Open-type capillary-assembled microchip for rapid, single-step, simultaneous multi-component analysis of serum sample†
Yusuke
Kimura
,
Terence G.
Henares
,
Shun-ichi
Funano
,
Tatsuro
Endo
and
Hideaki
Hisamoto
*
Graduate School of Engineering, Osaka Prefecture University, 1-1 Gakuen-cho, Naka-ku, Sakai City, Osaka 599-8531, Japan. E-mail: hisamoto@chem.osakafu-u.ac.jp; Fax: +81-72-254-9284; Tel: +81-72-254-9285
First published on 20th August 2012
Abstract
Here we present an open-type capillary-assembled microchip (CAs-CHIP), demonstrating its ease of fabrication, and use for rapid and simultaneous sensing of different serum components; viz., glucose, cholesterol, and alkaline phosphatase (ALP) and pH. Multiple square glass capillary sensors (outer diameter, 300 μm square) were embedded into a black polydimethylsiloxane (PDMS) microchannel array (300 μm width and depth) generating an open-type CAs-CHIP into which samples were introduced by capillary action. The open-type CAs-CHIP was then embedded into a PDMS reservoir chip where PDMS oil was dropped onto both ends of the capillary to avoid evaporation of the nanoliter sample inside the capillaries. We used fluorescein-based probes to achieve simultaneous multi-component sensing by using a single fluorescence filter and obtained accurate measurements with acceptable day-to-day precision (5.3–8.8%). Discrimination between control and analyte-spiked serum samples was simultaneously accomplished for 4 analytes within 10 min.
Introduction
Serum analysis is an important part of blood examination that is widely used for health diagnostics and infectious disease monitoring. As a component of blood, serum circulates to different organs of the body and contains different biomaterials that may reflect the state of health of a person. Current serum analysis requires the use of expensive reagents and cumbersome sample preparation, which may limit its implementation in clinics and small hospitals, thus, preventing early disease detection in small communities.1The development of a simple, easy-to-produce and inexpensive microdevice capable of rapid, single-step and simultaneous multi-component analysis is an important focus area in the field of analytical and bioanalytical science. Additionally, miniaturization of bioanalytical devices could shorten analysis time and minimize reagent and sample consumption.2 Such technology, if realized, would hold promise for serum analysis.
One of the important factors to consider in the fabrication of a multi-parametric biosensor is the immobilization of the chemical or biochemical material on a particular surface of the analytical device. The material that is immobilized determines the specificity, and typically involves enzymes,3–9 aptamers,10,11 antigens12 or antibodies.13–15 The oxidase enzyme is commonly used in multi-analyte sensing because of three important points (a) it uses a similar reagent to simultaneously detect various analytes; (b) it generates a sensitive electrochemical or optical response to H2O2, the reaction product; and (c) the use of glucose and cholesterol oxidase, in particular, in such analysis can provide significant data for diseases like diabetes and heart conditions, respectively. Many of the approaches reported for immobilizing oxidases are also amenable to miniaturization;3,6,8,9 however, these approaches require expensive and sophisticated methods for production, increasing the total cost of the device. Moreover, these immobilization techniques may only be applicable for metabolite sensing and may not be suitable for the detection of other biologically relevant biomolecules such as ions and proteins. Furthermore, simultaneous immobilization of various reagents on defined positions of a single microchip is challenging as it may require variation in the immobilization technique for each reagent, which may lead to cross-contamination among the different sensing materials (reagents) during the immobilization procedure.
