Microwave-assisted highly efficient transformation of ketose/aldose to 5-hydroxymethylfurfural (5-HMF) in a simple phosphate buffer system†
Jinhua
Lu
,
Yani
Yan
,
Yahong
Zhang
* and
Yi
Tang
Department of Chemistry, Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials and Laboratory of Advanced Material, Fudan University, Shanghai 200433, PR China. E-mail: zhangyh@fudan.edu.cn; Fax: +86-21-65641740
First published on 3rd July 2012
Abstract
An acidic phosphate buffer system is developed to highly-selectively catalyze ketoses/aldoses dehydrating into 5-HMF without undesirable rehydration under microwave irradiation.
The interest in renewable resources of energy and chemicals has been growing fast in recent years because of diminishing petrochemical resources, increasing energy demands and rising CO2 emissions.1 Abundant biomass resources are the most promising alternatives in the existing energy consumption mode.2 Among the various chemicals from biomass, 5-hydroxymethylfurfural (5-HMF) is believed to be a key renewable platform chemical because it can be easily converted into fuels and various valuable chemicals, which can be substituted for corresponding ones from fossil resources. Generally, 5-HMF is directly obtained from the acid-catalyzed dehydration of polysaccharides or hexoses, in which fructose has a much higher conversion and selectivity. Many types of acid catalysts have been used to study this process, containing mineral acids, organic acids, inorganic salts and solid acid catalysts.2–6 Commercial concerns favour homogenous acid catalysts for their high efficiency with a high fructose conversion and a desirable 5-HMF yield. In several main reaction solvents (organic solvent or organic–water mixture, water, ionic liquid), water is a convenient and green solvent for biomass, considering that it is much cheaper than ionic liquids, without a tendency to generate waste streams, and biomass contains much water.7 There are two main problems for aqueous processes, i.e. inevitable by-products comprised of insoluble/soluble polymeric compounds (humins) and undesirable levulinic acid (LA) and formic acid (FA) obtained from rehydration of 5-HMF. Organic solvents can suppress undesired rehydration products, as well as polymeric compounds. However, the product has to be separated from high boiling point solvents, such as dimethyl sulfoxide, significantly increasing the cost of product purification.
Although fructose (ketose) can be selectively converted into 5-HMF with various kinds of acid catalysts, including homogeneous as well as heterogeneous ones, the selective synthesis of 5-HMF is difficult from glucose (aldose), which is the most abundant saccharide in nature.8,9 So far, good results have been obtained for the direct conversion of glucose to 5-HMF in ionic liquids with catalysts of various inorganic salts.10 Moreover, some dual-function catalysts, such as solid base/acid catalysts11 and mixed oxides,12 were used to directly prepare 5-HMF from glucose in the organic phase or water/organic phase, presumably via glucose isomerization to fructose followed by dehydration. In this respect, the isomerization of glucose into fructose is a crucial step in the efficient production of 5-HMF from glucose.13 However, the maximum attainable degree (about 50%) of the conversion of glucose to fructose is governed by the thermodynamic equilibrium, which will limit the conversion of glucose in the two-step process of separate isomerization and dehydration. Davis et al.13 employed Sn-beta zeolite as a catalyst to achieve the isomerization of glucose in acidic conditions and so enabled Sn-Beta to combine isomerization with an acid-catalyzed dehydration reaction, which would promote the shift of the isomerization equilibrium of glucose. Huang et al.14 developed a two-step process associating acidic dehydration with borate-assisted isomerase treatment to obtain 5-HMF from glucose. Thus, a conversion of 88.2% and a HMF yield of 63.3% generated at a glucose solution of 5 wt% containing an extractant (1-butanol) in nearly 10 h. However, it is still highly desirable to develop a cost-efficient approach to transform various aldoses to 5-HMF in aqueous phase systems from the standpoint of their performances (high yield and selectivity), as well as practical procedures.
