General synthetic strategy for high-yield and uniform rare-earth oxysulfate (RE2O2SO4, RE = La, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Y, Ho, and Yb) hollow spheres†
Xiaohe
Liu
*a,
Dan
Zhang
a,
Jinghui
Jiang
a,
Ning
Zhang
a,
Renzhi
Ma
*b,
Haibo
Zeng
b,
Baoping
Jia
b,
Shoubao
Zhang
b and
Guanzhou
Qiu
a
aDepartment of Inorganic Materials, Central South University, Changsha, Hunan 410083, China. E-mail: liuxh@csu.edu.cn; Fax: +86-731-88879815; Tel: +86-731-88830543
bInternational Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science, Namiki 1-1, Tsukuba, Ibaraki 305-0044, Japan. E-mail: MA.Renzhi@nims.go.jp
First published on 9th August 2012
Abstract
A general and facile synthetic strategy for fabrication of a series of high-yield and uniform rare-earth oxysulfate hollow spheres is demonstrated as the first example via calcination corresponding rare-earth coordination compounds obtained using L-cysteine as a biomolecular template. Furthermore, the hollow spheres can also be successfully doped or formed in a double-shell form for property modification and technological applications.
Hollow spheres have been pursued intensively owing to the fundamental requirements in basic scientific research and potential technological applications in diverse fields, including selective catalysis, controlled release and delivery, and photonic building blocks, etc.1 A variety of chemical and physicochemical strategies such as template-assisted synthesis, Kirkendall diffusion effects, Ostwald ripening, and chemically induced self-transformation have been employed for the design and controlled fabrication of micro/nanospheres with hollow interiors.2 Among them, template-assisted synthesis has been demonstrated to be the most effective and versatile approach. Various templates, such as hard templates (e.g., silica, polymer, and carbon spheres) and soft templates (e.g., vesicles, emulsions, and surfactant), have been extensively utilized to fabricate hollow spheres.3 In particular, biomolecules, as life's basic building blocks, have been employed as templates for the design and synthesis of various micro/nanomaterials, including hollow spheres, due to the special structures and fascinating functions.4,5 Despite these successes, exploiting a facile and general procedure for the fabrication of hollow spheres with narrow size distribution still remains a highly sophisticated challenge.
Rare-earth oxysulfates (RE2O2SO4) have attracted increasing attention in recent years due to the unique luminescent and specific magnetic properties as well as significant applications in large volume oxygen storage, which offers 8 times higher oxygen storage capacity (OSC) than that of conventional cerium-based oxygen storage materials, such as CeO2-ZrO2.6–9 RE2O2SO4 could be obtained through a high-temperature treatment of the corresponding hydrous sulfates (RE2(SO4)3·nH2O) or layered hydroxides intercalated with dodecyl sulfate (DS) ions.10,11 Recently, layered rare-earth hydroxysulfate (RE2(OH)4SO4·nH2O) could also be transformed into RE2O2SO4 by a thermal-induced dehydroxylation process.12 However, most of RE2O2SO4 prepared by the above methods are bulk materials with irregular morphology. There is still no report on the production of hollow spheres until now. Herein, we report a general and facile procedure for production of a series of high-yield and uniform RE2O2SO4 hollow spheres via the calcination of corresponding rare-earth coordination compounds obtained by using L-cysteine as a biomolecular templates. Furthermore, doped and coated RE2O2SO4 hollow spheres have also been prepared for the property modification and technological applications.