Our group has introduced a microdevice for multi-analyte sensing called the capillary-assembled microchip (CAs-CHIP).16–18 This device involves integration of different independent sensing glass capillaries (outer diameter [OD], 300 μm square) embedded into a square PDMS microchannel (300 μm width and depth). We have developed various sensing capillaries for the sensitive detection of ions,17,18 metabolites,19 enzymes,20 and antigens21,22 by immobilizing different reagents. Therefore, this concept involves the possibility of universal integration of various different sensing functions, which has not been achieved in current DNA chip or protein chip technology. Once these sensing capillaries are placed into the PDMS microchannel, a PDMS pre-polymer which is spin-coated on an acrylic plate is used to cover the microchip for the final fabrication of the CAs-CHIP.16 Since the sensing capillaries are embedded independently, cross-talk or cross-contamination is completely avoided. Another useful aspect of the CAs-CHIP system is the “drop and sip” fluid-handling technique which uses a micropipette as a negative pressure pump to deliver a small volume of sample into the microchannel leading to an instantaneous sample introduction into each of the sensing capillaries via capillary action.17
Although, we achieved multiplexed sensing using the CAs-CHIP system,17,20 the final fabrication step, i.e., covering the microchip with the spin-coated PDMS pre-polymer, and fluid-handling nevertheless required an experienced researcher in order to achieve reproducible microdevice fabrication and operation, which, in turn, dictates the quality of the analytical results.
Here, we propose an open-type CAs-CHIP for the rapid simultaneous measurement of multiple analytes, viz., cholesterol, glucose, pH, and alkaline phosphatase. As with our original CAs-CHIP system, sample solutions are introduced via capillary action into the sensing capillaries embedding into the black PDMS channel array (Fig. 1A). However, the final covering with the PDMS pre-polymer spin-coated acrylic plate was avoided to achieve a more reproducible fabrication. In order to prevent evaporation of the sample solutions inside the capillary array, a PDMS reservoir (Fig. 1B) was independently prepared for simple addition of PDMS oil to both ends of the capillary array.
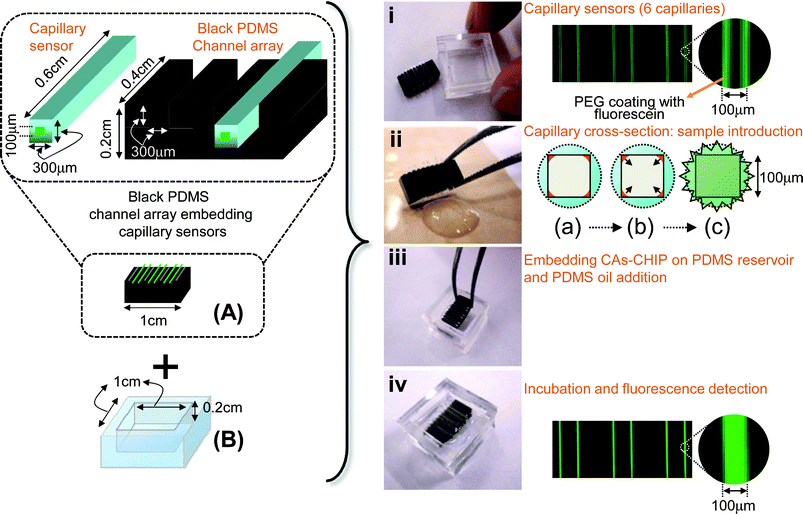 | ||
| Fig. 1 Basic concept of the open-type CAs-CHIP with the operational steps. Black PDMS channel array embedding capillary sensors (A). Sample introduction is achieved by capillary action (ii (a), (b)), then assembly with the reservoir (B) (iii) and PDMS oil is added to the reservoirs, then, finally measure the fluorescence intensity (iv, ii (c)). | ||
This open-type CAs-CHIP system is expected to be easily fabricated by the manufacturers and facilitate easy-handling for the users. The present concept is applicable for the development of multicomponent analysis devices for use in prognosis or diagnosis of various diseases. As a proof of concept, we demonstrate the fabrication of the device allowing four-component analysis, and application for the discrimination of the unusual concentration component in serum sample for the first screening use.