Here, we present a highly-active phosphate buffer system (PBS) for the transformation of highly-concentrated aqueous ketoses/aldoses (10–50 wt%) into 5-HMF. It is found that the rehydration of 5-HMF can be eliminated in such a buffer system, which is the key factor to highly-selectively achieve 5-HMF from saccharides in this buffer system. More importantly, this buffer system can not only achieve highly efficient dehydration of fructose/inulin, but also realize isomerization and dehydration of glucose/sucrose in one pot by using borate as a promoter. Such a simple and efficient one-pot system will not only open up the possibility of highly efficient transformation of fructose/glucose, as well as their mixture solution in the aqueous phase, but also allow their use on a practical scale.
The dehydrations of various saccharides were performed in a Teflon reactor under microwave irradiation and all of the reactions were directly carried out in one pot (†). The products collected at different reaction times were analyzed using a high performance liquid chromatography equipped with a refractive index detector (RID) and a Shodex SH1011 sugar column (†). Different mineral acid solutions with different acidic strengths (pH) were used to study the dehydration process of fructose and their products under microwave radiation in order to avoid the formation of LA, FA and polymeric compounds in the aqueous system (Table 1). It can be observed that product distribution and conversion are determined by the pH of the system. Too strong acids (pH < 2.0) will lead to the formation of LA and FA, which decreases the selectivity and yield of the resulting 5-HMF. Too weak acids (pH > 2.5) will result in low conversion. Moreover, the selectivity of 5-HMF decreases with increasing the pH from 2.7 to 4.7. The products will completely change at pH ≈ 7.0 (PBS6.8). Obviously, an appropriate pH is key for the preparation of 5-HMF with high selectivity and yield, and it is at pH ≈ 2.0 according to Table 1. LA and FA at this pH are effectively eliminated (Fig. S1†). Although dilute HCl solution (0.01 M, pH = 2) with an appropriate pH can also avoid the formation of LA and FA and result in the same selectivity of 5-HMF as the PBS2.1 system, the PBS2.1 system seems to be the better choice according to Table 1. Compared to the PBS2.1 system, the conversion of fructose in dilute HCl solution greatly slows because the low H3O+ concentration will cause the pH change during the reaction. The buffer solution with a suitable pH can not only provide a stable pH and ensure the steady formation of 5-HMF from fructose, but also avoid the rehydration of 5-HMF. This can also be proved by the fact that the commercial 5-HMF cannot be hydrated into LA and FA in PBS2.1, but other acid systems can lead to the formation of LA and FA (Table S1†).
| Sample | Catalysts | pH | Conv. (%) | YieldFA (%) | YieldLA (%) | Yield5-HMFa (%) |
|---|---|---|---|---|---|---|
| a The data in the parenthesis presents the selectivity of 5-HMF. b The reaction time is 30 min. c PBS1.4: 0.83 M H3PO4/0.17 M NaH2PO4. d PBS2.1: 0.5 M H3PO4/0.5 M NaH2PO4. e PBS2.8: 0.17 M H3PO4/0.83 M NaH2PO4. f PBS6.8: 0.5 M NaH2PO4/0.5 M Na2HPO4. g The reaction time is 90 min. | ||||||
| Fructoseb | H2SO4/0.25 M | 0.3 | 100 | 38 | 28 | 0 |
| HCl/0.25 M | 0.6 | 100 | 39 | 29 | 0 | |
| HCl/0.01 M | 2.0 | 29 | 0 | 0 | 20(69) | |
| H3PO4/1 M | 1.1 | 100 | 26 | 19 | 12(12) | |
| PBS1.4c | 1.4 | 99 | 9 | 6 | 34(34) | |
| PBS2.1d | 2.1 | 94 | 0 | 0 | 64(68) | |
| PBS2.8e | 2.8 | 83 | 3 | 0 | 46(55) | |
| NaH2PO4/1 M | 4.7 | 74 | 4 | 1 | 20(27) | |
| PBS6.8f | 6.8 | 90 | 28 | 2 | 0 | |
| Glucoseg | H2SO4/0.25 M | 0.3 | 61 | 21 | 15 | 1 |
