.75 the spectra presented are obtained in situ but not operando due to the low amount of iron in the electrode.
3. Results and discussion
XRD patterns of the obtained powders are given in Fig. 1a. As it can be seen, the powders are free of any crystalline impurity.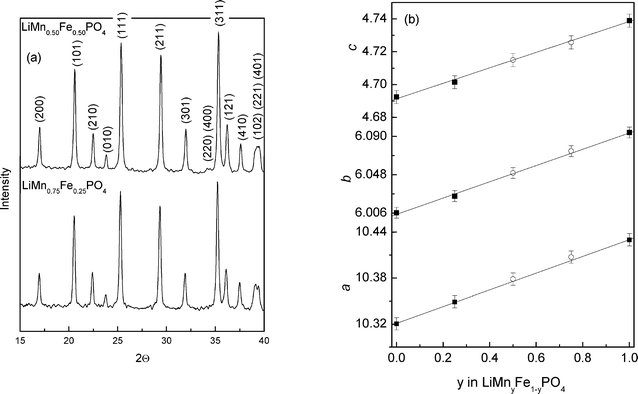 | ||
| Fig. 1 Powder X-ray diffraction patterns (a), and evolution of lattice parameters with manganese content of LiMnyFe1−yPO4/C (b). | ||
All the diffraction peaks can be indexed in the orthorhombic Pnma space group. Lebail method28 has been used to estimate the lattice parameters a = 10.368(1) Å, b = 6.050(1) Å, c = 4.710(1) Å for LiMn0.50Fe0.50PO4/C, and a = 10.397(1) Å, b = 6.073(1) Å, c = 4.726(1) Å for LiMn0.75Fe0.25PO4/C. These values are in good agreement with the literature.3 Average crystallite sizes of 120 nm have been obtained using the Scherrer formula.29 The residual carbon content was evaluated at 4.89% and 4.82% for LiMnyFe1−yPO4 (y = 0.50; 0.75), respectively.
Fig. 1b gives the variation of a, b and c for the olivine phases. All the lattice parameters increase linearly with manganese content. The main effect of the substitution of iron-manganese in LiMnyFe1−yPO4 is the expansion of the network that can be explained simply by the difference between the ionic radii of high spin Mn2+ (0.97 Å) and Fe2+ high spin (0.92 Å).
LiMnyFe1−yPO4/C (y = 0.50; 0.75) spectra (Fig. 2) consist of sharp doublets with an isomer shift of δ = 1.235(2) and a quadrupole splitting of Δ = 2.947(3) mm s−1 for y = 0.5 and an isomer shift of δ = 1.239(1) and a quadrupole splitting of Δ = 2.942(2) mm s−1 for y = 0.75 as discussed previously.1
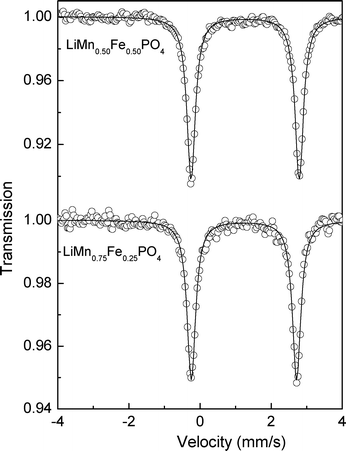 | ||
| Fig. 2 57Fe Mössbauer spectra for LiMnyFe1−yPO4/C, where y = 0.50 and y = 0.75. | ||
The voltage profiles of LiMnyFe1−yPO4 are shown in Fig. 3a. For LiFePO4, a flat plateau is observed at 3.4 V as previously reported. For the substituted phases, two pseudo plateaus are observed in agreement with the two redox reactions Fe3+/Fe2+ and Mn3+/Mn2+. The length of the Mn plateau is proportional to manganese content.