Fig. 1a exhibits a typical scanning electron microscopy (SEM) image of a La-coordination compound consisting of uniform spherical particles with smooth surfaces. The broken shell of an individual sphere confirms that the interior of the particles is hollow. This is consistent with the transmission electron microscopy (TEM) image shown in Fig. 1b, which also reveals the hollow nature of these spherical particles. No crystal fringes are observed in the corresponding high-resolution TEM (HRTEM) image (inset of Fig. 1b), which indicates that the hollow spheres are amorphous. The hollow spheres have a mean diameter of approximately 750 nm with a standard deviation of 70 nm and shell thickness in the range of 40–100 nm. Fig. 1c depicts a typical SEM image of La2O2SO4 obtained by thermolysis of corresponding La-coordination compounds at 600 °C for 2 h. It is obvious that the spherical shapes of the particles are still well retained. The surfaces, however, become relatively rough, which is different from the quite smooth surface of the precursory spheres. The average diameter of La2O2SO4 hollow spheres is estimated to be approximately 550 nm with a standard deviation of 40 nm, smaller than that of the corresponding La-coordination compound, implying the tendency to shrink after calcination.13 A typical TEM image of La2O2SO4 hollow spheres, given in Fig. 1d, also clearly displays almost 100% hollow spheres with a mean diameter of about 550 nm and shell thickness of about 60 nm, which again suggests that the hollow nature was perfectly inherited from La-coordination compound hollow spheres. The inset of Fig. 1d shows the corresponding HRTEM image, which provided further insight into the structure of La2O2SO4 hollow spheres. Lattice spacings were measured to be about 0.31 and 0.21 nm, which are consistent with the interplanar spacings between (310) and (020) crystallographic planes in monoclinic La2O2SO4, respectively. The evolution in crystal structure can also be interpreted from X-ray powder diffraction (XRD) results. The XRD pattern of the La-coordination compound is mainly featureless, indicating the non-crystalline or amorphous nature (Fig. 1e). After calcination, the crystalline form of La2O2SO4 was obtained. All the reflections can be indexed to the monoclinic structure of La2O2SO4 (space group: C2/c (No. 15)) with lattice constant a = 14.345 Å, b = 4.259 Å, c = 8.387 Å, and β = 107°. Fig. 1f depicts the structure of La2O2SO4, which consists of the alternating stacking of a La2O22+ layer and a layer of sulfate (SO42−) along the a-axis.6,14
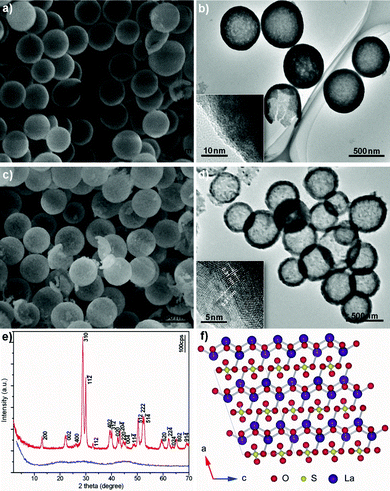 | ||
| Fig. 1 a) SEM and b) TEM images of La-coordination compound. c) SEM and d) TEM images of La2O2SO4 hollow spheres. The insets in b) and d) depict the corresponding HRTEM images. e) XRD patterns of La-coordination compound (blue) and La2O2SO4 (red). f) Crystal structure for La2O2SO4 projected along the b* axis. The line shows a two-dimensional unit cell. The O, S, and La species are represented by red, yellow, and violet balls, respectively. | ||
It is interesting and exciting that the current strategy, converting as-prepared La-coordination compounds into La2O2SO4 hollow spheres, can be extended to a series of rare-earth elements, such as Pr, Nd, Sm, Eu, Gd, Tb, Dy, Y, Ho, and Yb. All the reflection patterns of calcined products are found to be very similar to that of La2O2SO4 in monoclinic symmetry (Fig. S1, ESI†). Representative SEM images of light rare-earth oxysulfates such as Pr2O2SO4, Sm2O2SO4, and Eu2O2SO4 are shown in Fig. 2a, b and c, respectively, from which it can be seen that the products unexceptionally exhibit spherical morphologies with hollow interiors. For example, the selected areas marked by a quadrangle in Fig. 2a and c show Pr2O2SO4 and Eu2O2SO4 hollow spheres with broken shells. The inset clearly reveals the hollow nature. For heavy rare-earth oxysulfates, the morphological features are similar with the light rare-earth counterpart. Fig. 2d displays a typical SEM image of Tb2O2SO4 hollow spheres. Careful observation shows that the shells of hollow spheres are composed of small nanoparticles with hollow interiors (inset of Fig. 2d).