| HCl/0.25 M | 0.6 | 71 | 24 | 17 | 1 | |
| HCl/0.01 M | 2.0 | 10 | 0 | 0 | 2(20) | |
| H3PO4/1 M | 1.1 | 44 | 10 | 7 | 4 | |
| PBS2.1d | 2.1 | 33 | 0 | 0 | 19(58) | |
| NaH2PO4/1 M | 4.7 | 79 | 4 | 1 | 22(28) | |
Furthermore, the reaction temperature and the concentration of the buffer solution were studied to further optimize the selectivity of 5-HMF (Figs. S2 and S3†). A temperature of 150 °C and a PBS2.1 concentration of 0.5 M H3PO4/0.5 M NaH2PO4 were finally selected after considering both conversion of fructose and selectivity of 5-HMF. In such a simple PBS, fructose with different concentrations (10–50 wt%) are catalytically dehydrated into 5-HMF under microwave irradiation at 150 °C (Fig. 1). It is found that all of the fructose solutions can be highly selectively transformed into 5-HMF. The selectivity and yield of 5-HMF for the 10 wt% fructose system can reach up to 68% and 64% within 30 min at a 94% conversion of fructose. However, because of the fast formation of the polymeric compounds at higher substrate concentration (50 wt%), the selectivity of the system decreases with increasing the substrate concentration, although no LA or FA are observed during these reactions (Fig. 1).
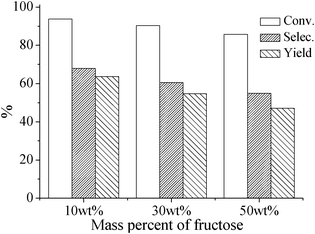 | ||
| Fig. 1 Conversion of fructose, as well as selectivity and yield of 5-HMF in the PBS2.1 system at 150 °C within 30 min under microwave irradiation. | ||
Interestingly, such a buffer system also applies to the transformation of glucose into 5-HMF. As shown in Table 1 and Fig. 2A, a 33% conversion of glucose and 58% selectivity of 5-HMF could be observed for the glucose of 10 wt% in this acidic PBS2.1 system. However, when the PBS2.1 system is replaced by a stronger acidic solution, such as H2SO4, HCl and H3PO4, there is only < 5% of 5-HMF in the reaction of 90 min, and FA and LA are produced with low selectivity (Table 1). The weaker acid (NaH2PO4, pH = 4.7) results in a high conversion of glucose, but a low selectivity of 5-HMF (Table 1). It is worth noting that a much lower glucose conversion (10%) and 5-HMF selectivity (20%) are obtained in dilute HCl solution (0.01 M, pH = 2) although it possesses an appropriate pH, which further indicates the necessity of the buffer system. It is well-known that the reactivity of glucose (aldose) is lower than fructose (ketose) because the enolization rate of glucose in solution is lower than fructose due to its very stable ring structure, which is the rate-determining step for 5-HMF formation.15 Thereby, compared to fructose, a longer time is needed in the dehydration of glucose. However, a long reaction time will facilitate the rehydration of 5-HMF, as well as other by-products.16 On the other hand, glucose is easy to form oligosaccharides and furthermore to cross-polymerize with reactive intermediates and 5-HMF, resulting in a lower selectivity of 5-HMF or LA.17 Therefore, the appropriate dehydration rate of glucose and good stability of 5-HMF in this PBS2.1 system are the two crucial factors to achieve 5-HMF from glucose with high selectivity. The facile cross-polymerization of glucose can also be testified by our results, i.e. the conversion of glucose increases while the selectivity of 5-HMF greatly decreases with the increasing glucose concentration (Fig. 2A). The selectivity of 5-HMF decreases from 58 to 25% with the increase of glucose concentration from 10 to 50 wt%. However, this decrease of 5-HMF selectivity is not sharp with the increasing fructose concentration (Fig. 1). Therefore, a high concentration of glucose is not appropriate to the highly-selective formation of 5-HMF in the acidic catalysis system.
