A versatile resolving agent for diffusion edited separation of enantiomers, complex mixtures and constitutional isomers†
Sachin Rama
Chaudhari
ab,
Srinivasa
a and
N.
Suryaprakash
ab
aNMR Research Center, Indian Institute of Science, Bangalore, India. E-mail: nsp@sif.iisc.ernet.in; Fax: ++91 80 23601550; Tel: ++91 80 22933300
bSolid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore, India
First published on 13th July 2012
Abstract
The study is the first report of the utilization of a crown ether as a new and versatile resolving agent for the diffusion edited separation of enantiomers, complex mixtures and constitutional isomers. As a consequence of different binding affinities of enantiomers of a chiral molecule and individual components of the complex mixtures with the crown ether, the molecules diffuse at different rates. The enhanced separation achieved due to matrix assisted diffusion permitted their separation in the diffusion dimension. The generality and wide utility of the new resolving agent and the methodology are demonstrated on diverse examples, such as an organic chiral molecule, constitutional isomers and complex mixture of molecules possessing different functional groups that possess nearly identical molecular weights.
Diffusion Ordered Spectroscopy (DOSY) has gained enormous importance in diverse fields, such as supramolecular, pharmaceutical and combinatorial chemistry, that deal with complex mixtures.1 DOSY methodology has been extensively employed in the identification of metabolites, characterization of aggregates, studies of hydrogen bonding, host–guest complexes, ion pairing in organometallic chemistry, supramolecular assemblies, etc..2 The pseudo 2D DOSY spectrum is constructed by displaying chemical shifts in the direct dimension and the measured diffusion coefficients in a direction orthogonal to it.3 The separation of the NMR spectrum of each molecule from a complex mixture is an urgent necessity for their spectral assignment and subsequent determination of structures. The high resolution (HR)-DOSY experiment permits such a separation, without any basic need for physical separation of individual components. But the essential requirements of DOSY are: the diffusion coefficients of the individual components of the mixture have to be substantially different and their NMR spectra must be well resolved.4 To minimize the problems encountered due to severe overlap of signals several data processing techniques, such as, multi-exponential fitting5 and multivariate methods6 have been reported. Nevertheless the best alternative would be to devise new and convenient experimental methodologies to circumvent such problems. One of the powerful ways of reducing the signal overlap is to invoke spectral dispersion by the incorporation of another dimension.7 The 3D technique, however, demands the investment of more instrument time. The 2D DOSY pure shift method, on the other hand, significantly improves the resolution by collapsing the multiplet pattern to a singlet, but at the cost of sensitivity.8 The severity of the problem arises when one is dealing with mixtures of chemical species that diffuse at the same rates, possess the same hydrodynamic radii and identical molecular weights, which the high-resolution DOSY fails to separate. Nevertheless there are studies to combat this problem by employing solid chromatographic media such as silica gel, where the separation is enhanced.9,10 The drawback of using such a solid medium is broadening of the signal and demand for a spectrometer with solid state capabilities. There are other studies wherein the introduction of a co-solute or co-solvent such as microemulsion,11 polymers,12 cyclodextrin13 and lanthanide agents,14 which interact with the components of the mixture with differential extent, giving increased separation. Micelles15 and cyclodextrin16 are the only co-solutes described to separate the signals of positional isomers and for chiral discrimination respectively. There is also a recent report on the separation of double bonded geometrical isomers using micelles and reverse micelles.17 In spite of enormous progress the challenge of resolving isomers continues to persist and there is a growing need for the development of convenient and efficient resolving agents for application in synthetic and combinatorial chemistry. The focus of the present study is to introduce a simple resolving agent for multipurpose applications, such as, enantiomer discrimination, resolution of constitutional isomers and to unscramble the complex mixtures, by enhancing the resolution in the diffusion dimension, which are otherwise impossible to achieve by high resolution DOSY. We are thus proposing the utilization of crown ether as a new resolving agent and have demonstrated it as a versatile co-solute. The utility of crown ether for the determination of association constants using diffusion coefficients has been demonstrated earlier.18
Crown ethers such as, diesters crown ethers, thiocrown ethers, azacrown ethers, and chiral crown ethers19,20 are well established for enantio-discrimination21 and have been applied in chromatography.22 They have also been exploited as a mobile phase or crown ether bonded silica column because of their special properties, such as, binding selectivity and strength towards a wide range of metal ions, non-metal ions,23 and neutral molecules.24 The mobility of the molecules gets substantially altered because of their differential binding affinities. This property can be exploited to derive benefit for the separation of the components of mixture and to amplify the resolution in the diffusion dimension of DOSY.