Experimental
Chemicals and reagents
The PDMS pre-polymer (SILPOT 184) and curing agent (SILPOT 184 CAT) were purchased from Dow Corning Toray Co. Ltd (Tokyo, Japan). Sodium hydroxide, BES–H2O2 fluorescent dye (cell impermeant), polyethylene glycol (PEG; Mw 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), D-(+)-glucose, and fluorescein diphosphate tetraammonium salt (FDP) were acquired from Wako Pure Chemical Industries, Ltd (Osaka, Japan). India ink (Bokuteki) was purchased from Kuretake Co., Ltd (Nara, Japan). Glucose oxidase (GOx) type X-S from Aspergillus niger, cholesterol oxidase (ChOx) from Streptomyces sp., cholesterol, Triton X-100 and alkaline phosphatase (ALP) were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA). Frozen control serum and fluorescein were procured from Nissui Pharmaceutical Co. Ltd (Tokyo, Japan) and Tokyo Kasei (Tokyo, Japan), respectively. All the reagents were used without further purification. The distilled and deionized water used had resistivity values of more than 1.7 × 107 Ω cm−1 at 25 °C.
000), D-(+)-glucose, and fluorescein diphosphate tetraammonium salt (FDP) were acquired from Wako Pure Chemical Industries, Ltd (Osaka, Japan). India ink (Bokuteki) was purchased from Kuretake Co., Ltd (Nara, Japan). Glucose oxidase (GOx) type X-S from Aspergillus niger, cholesterol oxidase (ChOx) from Streptomyces sp., cholesterol, Triton X-100 and alkaline phosphatase (ALP) were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA). Frozen control serum and fluorescein were procured from Nissui Pharmaceutical Co. Ltd (Tokyo, Japan) and Tokyo Kasei (Tokyo, Japan), respectively. All the reagents were used without further purification. The distilled and deionized water used had resistivity values of more than 1.7 × 107 Ω cm−1 at 25 °C.
Apparatus
Square capillaries with a 300 μm OD (flat to flat) and 100 μm inner diameters (flat to flat) were purchased from Polymicro (Phoenix, AZ, USA). The polyimide coating of these capillaries was removed by heating before use.PDMS substrates were prepared using a drying oven (DX300; Yamato Scientific Co. Ltd, Tokyo, Japan), planetary mixer (ARE250, Thinky Corporation, Tokyo, Japan), and vacuum pump (GLD-100; ULVAC, Inc., Kanagawa, Japan). Fluorescence was evaluated with a fluorescence microscope (Multi Viewer System VB-S20, Keyence Corporation, Osaka, Japan) and analyzed by Image J, a public-domain image processing program (version 1.43, National Institutes of Health, Bethesda, MD, USA).
Preparation of the capillary sensors
Using a 1 mL Hamilton syringe, all capillaries were first washed with a 1 M sodium hydroxide solution (30 min) and were then flushed with pure water and subsequently with acetone. Finally, the capillaries were vacuum-dried for 90 min prior to use.All four different capillary sensors were prepared using reagent cocktails containing 300 mg mL−1 of PEG and various probe reagents for sensing in Tris buffer (50 mM, pH 7.4 or 9.4). These reagents were introduced into the capillary. Then, the sensor coating on the four corners of the capillary was formed by introducing air, followed by a 3 h vacuum drying process. The amounts of probe material in the reagent cocktails introduced into each capillary were as follows: for pH sensing, 10−4 M fluorescein; for glucose sensing, 155 unit mL−1 GOx and 0.5% (v/v) BES–H2O2; for ALP sensing, 2.0 × 10−3 M FDP in Tris buffer (50 mM, pH 9.4); and for cholesterol sensing, 1 unit mL−1 ChOx and 0.5% (v/v) BES–H2O2.