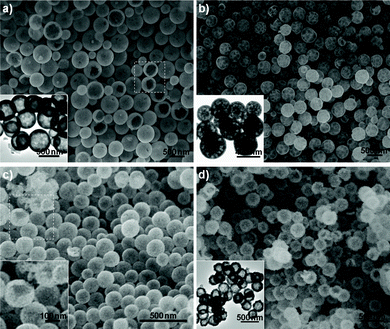 | ||
| Fig. 2 Typical SEM and TEM images of a) Pr2O2SO4, b) Sm2O2SO4, c) Eu2O2SO4, and d) Tb2O2SO4 hollow spheres. The inset in a), b), and d) depict the corresponding TEM images. The inset in c) is a high-magnification SEM image obtained from the selected area marked by a quadrangle. | ||
On the other hand, the rare-earth coordination compound can also be obtained under identical conditions for the elements of Ce, Er, and Tm, but they are crystallized into oxide forms after calcination. The XRD patterns prove the formation of pure CeO2, Er2O3, and Tm2O3 (Fig. S2, ESI†). Furthermore, the diffraction peaks of Y2O3, Ho2O3, and Yb2O3 can be found, as shown in Fig. S1, ESI.† It is well known that the radius of the trivalent rare-earth ions gradually decreases over the lanthanide series. In particular, the 4f1 electron configuration of Ce(III) ions (1.143 Å) can easily transform into the much steadier 4f0 configuration of Ce(IV) ions (0.970 Å), which results in the formation of CeO2. For Y(III) ions, although it is much lighter in weight than the heavy rare-earth ions, the ionic radius (1.019 Å) is very close to that of Ho(III) ions (1.015 Å) due to the lanthanide contraction.15 Thus, it is reasonable to believe that the formation of rare-earth oxides may be closely correlated to the structural stability and/or lanthanide contraction. Typical SEM images of CeO2 are shown in Fig. 3a, which distinctly reveals the hollow nature of the spherical particles, consistent with TEM results (inset of Fig. 3a). The diameter distribution of Er2O3 hollow spheres is relatively uniform, as shown in Fig. 3b. Fig. 3c depicts the TEM image of Tm2O3, which reveals the Tm2O3 hollow spheres have a rough surface with retained spherical contours.
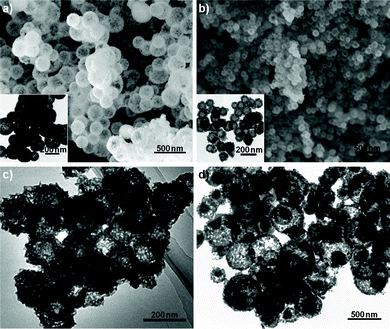 | ||
| Fig. 3 Typical SEM and TEM images of rare-earth oxide hollow spheres: a) CeO2, b) Er2O3, and c) Tm2O3 and d) double-shelled CeO2/ZnS hollow spheres. The insets in a) and b) are corresponding TEM images of as-prepared CeO2 and Er2O3 hollow spheres, respectively. | ||
Based on the experimental results, the possible scenario for the formation of hollow spheres is illustrated in Fig. S3, ESI.†L-cysteine plays an important role in the present synthesis procedure, which serves as both a biomolecular template and sulfide source. Firstly, L-cysteine is dispersed in deionized water and simultaneously capped by rare-earth ions due to the coordination of functional groups in the L-cysteine molecules, such as –NH2, –COOH, and –SH, which has a strong tendency to coordinate with inorganic cations. Subsequently the fresh rare-earth coordination compound is unstable due to the high surface energy and tends to aggregate into spherical products driven by the minimization of interfacial energy, which can then convert into hollow spheres due to the Ostwald ripening process.16 Finally, rare-earth oxysulfates or oxide hollow spheres can be achieved via the calcination of corresponding rare-earth coordination compounds.
It is worth noting that all the rare-earth oxysulfates and oxides can be further functionalized through doping different rare-earth ions or coating procedures for potential applications in various fields. The doped and coated rare-earth oxysulfates and oxides maintain the morphological features as the corresponding host materials. The typical images of Eu-doped La2O2SO4 and double-shelled La2O2SO4/ZnS hollow spheres are shown in Fig. S4, ESI,† which indicates the morphologies of Eu-doped La2O2SO4 resemble that of La2O2SO4 hollow spheres. Fig. 3d indicates the typical TEM image of double-shelled CeO2/ZnS hollow spheres. A hollow core of CeO2 and shell of ZnS can be clearly observed.