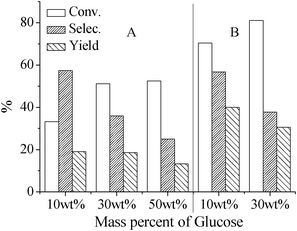 | ||
| Fig. 2 Conversion of glucose, selectivity and yield of 5-HMF in the PBS2.1 system containing borate as a promoter (B) or not (A) at 150 °C within 90 min under microwave irradiation. | ||
Considering that fructose is observed during transformation of the high-concentration glucose system (30 wt%) in the PBS2.1 system (Fig. S4†), it can be concluded that glucose dehydrates into 5-HMF via an isomerization step from glucose to fructose. Recently, Riisager et al.18 reported a dehydration of glucose into 5-HMF in ionic liquids with sodium borate as a promoter and showed that glucose could isomerize into fructose through combining with B(OH)4−. Therefore, a certain amount of sodium borate as a promoter is added into the above PBS2.1 system (†). As shown in Fig. 2B, the conversion of glucose is improved owing to the improvement of isomerization rate, but a stable selectivity of 5-HMF is observed. In this case, a 70.4% conversion of glucose at a 58% selectivity of 5-HMF is reached within 90 min, which is about twice that of a pure PBS2.1 system. This means that the dehydration of glucose indeed experiences an isomerisation process from glucose to fructose and the isomerization is the rate-determining step for 5-HMF formation. Moreover, when a glucose molecule interacts with borate, a proton will be released,19 which will influence the pH of the reaction system and so its selectivity. However, there is no visible pH change in our work because of the use of the buffer system, which also ensures a stable selectivity of 5-HMF with the reaction time.
Finally, a two-phase system was used to avoid the formation of polymeric compounds and so further improve the reaction selectivity by removing 5-HMF partly during the reaction. The results are listed in Table 2. Herein, an organic phase (methylisobutylketone and 2-butanol, volume ratio: 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) is employed as the extractor.2 When the extractor is used, the conversion of fructose/glucose changes little, but the selectivity of 5-HMF becomes higher. A 5-HMF selectivity of 93% is achieved at a fructose (10 wt%) conversion of 95% within 30 min, while a 5-HMF selectivity of 77% is obtained at a glucose (10 wt%) conversion of 83% within 2 h in the presence of borate. Additionally, inulin and sucrose were also applied to this PBS2.1 system. As shown in Table 2, inulin and sucrose can also be highly-selectively converted to 5-HMF in this simple buffer system, which provides a view for the direct conversion of other polysaccharides.
3) is employed as the extractor.2 When the extractor is used, the conversion of fructose/glucose changes little, but the selectivity of 5-HMF becomes higher. A 5-HMF selectivity of 93% is achieved at a fructose (10 wt%) conversion of 95% within 30 min, while a 5-HMF selectivity of 77% is obtained at a glucose (10 wt%) conversion of 83% within 2 h in the presence of borate. Additionally, inulin and sucrose were also applied to this PBS2.1 system. As shown in Table 2, inulin and sucrose can also be highly-selectively converted to 5-HMF in this simple buffer system, which provides a view for the direct conversion of other polysaccharides.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1
In conclusion, this work has proved that an aqueous acidic PBS system is a very suitable system for various ketoses/aldoses dehydrating to 5-HMF. The whole process can be carried out in one pot and is fit for the highly-concentrated saccharide solution. More importantly, rehydration of 5-HMF into LA and FA, which is always a challenge of 5-HMF production in the aqueous phase, is avoided in such a buffer system. Thanks to the two-phase system, a selectivity of 83% is generated for 30 wt% fructose and a selectivity of 77% is achieved for 10 wt% glucose separately for aqueous buffer phase with the same volume of organic phase. This simple one-pot acidic buffer system is not only easy to enlarge to industry scale with high efficiency, but can also be expected to extend to other polysaccharides, as well as their mixtures.