Preparation of PDMS substrates
The design of the PDMS platform is shown in Fig. 1. The platform was fabricated by combining two PDMS structures: the “black” PDMS channel array for embedding square capillaries (Fig. 1A) and the PDMS reservoirs (Fig. 1B). The PDMS channel array was prepared using a glass mold having 300 μm × 300 μm channels with a 0.8 mm pitch. PDMS was conventionally molded using the glass mold; subsequently, the PDMS was peeled off from this mold and used as the next PDMS mold to transfer the channel array structure from the glass mold. The black PDMS channel array was prepared using the primary PDMS mold. PDMS was modified in bulk by adding 3 wt% India ink and 10 wt% curing agent into the PDMS pre-polymer mixture, followed by mixing with the planetary mixer. Any remaining air bubbles were removed under vacuum for 2 h, followed by thermal curing for 2 h at 70 °C to obtain the final black PDMS channel array with 2 mm thickness. For the PDMS reservoir, a mold comprising a 1 × 1 × 0.2 cm acrylic plate fixed to a 5 × 5 cm acrylic plate with adhesive was used. Conventional PDMS molding was implemented to attain the final PDMS reservoir structure.Fabrication of open-type CAs-CHIP and operational procedure
Fig. 1 illustrates the fabrication of an open-type CAs-CHIP system and its operational steps. The open-type CAs-CHIP system was prepared by embedding 0.6 cm long glass capillary sensors (OD, 300 μm square) into the black PDMS (1 × 0.4 × 0.2 cm) microchannel array (300 μm width and depth). Since the length (0.6 cm) of the embedded capillary sensors was greater than the width of the black PDMS (0.4 cm), sample introduction by capillary action was easily achieved by dipping one end of the capillary sensor array into the sample solution. Then, the open-type CAs-CHIP containing sample solution was embedded into the PDMS reservoir (1 × 1 × 0.2 cm). Approximately 120 μL of PDMS oil was dropped onto the reservoir, sealing both ends of the capillary sensor array. The sample was allowed to incubate for 10 min, after which the green fluorescence response was measured using a fluorescence microscope with 470 ± 20 nm and 535 ± 25 nm excitation and emission filters, respectively.Preparation of serum sample
Spiked serum samples were prepared by adding 100 μL of Tris-HCl buffer (5 mM, pH 7.4) containing 4 mg mL−1 glucose, 7 mg mL−1 cholesterol and 2% Triton X-100, or 4 unit mL−1 ALP to 400 μL of control serum (total volume: 500 μL). The serum sample spiked with buffer served as a control that had 0.8 mg mL−1 glucose, 1.0 mg mL−1 cholesterol, and 0.13 unit mL−1 ALP. The serum samples, spiked with glucose, cholesterol or ALP, had final total concentrations of 1.6 mg mL−1, 2.4 mg mL−1, and 0.93 unit mL−1, respectively, of these substances. These spiking concentrations were chosen according to the concentrations typically present in disease-related serum samples.Results and discussion
Characterization of various capillary sensors
All square glass capillary sensors were designed such that their enzymatic reaction or protolytic products would yield a green fluorescence response. This strategy allows the use of a single fluorescence filter to simultaneously assess the cholesterol, glucose, and alkaline phosphatase levels and pH of a serum sample.The mechanism of detection for each capillary sensor is depicted in Fig. 2. For the glucose and cholesterol capillary sensors, GOx or ChOx was immobilized together with a fluorescent probe, BES–H2O2, inside the four corners of the square glass capillary with the aid of polyethylene glycol which acts as a scaffold. BES–H2O2 reacts with H2O2 with high specificity, in a non-oxidative manner, and does not require peroxidase enzyme.23 Samples containing analytes, like glucose or cholesterol, enter the capillary sensor via capillary action. These analytes then react with the oxidase enzyme released from the capillary, generating H2O2 as an enzyme reaction product. This peroxide then reacts with BES–H2O2 to liberate the difluorofluorescein dye, yielding a green fluorescence response. As a representative result, the analytical performance of a cholesterol-sensing capillary is shown in Fig. 3 (for other capillary sensors, please see the ESI†). In each case, sample solutions were introduced into independent capillary sensors and fluorescence intensities were recorded by fluorescence microscopy. The enzyme reaction time profile showed that rapid analysis could be achieved (within 8 min; Fig. 3A). This figure also shows that the initial fluorescence of the capillary sensor almost reached the saturation point. This implies fast H2O2 production through the ChOx–cholesterol reaction, which immediately reacts with the BES–H2O2. The fluorescence response should be a function of the cholesterol concentration, which dictates the rate of reaction; as expected, we observed that the fluorescence increased with cholesterol concentration (Fig. 3B). For the ALP-sensing capillary, the FDP is immobilized inside the capillary. The ALP cleaves the phosphate moiety of FDP and then liberates the fluorescein molecules. Moreover, the pH-sensing capillary contains a fluorescein molecule, which is a well-known pH-responsive fluorescent dye.24 Under acidic conditions, fluorescein is protonated, resulting in a decrease in fluorescence. In contrast, the anionic form of fluorescein yields a more intense green fluorescence at basic pH.