More interestingly, Eu-doped rare-earth oxysulfate and oxide hollow spheres display apparent differences in photoluminescence properties compared with the corresponding amorphous coordination compounds. For example, the emission spectra of the Eu-doped La2O2SO4 and CeO2 under the excitation wavelength of 278 and 344 nm originated from the excitation spectra (Fig. S5, ESI†) at room temperature are shown in Fig. 4a and b, respectively. It is striking that the emission maximums of the Eu-doped La2O2SO4 and CeO2 hollow spheres are 335 and 982 times higher than those of the amorphous precursory compounds, respectively, which exhibit a remarkable enhancement after calcination. The spectra are characteristic of the 5D0–7FJ (J = 0, 1, 2, 3, 4) line emissions of the 4f6 configuration of the Eu(III) ion. In particular, the major peaks positioned at 618 nm for Eu-doped La2O2SO4 and at 592 nm for Eu-doped CeO2 hollow spheres are attributed to 5D0–7F2 and 5D0–7F1 transitions, respectively. Generally, the 5D0–7F2 transition correlates well with the symmetry in local environments around Eu(III) ions, while the 5D0–7F1 transition is relatively insensitive to the local symmetry.17 The ratio of the 5D0–7F2 to 5D0–7F1 transition, known as the asymmetric ratio, is 10.8 for the Eu-doped La2O2SO4 and 0.8 for the Eu-doped CeO2, suggesting that Eu-doped La2O2SO4 hollow spheres have lower symmetry of the crystal field of the Eu(III) ions than Eu-doped CeO2 hollow spheres.17b,18 The reason may arise form the structural difference between the Eu-doped systems. As a result, the emission intensity of the Eu-doped La2O2SO4 was higher than that of the Eu-doped CeO2. These photoluminescent hollow spheres may be used as building blocks to construct thin film optical/display devices.
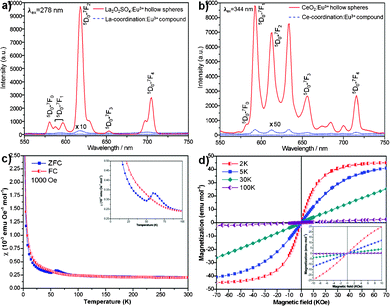 | ||
| Fig. 4 Room-temperature emission spectra of Eu-doped a) La2O2SO4, b) CeO2, and the corresponding coordination compounds. c) The ZFC and FC magnetic susceptibilities of La2O2SO4 measured under an applied field of 1000 Oe. The inset shows the enlarged plot in the low temperature region. d) Isothermal magnetization curves of La2O2SO4 at 2, 5, 30, and 100 K, respectively. The inset reveals the enlarged plot between −10 and 10 kOe. | ||
The magnetic properties of as-prepared La2O2SO4 hollow spheres were measured with the Magnetic Property Measurements (MPMS XP-5, SQUID). The temperature dependence of the zero-field-cooled (ZFC) and field-cooled (FC) magnetic susceptibilities of La2O2SO4 measured under an applied field of 1000 Oe is shown in Fig. 4c. There is a significant difference between ZFC and FC curves below about 100 K. The ZFC curve shows an obvious peak at around 60 K and a kink at about 50 K, which can be clearly observed from the inset of Fig. 4c. The feature indicates the possible presence of antiferromagnetic transitions, and such a behavior has also been observed in the case of Gd2O2SO4 single crystals.8aFig. 4d depicts the isothermal magnetization curves measured at various temperatures. The magnetization curves are linearly increased with magnetic field between −10 and 10 kOe, as shown in the inset of Fig. 4d. The magnetic moments at 2 K and 5 K depart from the linear behavior related to the magnetic field, and are close to saturation in high magnetic fields, which may derive from the canted local spin array owing to the edge effect. Furthermore, no hysteresis is discovered in these curves, revealing that ferromagnetic ordering component does not exist. These results further confirm that the antiferromagnetic behavior for La2O2SO4 at low temperature with a Néel temperature TN = 60 K may be a consequence of oxygen defects.
In summary, we have developed a general and facile synthetic strategy for fabrication of a series of high-yield and uniform rare-earth oxysulfates hollow spheres. Meanwhile, some oxide hollow spheres can be obtained due to structural stability and/or lanthanide contraction. Furthermore, doped and coated rare-earth oxysulfates and oxide hollow spheres could also be successfully prepared, which will greatly endow the potential application in various fields. It is believed that the synthetic strategy may provide a general and effective route to synthesize and rationally design other inorganic micro/nanospheres with hollow interiors. These rare-earth oxysulfate and oxide hollow spheres, in particular rare-earth oxysulfates, are expected to bring new opportunities for further fundamental research, as well as for technological applications in optical/display devices, nanoscale reactors, magnetic devices, and oxygen storage materials.