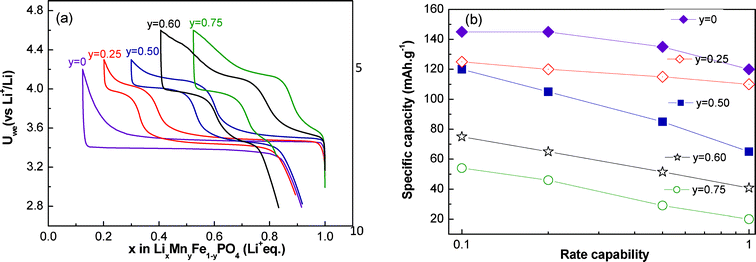 | ||
| Fig. 3 Electrochemical curves (first cycle) (a) and rate capability (b) of LiMnyFe1−yPO4. | ||
Calculated capacities with the cycle rate for LixMnyFe1−yPO4/C (y = 0.25, 0.50, 0.60, 0.75) are summarized in Fig. 3b. This representation allows direct visualization of the reversible capacity of the material depending on the rate of cycling. The capacity is found to be a function of both cycling rate and Mn content. For our synthesis conditions, the material with the best performance is the composition LiMn0.25Fe0.75PO4/C. The loss of capacity between C/10 and C is 12% and 62%, respectively, for the compositions LiMn0.50Fe0.50PO4/C and LiMn0.75Fe0.25PO4/C. The electrochemical performance decreases with the manganese content: for the phase LiMn0.75Fe0.25PO4/C the reversible capacity calculated for a cycle rate of C/10 is 56 mAh.g−1 against 125 mAh.g−1 for the substituted phase with less manganese content LiMn0.50Fe0.50PO4/C.
The redox potentials have been obtained from the derivative curves (Fig. 4). These potentials associated with the redox couples Fe3+/Fe2+ and Mn3+/Mn2+ undergo an increase with the manganese content. Kobayashi et al.30 explain this with an expansion of the volume of all compositions. These variations are sufficient to increase the average potential of the couple Fe3+/Fe2+ from 3.45 V to 3.56 V vs. Li+/Li0 respectively for y = 0 and y = 0.75. This variation in potential of 0.11 V may appear negligible in the case of a battery with one cell, however, it can be important in the case of batteries with many cells mounted in parallel that can be used, for example, for electrical vehicles.
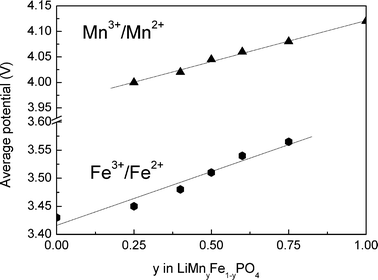 | ||
| Fig. 4 Average potential of redox reactions in LiMnyFe1−yPO4. | ||
To better understand the reaction mechanisms, operando analyses have been performed.