For investigation purposes we have chosen the prepared mixtures of positional isomers of ortho-, meta- and para- chloroaniline (please see the ESI).† It may be mentioned that the aromatic protons of these molecules resonate over a narrow chemical shift range and their 1H NMR spectra are severely overlapped rendering it difficult to identify peaks to individual species. The proposed crown ether has therefore been employed as a new resolving agent. The 1H detected DOSY spectra of the solution containing the mixture of isomers of chloroaniline, without and with crown ether, are reported in Fig. 1a and 1b respectively.
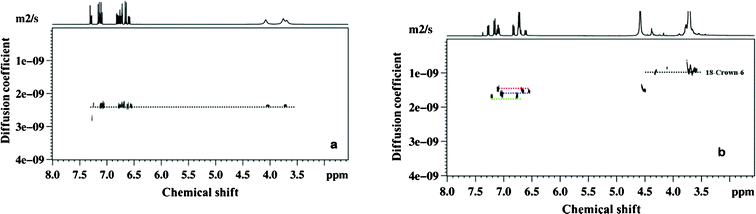 | ||
| Fig. 1 400 MHz 1H DOSY spectra of a mixture of ortho-, meta- and para- chloroaniline, a) sample 1 and b) sample 2, obtained at 298 K. Red, blue and green dotted lines in Fig. 1b represent para-, meta- and ortho- chloroaniline respectively. | ||
It is clearly evident from Fig. 1a that isomers are unresolved consequent to their identical diffusion rates, implying that they are indistinguishable under standard DOSY conditions. The introduction of crown ether, on the other hand, resulted in a remarkable difference in their diffusion rates due to different association of the molecules with crown ether enabling their separation in the diffusion dimension and permitting the unambiguous assignment of the spectra. This is clearly evident from Fig. 1b. The small changes in the chemical shifts for each isomer were also observed due to host–guest interaction. The variation of mobility sequence detected with the addition of crown ether was p-<m-<o- chloroaniline, indicating that interaction of p-chloroaniline is the strongest and that of o-chloroaniline is the weakest. These results are in agreement with those published using chromatographic methods.25 As far as the mechanisms of interactions are concerned, there are two possible types, viz. hydrogen bonding and ion–dipolar interaction.26 In the case of o-chloro aniline the steric interaction with 18-crown-6 is hindered by chlorine group causing the binding strength to become weaker and also the suppression of complex formation due to hydrophobicity. On the other hand in the case of m-chloroaniline the interactions are intermediate and in p-chloroaniline the steric interaction is a remote possibility.
For demonstrating the wide utility of this resolving agent another application involving the resolution of chemical species that possess nearly identical molecular weights was explored. The mixture of 2-trifluoromethylaniline and 2-trifluoromethylphenol which have nearly identical molecular weights, whose 1H spectrum exhibit severe overlap, was therefore chosen as the test molecules. The DOSY spectra of the solution containing these mixtures are reported in Fig. 2a and 2b without and with crown ether respectively. In Fig. 2a the spectrum without crown ether shows a huge overlap of peaks, which is impossible to differentiate because of their nearly identical rates of diffusion. The DOSY spectrum with the addition of crown ether, on the other hand, displays an excellent resolution in the diffusion dimension and also resulted in the dispersion of the chemical shifts in the detection dimension. The diffusion rate is found to be very slow for 2-trifluoromethylaniline compared to 2-trifluoromethylphenol. The very slow diffusion for 2-trifluoromethylaniline is due to the stronger binding of amine group with crown ether compared to 2-trifluoromethylphenol. Thus crown ether molecules appear to interact specifically with basic sites providing selectivity. From the mobility pattern, it is obvious that 2-trifluoromethylaniline forms stronger host guest complex than that of 2-trifluoromethylphenol. The 19F DOSY spectrum of this mixture with crown ether also gave similar results (please see supporting information).