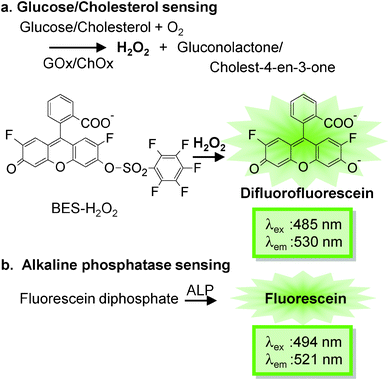 | ||
| Fig. 2 Enzymatic reactions in various sensing capillaries containing different enzymes like (a) glucose oxidase (GOx), cholesterol oxidase (ChOx), and (b) alkaline phosphatase (ALP). | ||
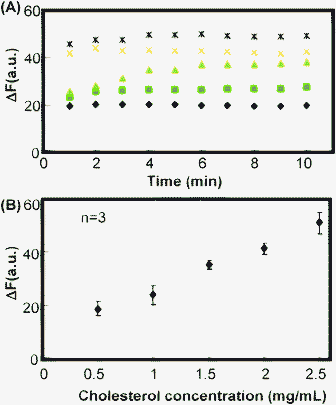 | ||
Fig. 3 Representative result of the bioanalytical performance of a capillary sensor. (A) Enzyme reaction time profile of the capillary cholesterol sensor with various cholesterol concentrations (mg mL−1):  0.5, 0.5,  1.0, 1.0,  1.5, 1.5,  2.0 and 2.0 and  2.5. (B) Calibration curve derived after 10 min of enzyme reaction. 2.5. (B) Calibration curve derived after 10 min of enzyme reaction. | ||
Table 1 shows some of the basic properties of the capillary sensors developed in this study. Rapid determination of four analytes could be achieved within 10 min. All the analyte concentrations in the serum were within the response range. Furthermore, the capillary sensors displayed good day-to-day reproducibility, with coefficients of variation ranging from 5.3% to 8.8%. It is therefore expected that these multiple capillary sensors, once integrated into the chip, will result in rapid, precise, and accurate measurement of analytes.