For y = 0.50 the Mössbauer spectra corresponding to the first cycle and the parameters associated with the first charge are represented, respectively, in Fig. 5a and 5b. The diffractograms corresponding to the first cycle of phase LiMn0.50Fe0.50PO4 are shown in Fig. 6a. At the beginning of the charge (blue area) (0.70 ≤ x < 0.95) a new phase appears in both XRD patterns and Mössbauer data. The (020) peak of the pristine material vanishes at the expense of a new peak located at 29.3°. In this domain, the Mössbauer spectra (Fig. 7) indicates the transformation of initial Fe2+ into two components: the first is associated with Fe3+ with the parameters (δ = 0.42 mm s−1 and Δ = 1.12 mm s−1) and a second component associated with a new Fe2+ component (δ = 1.26 mm s−1 and Δ = 2.72 mm s−1). The quadrupole splitting of this new Fe2+ is lower than that of the initial Fe2+ indicating an increase of the distortion of the iron environment31 caused by the deintercalation of Li+ and iron oxidation. This observation allows us to conclude the formation of a mixed valence phase with the following composition: Li0.70MnII0.50FeII0.20FeIII0.30PO4. It is worth noting that such phases (called ferrisicklerites Lix<1(Fe,Mn)PO4) have been reported by mineralogists and attributed to natural oxidation of triphylite Li(Fe,Mn)PO4.32–34 From the study of 45 ferrisicklerite minerals, Fanton et al. have concluded on the absence of any structural order of the Mn and Fe cations in this family of materials.32
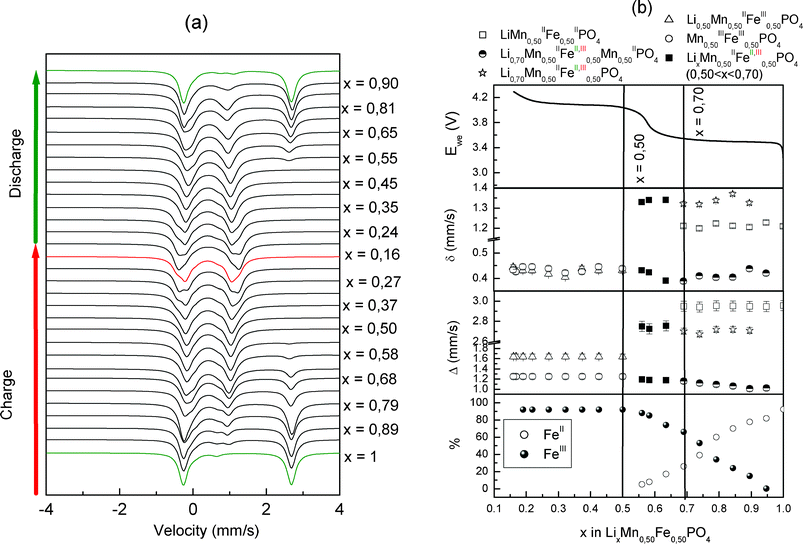 | ||
| Fig. 5 Evolution of the operando Mössbauer spectra (a), and hyperfine parameters of FeII and FeIII species (b), of LixMn0.5Fe0.5PO4/C recorded during the electrochemical process recorded at C/40 rate. | ||
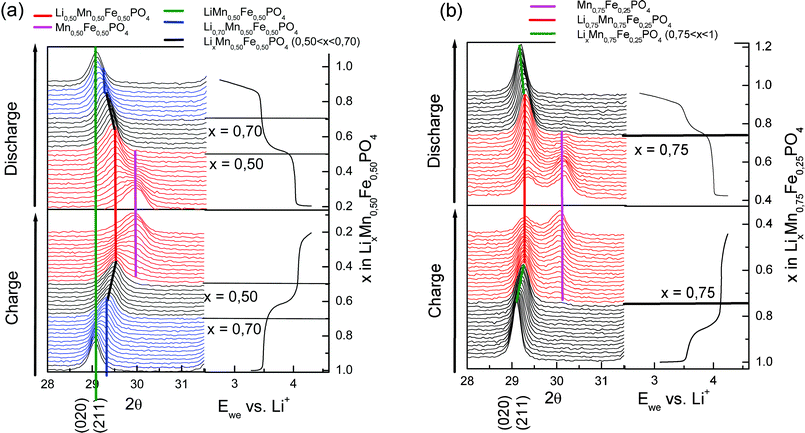 | ||
| Fig. 6 Operando X-ray diffractograms of LiMn0.50Fe0.50PO4/C (a), and LiMn0.75Fe0.25PO4/C (b). | ||
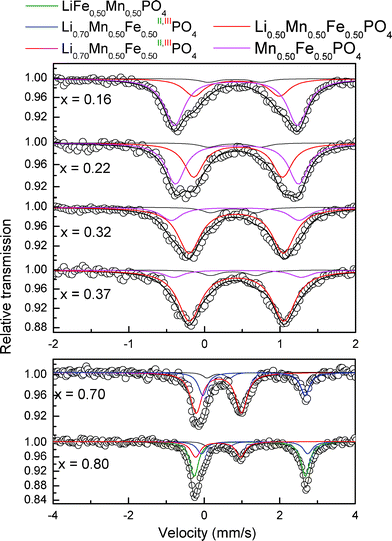 | ||
| Fig. 7 Mössbauer spectra of LixMn0.50Fe0.50PO4/C obtained at the indicated x values. | ||
From these observations we conclude a biphasic mechanism with the coexistence of LiMnII0.50FeII0.50PO4 and Li0.70MnII0.50FeII,III0.50PO4 for the charge and discharge in the range 0.70 ≤ x < 0.95.