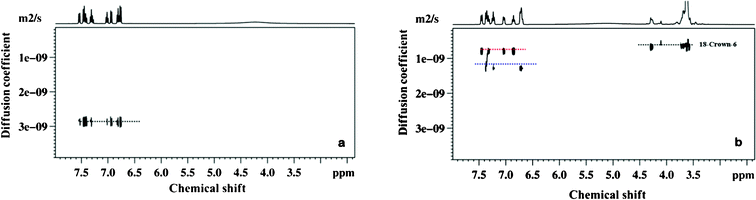 | ||
| Fig. 2 400 MHz 1H DOSY spectra of a mixture of 2-trifluoromethylaniline and 2-trifluoromethylphenol; a) sample 3 and b) sample 4 obtained at 298 K. Red and blue dotted lines represent 2-trifluoromethylaniline, 2-trifluoromethylphenol respectively. | ||
Another exciting application of using this resolving agent is the enantiomer discrimination of chiral molecules in the diffusion dimension. For demonstration purposes we have chosen chiral crown ether 18-C-6-TCA as the shift reagent and 2-methylpiperidine as a chiral solute. The 1H DOSY spectra of 2-methylpiperidine without and with chiral crown ether are given in Fig. 3. In the DOSY spectrum without crown-ether (Fig. 3a) the peaks belonging to both the enantiomers of 2-methylpiperidine are indistinguishably overlapped. On addition of chiral crown ether, the discrimination of R and S isomers is achieved both in the detection and the diffusion dimensions (Fig. 3b). The peaks pertaining to two diastereomers are indicated by red and blue dashed lines.
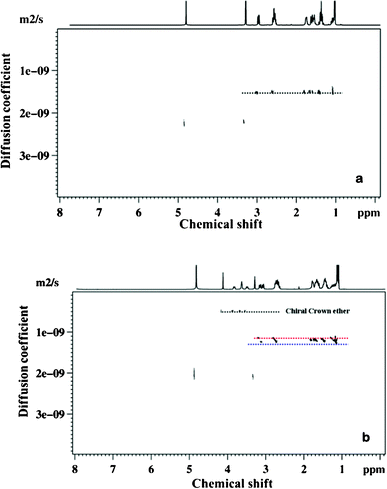 | ||
| Fig. 3 400 MHz 1H DOSY spectrum of 2-methylpiperidine, sample 5 and sample 6 obtained at 298 K. Black line pertains to the crown ether, red and blue lines pertain to R/S enantiomers. | ||
There are various advantages of utilization of crown ether as a resolving agent over other existing resolving agents, such as; i) there is no line broadening unlike in the case of silica and polymers,6,8 ii) the crown ether is soluble in a wide variety of solvents as compared to cyclodextrin and micelles, iii) due to very few peaks for crown ether there will not be much of overlap of the signals from the co-solute and thus the utilization of deuterated or fluorinated resolving agents as in the case of micelles and reverse micelles11 can be avoided, and iv) the crown ether interacts with broad functionality and can be utilised in varieties of applications. We believe this study might be extended for peptides, natural products and structural conformers which are usually not possible to resolve by high resolution DOSY techniques.
In conclusion, we have demonstrated the application of crown ether assisted DOSY for the unambiguous discrimination of enantiomers, enhancing the separation of constitutional isomers and complex mixtures by imposing difference in their rates of diffusion. The addition of crown ether improves the selectivity for structural isomers and permitted their separation. The resolving agent also permitted the unravelling of the spectrum corresponding to individual enantiomers and provided dispersion in the chemical shifts. The influence of crown ether is convincingly demonstrated on the mixture of isomers of chloroaniline, which yielded good resolution in the diffusion dimension consequent to differential diffusion rates imposed on them. The results of the present study provide conclusive evidence for the flexibility and robustness of matrix assisted DOSY methodology in the analysis of combinatorial mixtures using crown ether as a co-solute. The crown ether interacts with broad functionality and may have potential applications in the study of varieties of organic compounds.