Acknowledgements
This work is supported by NSFC (20721063, 30828010, 21171041 and 20890122), STCSM (10QH1400300, 08DZ2270500 and 09DZ2271500) and 973 programs (2009CB930403, 2009CB623506).References
- T. Tuercke, S. Panic and S. Loebbecke, Chem. Eng. Technol., 2009, 32, 1815 CrossRef CAS.
- Y. Roman-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933 CrossRef CAS.
- M. L. J. Mednick, J. Org. Chem., 1962, 27, 398 CrossRef CAS.
- F. S. Asghari and H. Yoshida, Ind. Eng. Chem. Res., 2006, 45, 2163 CrossRef CAS.
- M. Bicker, J. Hirth and H. Vogel, Green Chem., 2003, 5, 280 RSC.
- C. Moreau, R. Durand, S. Razigade, J. Duhamet, P. Faugeras, P. Rivalier, P. Ros and G. Avignon, Appl. Catal., A, 1996, 145, 211 CrossRef CAS.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith, Catal. Commun., 2008, 9, 2244 CrossRef CAS.
- B. F. M. Kuster, Starch/Staerke, 1990, 42, 314 CrossRef CAS.
- H. E. Vandam, A. P. G. Kieboom and H. Vanbekkum, Starch/Staerke, 1986, 38, 1921 Search PubMed.
- (a) H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597 CrossRef CAS; (b) G. Yong, Y. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2008, 47, 9345 CrossRef CAS; (c) J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979 CrossRef CAS; (d) S. Q. Hu, Z. F. Zhang, J. L. Song, Y. X. Zhou and B. X. Han, Green Chem., 2009, 11, 1746 RSC; (e) T. Ståhlberg, M. G. Søensen and A. Riisager, Green Chem., 2010, 12, 321 RSC.
- A. Takagaki, M. Ohara, S. Nishimura and K. Ebitani, Chem. Commun., 2009, 41, 6276 RSC.
- (a) X. H. Qi, M. Watanabe, T. M. Aida and R. L. Smith Jr., Catal. Commun., 2008, 9, 2244 CrossRef CAS; (b) H. Yan, Y. Yang, D. Tong, X. Xiang and C. Hu, Catal. Commun., 2009, 10, 1558 CrossRef CAS; (c) K. Yamaguchi, T. Sakurada, Y. Ogasawara and N. Mizuno, Chem. Lett., 2011, 40, 542 CrossRef CAS.
- M. Moliner, Y. Roman-Leshkov and M. E. Davis, Proc. Natl. Acad. Sci. U. S. A., 2010, 14, 6164 CrossRef.
- R. Huang, W. Qi, R. Su and Z. He, Chem. Commun., 2010, 46, 1115 RSC.
- S. Bhosale, M. Rao and V. Deshpande, Microbiol Rev., 1996, 60, 280 CAS.
- H. M. Pilath, M. R. Nimlos, A. Mittal, M. E. Himmel and D. K. Johnson, J. Agric. Food Chem., 2010, 58, 6131 CrossRef CAS.
- A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754 RSC.
- T. Stahlberg, S. Rodriguez- Rodriguez, P. Fristrup and A. Riisager, Chem.–Eur. J., 2011, 17, 1456 CrossRef CAS.
- T. S. Hansen, J. Mielby and A. Riisager, Green Chem., 2011, 13, 109 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available: The detailed experimental and product analysis process. The HPLC results of dehydration of fructose and glucose at different systems. The results of hydration of 5-HMF (1 wt%) in different acid solutions and PBS2.1 system at 150 °C under microwave radiation. The impact of PBS2.1 concentration and temperature on the conversion of fructose and selectivity of 5-HMF. See DOI: 10.1039/c2ra21011h/ |
| This journal is © The Royal Society of Chemistry 2012 |