The electrochemical reaction in this region can be written:
| Li1.0MnII0.50FeII0.50PO4 ↔ (α)Li0.70MnII0.50FeII0.20FeIII0.30PO4 + (1 − α)Li1.0MnII0.50FeII0.50PO4 + 0.30 αLi+ 0.30αe− (where 0 < α ≤ 1) |
In the second region (0.50 ≤ x ≤ 0.70), shown in black in Fig. 6a, the diffraction lines corresponding to the Li0.70Mn0.50Fe0.50PO4 phase are shifted to upper angles, indicating the existence of a solid solution domain in charge and discharge. This also is supported by gradual increase (∼21%) of the quadrupole splitting of Fe3+ with the oxidation of iron. Therefore, in this area the electrochemical reaction can be written as follows:
| Li0.70MnII0.50FeII0.20FeIII0.30PO4 ↔ Li0.70−αMnII0.50FeII0.20−αFeIII0.30+αPO4 + αLi+ + αe− (where 0 ≤ α ≤ 0.2) |
In the third region (∼0.16 ≤ x < 0.5, shown in red in Fig. 6a, the two-phase character is more evident. A new phase appears from x = 0.50 (Li0.50MnIII0.50FeIII0.50PO4) with diffraction peaks located at angles higher than the initial phase:
| Li0.50MnII0.50FeIII0.50PO4 ↔ βMnIII0.50FeIII0.50PO4 + (1 − β)Li0.50MnII0.50FeIII0.50PO4 + 0.50βLi+ + 0.50βe− (where 0 < β ≤ 1) |
All the Fe2+ was oxidized into Fe3+ at the end of the second region, however, the Mössbauer spectra continue to evolve (Fig. 7), indicating that the environment of iron is affected by the oxidation of Mn2+ in Mn3+ as already observed for the phase LiMn0.25Fe0.75PO4.1
From x ≤ 0.50 the spectra (Fig. 7) are refined by adding a new Fe3+ doublet shown in magenta. The new doublet corresponding to Fe3+ phase MnIII0.50FeIII0.50PO4 exhibits a quadrupole splitting of 1.65 mm s−1, which is higher than 1.55 mm s−1 observed for MnIII0.25FeIII0.75PO4, and this further confirms that the Fe3+ is very sensitive to the oxidation state of Mn since an increase of the amount of Mn3+ around the Fe3+ site leads to a significant increase of the quadrupole splitting.31 Similar spectra have been reported for natural purpurite (Fe,Mn)PO4.31 The electromechanism of the charge and discharge of LiMn0.50Fe0.50PO4/C is done in three steps as the LiMn0.25Fe0.75PO4/C phase. At the beginning, the mechanism is biphasic, with the coexistence of LiMnII0.50FeII0.50PO4 and a mixed composition Li0.70MnII0.50FeII0.20FeIII0.30PO4 up to x = 0.50. From x = 0.50, the second domain is a solid solution where the phase LixMnII0.50FeII0.50FeIII0.30PO4 oxidizes up to x = 0.16. The last domain is biphasic between Li0.50MnII0.50FeIII0.50PO4 and MnII0.50FeIII0.50PO4.
The electrochemical mechanism corresponding to the richest manganese phase LiMn0.75Fe0.25PO4/C was also studied. The diffractograms corresponding to the first cycle with the galvanostatic curves are shown in Fig. 6b. The three 57Fe Mössbauer spectra (Fig. 8) do not match the operando mode, indeed the low iron content of this phase would not have sufficient quality for data processing. They were, however, recorded in situ with the battery paused at key points.
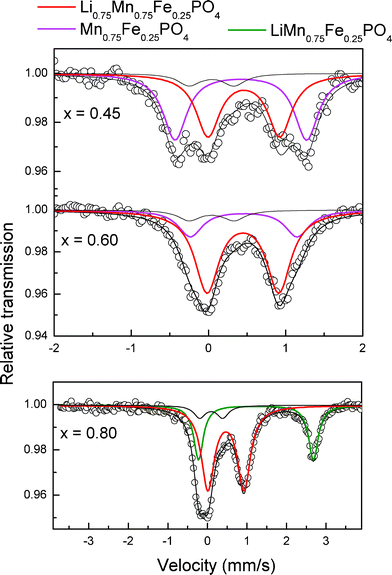 | ||
| Fig. 8 Mössbauer spectra of LixMn0.75Fe0.25PO4/C obtained at the indicated x values. | ||
From x = 0.95 the diffraction line corresponding to the plane (020) phase LiMn0.75Fe0.25PO4/C (29.2° (2θ)) shifts to higher angles up to x = 0.75 (black area of Fig. 6b). The reaction corresponding to the oxidation of iron is single phase in this area (0.45 ≤ x < 0.75) for the charge and discharge:
| Li1.0MnII0.75FeII0.25PO4 ↔ Li1−αMnII0.75FeII0.25+αFeIIIαPO4 +αLi+ + αe−(where 0 < α ≤ 0.25) |
After x = 0.75, the Mössbauer spectra show clearly that all the Fe2+ has been oxidized to Fe3+ (Fig. 8).
From x = 0.75, the potential is 4.15 V, an olivine-type phase appeared, the main diffraction peak (2θ ∼ 30.2°) corresponding to this new phase is shown in Fig. 6b. The coexistence of two phases in charge and discharge is established for the oxidation-reduction of Mn3+/Mn2+ in the area shown in red. The electrochemical reaction in (0.45 ≤ x < 0.75) can be written:
| Li0.75MnII0.75FeIII0.25PO4 ↔ βMnIII0.75FeIII0.25PO4 + (1 − β) Li0.75MnII0.75FeIII0.25PO4 + 0.25βLi+ + 0.25βe− (where 0 < β ≤ 1) |
Mössbauer spectra shown in Fig. 8 (where x = 0.60 and 0.45) correspond to the spectra of Fe3+ on the “plateau” of redox reaction Mn3+/Mn2+. Indeed, it was interesting to see the indirect effect of Mn3+ on the environment of Fe3+ as in previous phases with less manganese. The spectra cannot be refined with a single component in this area, we must add a second doublet for a correct refinement. Mössbauer parameters for the two Fe3+ components (Li0.75Mn0.75Fe0.25PO4 and Mn0.75Fe0.25PO4) are shown in Table 1. Component Mn0.75Fe0.25PO4 is characterized by a quadrupole splitting of 1.705 mm s−1.