Open-type CAs-CHIP for serum analysis of various analytes
The open-type CAs-CHIP is composed of the black PDMS microchannel array embedding capillary sensors, and PDMS reservoir (Fig. 1). The use of a black PDMS has been previously reported by our group for reducing background interference.25 In this study, bare capillaries were embedded in the black PDMS or native PDMS microchannel, and the sample (10−7 and 10−6 M fluorescein solutions) was then introduced via capillary action. When comparing the fluorescence intensities of the capillaries on the native and the black PDMS substrates, the black PDMS showed relatively higher sensitivity, with approximately 1.3 times more fluorescence (Fig. 4). This is consistent with the results we obtained previously,25 where the black PDMS absorbed the scattered light. Moreover, successful sealing of both ends of the capillary sensors was achieved using the PDMS oil. Fig. 5 shows that fluorescence intensity was maintained and that there was no visible leakage for up to 90 min. This result implies that reagent diffusion from the capillary sensor to the next capillary sensor via PDMS oil was minimal over a period of 1.5 h. Therefore, this device can be used even in approaches involving a long incubation time, avoiding concerns over evaporation and solution leakage.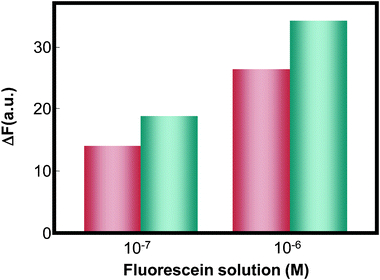 | ||
Fig. 4 Comparison of the fluorescence response of the glass capillary containing fluorescein solution, embedded on the native PDMS ( ) and black PDMS substrates ( ) and black PDMS substrates (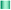 ). ). | ||
 | ||
| Fig. 5 Effectiveness of PDMS oil sealing of the capillary over different time periods. | ||
Finally, simultaneous determination of important analytes, such as glucose, cholesterol and ALP, and pH in the serum sample was demonstrated using this simple microchip. Elevated serum levels of glucose, cholesterol, or ALP are good indicators of various diseases, such as diabetes, cardiovascular conditions, and cancer,26 respectively. Fig. 6 shows the fluorescence intensity of spiked serum samples relative to that of control serum samples. The former values were larger than those of control as expected. However, changes in relative fluorescence intensity were smaller than the expected values based on spiked concentrations and calibration curves shown in Fig. 3 and the ESI.† This may be caused by the interference of serum components, thus further improvement related with quantitation such as standard addition is necessary. However, the single-step nature of the multiplex analysis contributes to the practical potential of this microdevice for the first screening of biomedical application, since the unusual concentration component was discriminated as shown in Fig. 6.
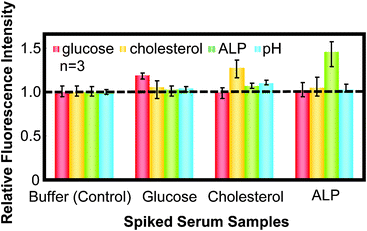 | ||
| Fig. 6 Comparison of the detection of various analytes in spiked serum, relative to the control serum using the open-type CAs-CHIP. | ||
Conclusions
Here, we demonstrated the fabrication of a simple microdevice composed of a black PDMS channel array embedding capillary sensors and a PDMS reservoir, for the rapid simultaneous multiplex measurement of glucose, cholesterol, ALP, and pH. Quantitative measurement of each analyte can be achieved using individual capillary sensors. All the developed sensing capillaries yielded the same green fluorescence response upon reaction with an analyte permitting the use of a single fluorescence filter. Thus, simultaneous multicomponent measurement was achieved. Moreover, the use of a black PDMS channel array improved the relative sensitivity of the sensors. Although further improvement is necessary, this open-type CAs-CHIP could discriminate between normal and high serum levels of various analytes with a simple, single-step operation. Thus, this bioanalytical tool is expected to be applied in assessing disease prognosis or diagnosis.Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Funding Program for Next Generation World-Leading Researchers from the Government of Japan.References
- P. B. Luppa, C. Muller, A. Schlichtiger and H. Schlebusch, TrAC, Trends Anal. Chem., 2011, 30, 887–898 CrossRef CAS.
- D. R. Reyers, D. Iossifidis, P. A. Auroux and A. Manz, Anal. Chem., 2002, 74, 2623–2636 CrossRef.
- J. Yuan, V. A. Pedrosa, A. L. Simonian and A. Revzin, ACS Appl. Mater. Interfaces, 2010, 2, 748–755 Search PubMed.
- Y. Cai, R. Shinar, Z. Zhou and J. Shinar, Sens. Actuators, B, 2008, 134, 727–735 CrossRef.
- B. P. Corgier, C. A. Marquette and L. J. Blum, Anal. Chim. Acta, 2005, 538, 1–7 CrossRef CAS.
- M. Yang, Y. Yang, Y. Yang, G. Shen and R. Yu, Anal. Chim. Acta, 2005, 530, 205–211 CrossRef CAS.
- C. Boero, S. Carrara, G. Del Vecchio, L. Calza and G. De Micheli, Sens. Actuators, B, 2011, 157, 216–224 CrossRef CAS.