Acknowledgements
NS gratefully acknowledges the financial aid for this work by the Science and Engineering Research Board, New Delhi (grant No. SR/S1/PC-42/2011) and SRC thanks UGC, India, for Senior Research Fellowship.References
-
(a) C. S. Johnson Jr, Prog. Nucl. Magn. Reson. Spectrosc., 1999, 34, 203 CrossRef
; (b) G. A. Morris in Encyclopedia of Nuclear Magnetic Resonance, ed. D. M. Grant and R. K. Harris, John Wiley & Sons Ltd., Chichester, 2002, Vol. 9, Advances in NMR, pp.35 Search PubMed
; (c) B. Antalek, Concepts Magn. Reson., 2002, 14, 225 CrossRef CAS
.
-
(a) Y. Cohen, L. Avram and L. Frish, Angew. Chem., Int. Ed., 2005, 44, 520 CrossRef CAS
and references therein.; (b) R. Novoa-Carballal, E. Fernandez-Megia, C. Jimenez and R. Riguera, Nat. Prod. Rep., 2011, 28, 78 RSC
; (c) S. Caldarelli, Magn. Reson. Chem., 2007, 45, S48 CrossRef CAS
; (d) J. Stchedroff, A. M. Keenwright, G. A. Morris, M. Nilsson and R. K. Harris, Phys. Chem. Chem. Phys., 2004, 6, 3221 RSC
; (e) W. S. Pricein Encyclopedia of Nuclear Magnetic Resonance, ed. D.M.Grant and R. K. Harris, John Wiley & Sons Ltd., Chichester, 2002, vol. 9: Advances in NMR, pp. 364 Search PubMed
.
- K. F. Morris and C. S. Johnson Jr., J. Am. Chem. Soc., 1992, 114, 3139 CrossRef CAS
.
- H. Barjat, G. A. Morris, S. Smart, A. G. Swanson and S. C. R. Williams, J. Magn. Reson., Ser. B, 1995, 108, 170 CrossRef CAS
.
- M. Nilsson, M. A. Connell, A. L. Davis and G. A. Morris, Anal. Chem., 2006, 78, 3040 CrossRef CAS
.
-
(a) M. Nilsson and G. A. Morris, Anal. Chem., 2008, 80, 3777 CrossRef CAS
; (b) P. Stilbs and K. Paulsen, Rev. Sci. Instrum., 1996, 67, 4380 CrossRef CAS
; (c) W. Windig, J. P. Hornak and B. Antalek, J. Magn. Reson., 1998, 132, 298 CrossRef CAS
; (d) M. Nilsson, J. Magn. Reson., 2009, 200, 296 CrossRef CAS
.
-
(a) D. Wu, A. Chen and C. S. Johnson, J. Magn. Reson., Ser. A, 1996, 121, 88 CrossRef CAS
; (b) M. Nilsson, A. M. Gil, I. Delagadillo and G. A. Morris, Chem. Commun., 2005, 1737 RSC
; (c) S. Viel and S. Caldarelli, Chem. Commun., 2008, 2013 RSC
.
-
(a) M. Nilsson and G. A. Morris, Chem. Commun., 2007, 93 Search PubMed
; (b) J. A. Aguilar, S. Faulkner, M. Nilsson and G. A. Morris, Angew. Chem., Int. Ed., 2010, 49, 3901 CrossRef CAS
.
- S. Viel, F. Ziarelli and S. Caldarelli, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 9696 CrossRef CAS
.
- G. Pages, C. Delaurent and S. Caldarelli, Angew. Chem., Int. Ed., 2006, 45, 5950 CrossRef CAS
.
- C. Pemberton, R. E. Hoffman and A. Aserin, Langmuir, 2011, 27, 4497 CrossRef CAS
.
-
(a) J. S. Kavakka, I. Kilpeläinen and S. Heikkinen, Org. Lett., 2009, 6, 1349 CrossRef
; (b) J. S. Kavakka, V. Parviainen, K. Wahala, I. Kilpeläinen and S. Heikkinen, Magn. Reson. Chem., 2010, 48, 777 CrossRef CAS
.