| Composition | δ | Δ |
|---|---|---|
| mm s−1 | mm s−1 | |
| Li0.70MnII0.50FeII,III0.50PO4 | 1.32 | 2.72 |
| 0.43 | 1.02 | |
| Li0.50MnII0.50FeII,III0.50PO4 | 0.43 | 1.25 |
| MnIII0.50FeIII0.50PO4 | 0.43 | 1.64 |
| Li0.55MnII0.75FeII,III0.25PO4 | 1.28 | 2.71 |
| 0.44 | 1.08 | |
| Li0.25MnII0.75FeIII0.25PO4 | 0.41 | 1.30 |
| MnIII0.75FeIII0.25PO4 | 0.41 | 1.52 |
In conclusion, the electrochemical mechanism of phase LixMn0.75Fe0.25PO4/C differs from the three previously studied compositions. The first part corresponding to the domain (0.75 ≤ x < 0.95) is single-phase, the phase LiMn0.75Fe0.25PO4 evolves to the composition Li0.75Mn0.75Fe0.25PO4. The second area (0.45 ≤ x < 0.75) which is located at the “plateau” of the reaction Mn3+/Mn2+ is biphasic between the two compositions Li0.75Mn0.75Fe0.25PO4 and Mn0.75Fe0.25PO4 for the charge and discharge.
To summarize, all the mechanisms observed for these substituted phases LiMnyFe1−yPO4/C in charge are respectively represented in Fig. 9. Other compositions LiMnyFe1−yPO4/C (y = 0.40, 0.60) prepared under the same conditions have also been studied and can complete the graph of Fig. 9. The regions corresponding to different types of mechanism are shown as a function of lithium content:
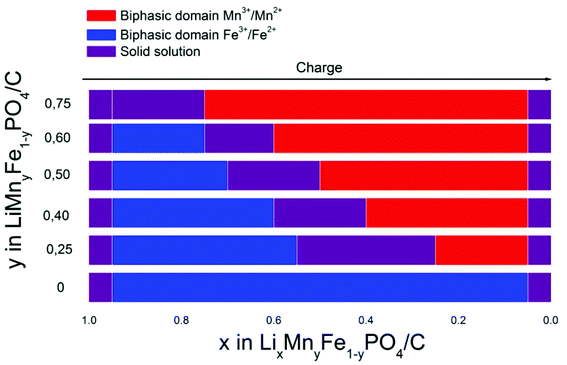 | ||
| Fig. 9 Schematic diagram of the different mechanisms observed during the first charge. | ||
-The beginning of the charge corresponding to the reaction Fe2+ → Fe3+ is biphasic for all compositions, except LiMn0.75Fe0.25PO4/C where the reaction is a single-phase.
-A domain of solid solution is also observed for all compositions, except LiMn0.75Fe0.25PO4/C between the two “plateaus” corresponding to the two redox reactions, this domain decreases with the manganese content.
-The end of the charge where the potential is around 4.2 V corresponding to the Mn3+/Mn2+ reaction is biphasic for all the compositions.
An inductive effect has already been established in some intercalation compounds polyanionic lithium,35 it can result in changes in potential of more than 1 V. In the case of compounds LiMnyFe1−yPO4/C, the ionicity or covalent bonds (Fe, Mn–O) are controlled by the size of the network (the length of these bonds). It was therefore interesting to compare the values of the isomer shifts of phases LiMnyFe1−yPO4/C with the values of potentials associated with the couple Fe3+/Fe2+ (Fig. 10). Indeed, there is a correlation between the inductive effect and value of the isomer shift as demonstrated by Menil.36 The representation of values of δ and potentials in Fig. 10 clearly shows a very similar tendency of the two parameters. This interesting result suggests that it is possible to predict the reaction potential from iron Mössbauer data as reported by Naille et al. for tin compounds.37
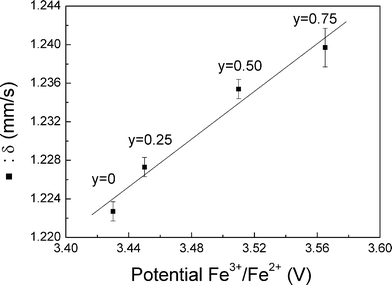 | ||
| Fig. 10 Evolution of the isomer shift vs. average potential. | ||
Finally we observed an excellent capacity retention over 100 cycles for all the studies compositions (see Add. Data). Although the substitution of iron with manganese does not improve the capacity (since it decreases with the manganese content), the possibility of tuning the working voltage may be useful for some applications as electric hybrid cars.