- I. Moser, G. Jobst and G. A. Urban, Biosens. Bioelectron., 2002, 17, 297–302 CrossRef CAS.
- C. A. Marquette, A. Degiuli and L. J. Blum, Biosens. Bioelectron., 2003, 19, 433–439 CrossRef CAS.
- J. Liu, J. H. Lee and Y. Lu, Anal. Chem., 2007, 79, 4120–4125 CrossRef CAS.
- H. Zhang, B. Jiang, Y. Xiang, Y. Zhang, Y. Chai and R. Yuan, Anal. Chim. Acta, 2011, 688, 99–103 CrossRef CAS.
- J. Adrian, S. Pasche, D. G. Pinacho, H. Font, J.-M. Diserens, F. Sachez-Baeza, B. Granier, G. Voirin and M.-P. Marco, TrAC, Trends Anal. Chem., 2009, 28, 769–777 CrossRef CAS.
- D. Bhatta, A. A. Michel, M. M. Villalba, G. D. Emmerson, I. J. G. Sparrow and E. A. Perkins, Biosens. Bioelectron., 2011, 30, 78–86 CrossRef CAS.
- G. Marusov, A. Swett, K. Pietrosimone, D. Benson, S. J. Geary, L. K. Silbart, S. Challa, J. Lagoy, D.A. Lawrence and M. A. Lynes, Environ. Sci. Technol., 2012, 46, 348–359 CrossRef CAS.
- D. Bhatta, E. Stadden, E. Hashem, I. J. G. Sparrow and G. D. Emmerson, Sens. Actuators, B, 2010, 149, 233–238 CrossRef.
- H. Hisamoto, Y. Nakashima, C. Kitamura, S.-i. Funano, M. Yasuoka, K. Morishima, Y. Kikutani, T. Kitamori and S. Terabe, Anal. Chem., 2004, 76, 3222–3228 CrossRef CAS.
- T. G. Henares, M. Takaishi, N. Yoshida, S. Terabe, F. Mizutani, R. Sekizawa and H. Hisamoto, Anal. Chem., 2007, 79, 908–915 CrossRef CAS.
- H. Hisamoto, M. Yasuoka and S. Terabe, Anal. Chim. Acta, 2006, 556, 164–170 CrossRef CAS.
- T. G. Henares, E. Maekawa, F. Okubo, F. Mizutani, T. Yao, R. Sekizawa and H. Hisamoto, Anal. Sci., 2009, 25, 1025–1028 CrossRef CAS.
- T. G. Henares, F. Mizutani, R. Sekizawa and H. Hisamoto, Anal. Bioanal. Chem., 2008, 391, 2507–2512 CrossRef CAS.
- T. G. Henares, E. Tsutsumi, H. Yoshimura, K. Kawamura, T. Yao and H. Hisamoto, Sens. Actuators, B, 2010, 149, 319–324 CrossRef.
- E. Tsutsumi, T. G. Henares, H. Yoshimura, S.-i. Funano, K. Kawamura, T. Endo and H. Hisamoto, Anal. Sci., 2012, 28, 51–56 CrossRef CAS.
- H. Maeda, Y. Fukuyasu, S. Yoshida, M. Fukuda, K. Saeki, H. Matsuno, Y. Yamauchi, K. Yoshida, K. Hirata and K. Miyamoto, Angew. Chem., Int. Ed., 2004, 43, 2389–2391 CrossRef CAS.
- M. M. Martin and L. Lindqvist, J. Lumin., 1975, 10, 381–390 CrossRef CAS.
- Y. Fujii, T. G. Henares, K. Kawamura, T. Endo and H. Hisamoto, Lab Chip, 2012, 12, 1522–1526 RSC.
- J. Han, B. Yong, C. Luo, P. Tan, T. Peng and J. Shen, World J. Surg. Oncol., 2012, 10, 1–10 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c2ra21843g |
| This journal is © The Royal Society of Chemistry 2012 |