Experimental
In a typical procedure, hydrated lanthanum nitrate (1.0 mmol), L-cysteine (2.0 mmol) and polyvinylpyrrolidone (0.3 g) were dissolved in 40 ml deionized water under vigorous magnetic stirring. The solution was then transferred into a Teflon-lined autoclave of 50 ml capacity and was sealed and maintained at 140 °C for 24 h. Subsequently, the system was allowed to cool to room temperature. The resulting La-coordination compound was collected by filtration and washed with ethanol and distilled water several times and dried at 50 °C for 4 h, which can be transformed into La2O2SO4 by calcination at 600 °C for 2 h. The yield of La2O2SO4 is about 85% based on the calculation of lanthanum nitrate. The obtained La-coordination compounds (1 mmol) were redispersed into 100 ml thioacetamide (0.5 mmol) and zinc nitrate (0.5 mmol) solution, which were then ultrasonically treated for 30 min and calcinated at 600 °C for 2 h to fabricate ZnS-coated La2O2SO4 hollow spheres (see ESI† for details).XRD data were collected by a D/max2550 VB+ diffractometer with Cu-Kα radiation (λ = 0.15405 nm). Morphology of the synthesized products was examined using a JEOL JSM–6700F field emission scanning SEM. TEM was performed on a JEOL JEM–3100F energy-filtering (Omega type) transmission microscope. The photoluminescence excitation and emission spectra were measured on a Hitachi F-4500 fluorescence spectrophotometer at room temperature. Magnetic measurements were conducted using a Quantum Design MPMS XP-5 superconducting quantum interference device (SQUID).
Acknowledgements
This work was supported by Fundamental Research Funds for the Central Universities of China (No. 2010QZZD008) and National Natural Science Foundation of China (No. 50504017).References
- (a) D. Mertz, P. Tan, Y. Wang, T. K. Goh, A. Blencowe and F. Caruso, Adv. Mater., 2011, 23, 5668 CrossRef CAS; (b) B. Wang, J. S. Chen, H. B. Wu, Z. Wang and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 17146 CrossRef CAS; (c) D. Kim, E. Kim, J. Lee, S. Hong, W. Sung, N. Lim, C. G. Park and K. Kim, J. Am. Chem. Soc., 2010, 132, 9908 CrossRef CAS; (d) Y. Zhao and L. Jiang, Adv. Mater., 2009, 21, 3621 CrossRef CAS; (e) Z.-R. Shen, J.-G. Wang, P.-C. Sun, D.-T. Ding and T.-H. Chen, Chem. Commun., 2009, 1742 RSC; (f) X. W. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987 CrossRef CAS; (g) X. Liang, B. Xu, S. M. Kuang and X. Wang, Adv. Mater., 2008, 20, 3739 CrossRef CAS; (h) H. B. Zeng, W. P. Cai, P. S. Liu, X. X. Xu, H. J. Zhou, C. Klingshirn and H. Kalt, ACS Nano, 2008, 2, 1661 CrossRef CAS; (i) S. H. Im, U. Jeong and Y. N. Xia, Nat. Mater., 2005, 4, 671 CrossRef.
- (a) E. Gonzalez, J. Arbiol and V. F. Puntes, Science, 2011, 334, 1377 CrossRef CAS; (b) G. Réthoré and A. Pandit, Small, 2010, 6, 488 CrossRef; (c) R. Yi, R. R. Shi, G. H. Gao, N. Zhang, X. M. Cui, Y. H. He and X. H. Liu, J. Phys. Chem. C, 2009, 113, 1222 CrossRef CAS; (d) Y. D. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Science, 2004, 304, 711 CrossRef CAS.
- (a) J. Liu, F. Liu, K. Gao, J. S. Wu and D. F. Xue, J. Mater. Chem., 2009, 19, 6073 RSC; (b) H. L. Xu and W. Z. Wang, Angew. Chem., Int. Ed., 2007, 46, 1489 CrossRef CAS; (c) X. M. Sun, J. F. Liu and Y. D. Li, Chem.–Eur. J., 2006, 12, 2039 CrossRef CAS; (d) Z. Z. Yang, Z. W. Niu, Y. F. Lu, Z. B. Hu and C. C. Han, Angew. Chem., Int. Ed., 2003, 42, 1943 CrossRef CAS; (e) S.-W. Kim, M. Kim, W. Y. Lee and T. Hyeon, J. Am. Chem. Soc., 2002, 124, 7642 CrossRef CAS; (f) Z. Y. Zhong, Y. D. Yin, B. Gates and Y. N. Xia, Adv. Mater., 2000, 12, 206 CrossRef CAS.