4. Conclusion
In this paper we report a comparative operando study for LiMnyFe1−yPO4/C for y = 0.50 and y = 0.75 combining XRD and 57Fe Mössbauer spectroscopy. The electrochemical mechanisms have been studied by monitoring the evolution of the local electronic environment (Mössbauer probe) and the lattice changes (XRD). The solid solution phase LiMn0.50Fe0.50PO4 exhibits a complex three steps mechanism during the charge. First, a biphasic reaction in the domain (0.70 ≤ x < 0.95) between LiMnII0.50FeII0.50PO4 and Li0.70MnII0.50FeII0.20FeIII0.30PO4, the Mössbauer probe helps to determine the composition of this mixed valence phase. The reaction is followed by a solid solution in the domain (0.50 ≤ x ≤ 0.70). The third step is described by a biphasic reaction between Li0.50MnII0.50FeIII0.50PO4 and MnIII0.50FeIII0.50PO4 until x ∼ 0.1. In conclusion, the electrochemical mechanism of phase LixMn0.75Fe0.25PO4/C differs from the three previously studied compositions. The first part corresponding to the domain (0.75 ≤ x < 0.95) is single-phase, the phase LiMn0.75Fe0.25PO4 evolves to the composition Li0.75Mn0.75Fe0.25PO4 followed by a two phase mechanism with LiMn0.25Fe0.75PO4 and LiMn0.50Fe0.50PO4. The second area (0.45 ≤ x < 0.75) which is located at the “plateau” of the reaction Mn3+/Mn2+ is biphasic between the two compositions Li0.75Mn0.75Fe0.25PO4 and Mn0.75Fe0.25PO4 for the charge and discharge. An interesting correlation has been established between the isomer shift and the average potential of the Fe3+/Fe2+ redox reaction, which can be used to predict the potential indirectly from Mössbauer data.Acknowledgements
The authors would like to thank SAFT Company and CNRS (contract SAFT/CNRS No. 029888) through the Ph.D. grant of Alexis Perea and ANR PHOSPHALION (National Research Agency) Stock-E program (ANR-09-STOCK-E-07) for financial support and Manfred Womes for fruitful discussions. Région Languedoc-Roussillon is gratefully acknowledged for the financial support to X-rays and gamma-rays platform (Contracts No. 2006 Q-086 and 2008 094192).References
- A. Perea, M. T. Sougrati, C. M. Ionica-Bousquet, B. Fraisse, C. Tessier, L. Aldon and J.-C. Jumas, Operando 57Fe Mossbauer and XRD investigation of LixMnyFe1-yPO4/C composites (y = 0; 0.25), RSC Adv., 2012, 2, 2080–2086 RSC.
- C. Delacourt, P. Poizot, M. Morcrette, J. M. Tarascon and C. Masquelier, One-step low-temperature route for the preparation of electrochemically active LiMnPO4 powders, Chem. Mater., 2004, 16(1), 93–99 CrossRef CAS.
- A. K. Padhi, K. S. Nanjundaswamy and J. B. Goodenough, Phospho-olivines as positive-electrode materials for rechargeable lithium batteries, J. Electrochem. Soc., 1997, 144(4), 1188–1194 CrossRef CAS.
- C. Delacourt, L. Laffont, R. Bouchet, C. Wurm, J. B. Leriche, M. Morcrette, J. M. Tarascon and C. Masquelier, Toward understanding of electrical limitations (electronic, ionic) in LiMPO4 (M = Fe, Mn) electrode materials, J. Electrochem. Soc., 2005, 152, A913–A921 CrossRef CAS.
- C. Delmas, M. Maccario, L. Croguennec, F. Le Cras and F. Weill, Lithium deintercalation in LiFePO4 nanoparticles via a domino-cascade model, Nat. Mater., 2008, 7(8), 665–671 CrossRef CAS.