-
(a) J. Xu, T. Tan, L. Kenne and C. Sandstrom, New J. Chem., 2009, 33, 1057 RSC
; (b) M. Lin, D. A. Jayawickrama, R. A. Rose, J. A. DelViscio and C. K. Larive, Anal. Chim. Acta, 1995, 307, 449 CrossRef CAS
.
- A. K. Rogerson, J. A. Aguilar, M. Nilsson and G. A. Morris, Chem. Commun., 2011, 47, 7063 RSC
.
-
(a) R. Evans, S. Haiber, M. Nilsson and G. A. Morris, Anal. Chem., 2009, 81, 4548 CrossRef CAS
; (b) C. F. Tormena, R. Evans, S. Haiber, M. Nilsson and G. A. Morris, Magn. Reson. Chem., 2010, 48, 550 CrossRef CAS
.
- R. W. Adams, J. A. Aguilar, J. Cassani, G. A. Morris and M. Nilsson, Org. Biomol. Chem., 2011, 9, 7062 CAS
.
- S. R. Chaudhari and N. Suryaprakash, J. Mol. Struct., 2012, 1017, 106 CrossRef CAS
.
- O. Mayzel and Y. Cohen, J. Chem. Soc., Chem. Commun., 1994, 1901 RSC
.
- J. S. Bradshaw, R. M. Izatt, A. V. Bordunov, C. Y. Zhu and J. K. Hathaway, Crown Ethers; Pergamon, 1997, 1, 35 Search PubMed
.
-
G. W. Gokel, Crown Ethers and Cryptands; The Royal Society of Chemistry :Cambridge, 1991 Search PubMed
.
-
(a)
T. J. Wenzel, Discrimination of Chiral Compounds Using NMR Spectroscopy, Wiley, Hoboken, 2007 Search PubMed
; (b) T. J. Wenzel and C. D. Chisholm, Prog. Nucl. Magn. Reson. Spectrosc., 2011, 59, 1 CrossRef CAS
.
-
(a) T. Nakagawa, A. Shibukawa, A. Kaihara, T. Itamcochi and H. Tanaka, J. Chromatogr., A, 1986, 353, 399 CrossRef CAS
; (b) T. Nakagawa, A. Shibukawa and T. Uno, J. Chromatogr., A, 1982, 239, 695 CrossRef CAS
; (c) T. Nakagawa and A. Shibukawa, J. Chromatogr., A, 1983, 254, 27 CrossRef CAS
; (d) M. Mori, H. Tsue and S. Tanaka, J. Chromatogr., A, 2001, 922, 399 CrossRef CAS
.
- J. S. Bradshaw and R. M. Izatt, Acc. Chem. Res., 1997, 30, 338 CrossRef CAS
.
-
(a) C. J. van Staveren, M. L. Veronika, J. Aarts, P. D. J. Grootenhuis, J. van Eerden, S. Harkema and D. N. Reinhoudt, J. Am. Chem. Soc., 1986, 108, 5271 CrossRef CAS
; (b) A. Elbasyouny, H. J. Brugge, K. von Deuten, M. Dickel, A. Knochel, K. U. Koch, J. Kopf, D. Melzer and G. Rudolph, J. Am. Chem. Soc., 1983, 105, 6568 CrossRef CAS
.
-
(a) A. Shibukawa, T. Nakagawa, A. Kaihara, K. Yagi and H. Tanaka, Anal. Chem., 1987, 59, 2496 CrossRef CAS
; (b) Xi-C. Zhou, C. Y. Wu, X. R. Lu and Y. Y. Chen, J. Chromatogr., A, 1994, 662, 203 CrossRef CAS
.
- D. J. Cram and J. M. Lehn, Science, 1974, 183, 803 CAS
.
Footnote |
| † Electronic Supplementary Information (ESI) available: experimental details, sample preparation and 19F DOSY spectra. See DOI: 10.1039/c2ra20982a/ |
| This journal is © The Royal Society of Chemistry 2012 |