- (a) Q. Y. Lu, F. Gao and S. Komarneni, J. Am. Chem. Soc., 2004, 126, 54 CrossRef CAS; (b) M. Knez, A. M. Bittner, F. Boes, C. Wege, H. Jeske, E. Maiβ and K. Kern, Nano Lett., 2003, 3, 1079 CrossRef CAS.
- (a) B. X. Li, Y. Xie and Y. Xue, J. Phys. Chem. C, 2007, 111, 12181 CrossRef CAS; (b) P. T. Zhao, T. Huang and K. X. Huang, J. Phys. Chem. C, 2007, 111, 12890 CrossRef CAS.
- S. Zhukov, A. Yatsenko, V. Chernyshev, V. Trunov, E. Tserkovnaya, O. Antson, J. Hölsä, P. Baulés and H. Schenk, Mater. Res. Bull., 1997, 32, 43 CrossRef CAS.
- T. Kijima, T. Shinbori, M. Sekita, M. Uota and G. Sakai, J. Lumin., 2008, 128, 311 CrossRef CAS.
- (a) W. Paul, J. Magn. Magn. Mater., 1990, 87, 23 CrossRef CAS; (b) H. G. Kahle and A. Kasten, J. Magn. Magn. Mater., 1983, 31–34, 1081 CrossRef CAS; (c) H. Hülsing, H. G. Kahle, M. Schwab and H. J. Schwarzbauer, J. Magn. Magn. Mater., 1978, 9, 68 CrossRef.
- (a) D. J. Zhang, F. Yoshioka, K. Ikeue and M. Machida, Chem. Mater., 2008, 20, 6697 CrossRef CAS; (b) M. Machida, K. Kawamura, T. Kawano, D. J. Zhang and K. Ikeue, J. Mater. Chem., 2006, 16, 3084 RSC.
- (a) H. G. Kahle and A. Kasten, J. Magn. Magn. Mater., 1983, 31–34, 1081 CrossRef CAS; (b) H. Hülsing, H. G. Kahle and A. Kasten, J. Magn. Magn. Mater., 1980, 15–18, 512 CrossRef.
- (a) M. Machida, K. Kawamura, K. Ito and K. Ikeue, Chem. Mater., 2005, 17, 1487 CrossRef CAS; (b) M. Machida, K. Kawamura and K. Ito, Chem. Commun., 2004, 662 RSC.
- J. B. Liang, R. Z. Ma, F. X. Geng, Y. Ebina and T. Sasaki, Chem. Mater., 2010, 22, 6001 CrossRef CAS.
- (a) X. H. Liu, R. Z. Ma, Y. Bando and T. Sasaki, Angew. Chem., Int. Ed., 2010, 49, 8253 CrossRef CAS; (b) X. H. Liu, R. Yi, N. Zhang, R. R. Shi, X. G. Li and G. Z. Qiu, Chem.–Asian J., 2008, 3, 732 CrossRef CAS.
- M. Machida, T. Kawano, M. Eto, D. J. Zhang and K. Ikeue, Chem. Mater., 2007, 19, 954 CrossRef CAS.
- (a) M. J. Martínez-Lope, J. A. Alonso, M. Retuerto and M. T. Fernández-Díaz, Inorg. Chem., 2008, 47, 2634 CrossRef; (b) R. X. Yan, X. M. Sun, X. Wang, Q. Peng and Y. D. Li, Chem.–Eur. J., 2005, 11, 2183 CrossRef CAS; (c) H.-L. Sun, C.-H. Ye, X.-Y. Wang, J.-R. Li, S. Gao and K.-B. Yu, J. Mol. Struct., 2004, 702, 77 CrossRef CAS.
- J. Li and H. C. Zeng, J. Am. Chem. Soc., 2007, 129, 15839 CrossRef CAS.
- (a) S. Ida, C. Ogata, M. Eguchi, W. J. Youngblood, T. E. Mallouk and Y. Matsumoto, J. Am. Chem. Soc., 2008, 130, 7052 CrossRef CAS; (b) H.-X. Mai, Y.-W. Zhang, R. Si, Z.-G. Yan, L.-D. Sun, L.-P. You and C.-H. Yan, J. Am. Chem. Soc., 2006, 128, 6426 CrossRef CAS.
- R. Si, Y.-W. Zhang, L.-P. You and C.-H. Yan, Angew. Chem., Int. Ed., 2005, 44, 3256 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Typical SEM, EDS and TEM images and XRD patterns of as-prepared hollow spheres. See DOI: 10.1039/c2ra21007j |
| This journal is © The Royal Society of Chemistry 2012 |