- R. Dedryvere, M. Maccario, L. Croguennec, F. Le Cras, C. Delmas and D. Gonbeau, X-Ray Photoelectron Spectroscopy Investigations of Carbon-Coated LixFePO4 Materials, Chem. Mater., 2008, 20(22), 7164–7170 CrossRef CAS.
- C. Delacourt, J. Rodriguez-Carvajal, B. Schmitt, J. M. Tarascon and C. Masquelier, Crystal chemistry of the olivine-type LixFePO4 system (0 ≤ x ≤ 1) between 25 and 370 degrees C, Solid State Sci., 2005, 7(12), 1506–1516 CrossRef CAS.
- A. Yamada, H. Koizumi, N. Sonoyama and R. Kanno, Phase change in LixFePO4, Electrochem. Solid-State Lett., 2005, 8(8), A409–A413 CrossRef CAS.
- L. Aldon, A. Perea, M. Womes, C. M. Ionica-Bousquet and J. C. Jumas, Determination of the Lamb-Mossbauer factors of LiFePO4 and FePO4 for electrochemical in situ and operando measurements in Li-ion batteries, J. Solid State Chem., 2010, 183(1), 218–222 CrossRef CAS.
- F. Zhou, K. S. Kang, T. Maxisch, G. Ceder and D. Morgan, The electronic structure and band gap of LiFePO4 and LiMnPO4, Solid State Commun., 2004, 132((3–4)), 181–186 CrossRef CAS.
- G. H. Li, H. Azuma and M. Tohda, LiMnPO4 as the cathode for lithium batteries, Electrochem. Solid-State Lett., 2002, 5(6), A135–A137 CrossRef CAS.
- N. H. Kwon, T. Drezen, I. Exnar, I. Teerlinck, M. Isono and M. Graetzel, Enhanced electrochemical performance of mesoparticulate LiMnPO4 for lithium ion batteries, Electrochem. Solid-State Lett., 2006, 9(6), A277–A280 CrossRef CAS.
- C. Wang, S. Lu, S. Kan, J. Pang, W. Jin and X. Zhang, Enhanced capacity retention of Co and Li doubly doped LiMn2O4, J. Power Sources, 2009, 189(1), 607–610 CrossRef CAS.
- M. Yonemura, A. Yamada, Y. Takei, N. Sonoyama and R. Kanno, Comparative kinetic study of olivine LixMPO4 (M = Fe, Mn), J. Electrochem. Soc., 2004, 151(9), A1352–A1356 CrossRef CAS.
- A. Yamada, Y. Kudo and K. Y. Liu, Reaction mechanism of the olivine-type Lix(Mn0.6Fe0.4)PO4 (0 ≤ x ≤ 1), J. Electrochem. Soc., 2001, 148(7), A747–A754 CrossRef CAS.
- J. S. Yang and J. J. Xu, Synthesis and characterization of carbon-coated lithium transition metal phosphates LiMPO4 (M = Fe, Mn, Co, Ni) prepared via a nonaqueous sol–gel route, J. Electrochem. Soc., 2006, 153(4), A716–A723 CrossRef CAS.
- S. W. Kim, J. Kim, H. Gwon and K. Kang, Phase Stability Study of Li1-xMnPO4 (0 ≤ x ≤ 1) Cathode for Li Rechargeable Battery, J. Electrochem. Soc., 2009, 156(8), A635–A638 CrossRef CAS.
- A. Yamada, M. Hosoya, S. C. Chung, Y. Kudo, K. Hinokuma, K. Y. Liu and Y. Nishi, Olivine-type cathodes achievements and problems, J. Power Sources, 2003, 119, 232–238 CrossRef.
- Y. J. Shin, J. K. Kim, G. Cheruvally, J. H. Ahn and K. W. Kim, Li(Mn0.4Fe0.6)PO4 cathode active material: Synthesis and electrochemical performance evaluation, J. Phys. Chem. Solids, 2008, 69((5–6)), 1253–1256 CrossRef CAS.
- J. Xu, G. Chen, H. J. Li and Z. S. Lv, Direct-hydrothermal synthesis of LiFe1-xMnxPO4 cathode materials, J. Appl. Electrochem., 2010, 40(3), 575–580 CrossRef CAS.
- X. Y. Chang, Z. X. Wang, X. H. Li, L. Zhang, H. J. Guo and W. J. Peng, Synthesis and performance of LiMn0.7Fe0.3PO4 cathode material for lithium ion batteries, Mater. Res. Bull., 2005, 40(9), 1513–1520 CrossRef CAS.
- G. Kobayashi, A. Yamada, S. Nishimura, R. Kanno, Y. Kobayashi, S. Seki, Y. Ohno and H. Miyashiro, Shift of redox potential and kinetics in Lix(MnyFe1-y)PO4, J. Power Sources, 2009, 189(1), 397–401 CrossRef CAS.
- K. W. Nam, W. S. Yoon, K. Zaghib, K. Y. Chung and X. Q. Yang, The phase transition behaviors of Li1-xMn0.5Fe0.5PO4 during lithium extraction studied by in situ X-ray absorption and diffraction techniques, Electrochem. Commun., 2009, 11(10), 2023–2026 CrossRef CAS.
- T. Nedoseykina, M. G. Kim, S. A. Park, H. S. Kim, S. B. Kim, J. Cho and Y. Lee, In situ X-ray absorption spectroscopic study for the electrochemical delithiation of a cathode LiFe0.4Mn0.6PO4 material, Electrochim. Acta, 2010, 55(28), 8876–8882 CrossRef CAS.
- Y. C. Chen, J. M. Chen, C. H. Hsu, J. F. Lee, J. W. Yeh and H. C. Shih, In situ synchrotron X-ray absorption studies of LiMn0.25Fe0.75PO4 as a cathode material for lithium ion batteries, Solid State Ionics, 2009, 180((20–22)), 1215–1219 CrossRef CAS.
- L. Aldon and A. Perea, 2D-correlation analysis applied to in situ and operando Mossbauer spectroscopy, J. Power Sources, 196(3), 1342–1348 CrossRef CAS.
- J. B. Leriche, S. Hamelet, J. Shu, M. Morcrette, C. Masquelier, G. Ouvrard, M. Zerrouki, P. Soudan, S. Belin, E. Elkaim and F. Baudelet, An Electrochemical Cell for Operando Study of Lithium Batteries Using Synchrotron Radiation, J. Electrochem. Soc., 2010, 157(5), A606–A610 CrossRef CAS.
- J. Rodríguez-Carvajal, Recent advances in magnetic structure determination by neutron powder diffraction, Phys. B, 1993, 192((1–2)), 55–69 CrossRef.
- J. P. Eberhart, Méthodes physiques d′étude des minéraux et des matériaux solides, DOIN edn, p. 1976 Search PubMed.
- G. Kobayashi, A. Yamada, S. i. Nishimura, R. Kanno, Y. Kobayashi, S. Seki, Y. Ohno and H. Miyashiro, Shift of redox potential and kinetics in Lix(MnyFe1-y)PO4, J. Power Sources, 2009, 189(1), 397–401 CrossRef CAS.
- Z. G. Liu, D. W. Dong, M. L. Liu, Y. Sui, W. H. Su, Z. N. Qian and Z. Li, Quadrupole splitting distributions in purpurite and related minerals, Hyperfine Interact., 2005, 163((1–4)), 13–27 CAS.
- F. Fontan, P. Huvelin, M. Orliac and F. Permingeat, Ferrisicklerite of sidi bou othmane pegmatites (jebilet, morokko) and mineral group with a triphylite structure, Bulletin De La Societe Francaise Mineralogie Et De Cristallographie, 1976, 99(5), 274–286 CAS.
- Z. Liu, D. Dong, M. Liu, Y. Sui, W. Su, Z. Qian and Z. Li, Quadrupole Splitting Distributions in Purpurite and Related Minerals, Hyperfine Interact., 2005, 163(1), 13–27 CrossRef CAS.
- A. Alberti, The crystal structure of ferrisicklerite, Lix≤1(Fe3+, Mn2+)PO4, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1976, 32(OCT15), 2761–2764 CrossRef.
- A. K. Padhi, K. S. Nanjundaswamy, C. Masquelier, S. Okada and J. B. Goodenough, Effect of structure on the Fe3+/Fe2+ redox couple in iron phosphates, J. Electrochem. Soc., 1997, 144(5), 1609–1613 CrossRef CAS.
- F. Menil, Systematic trends of the 57Fe Mössbauer isomer shifts in (FeOn) and (FeFn) polyhedra. Evidence of a new correlation between the isomer shift and the inductive effect of the competing bond T-X (→Fe) (where X is O or F and T any element with a formal positive charge), J. Phys. Chem. Solids, 1985, 46(7), 763–789 CrossRef CAS.
- S. Naille, J. C. Jumas, P. E. Lippens and J. Olivier-Fourcade, 119Sn Mössbauer parameters as predictive tool for future Sn-based negative electrode materials, J. Power Sources, 2009, 189(1), 814–817 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20949g/ |
| This journal is © The Royal Society of Chemistry 2012 |
