Atmospheric plasma polymer films as templates for inorganic synthesis to yield functional hybrid coatings†
Julien
Petersen
ab,
Marc
Michel
a,
Valérie
Toniazzo
a,
David
Ruch
a,
Guy
Schmerber
b,
Dris
Ihiawakrim
b,
Dominique
Muller
*c,
Aziz
Dinia
*b and
Vincent
Ball
*a
aAdvanced Materials and Structures, Centre de Recherche Public Henri Tudor, 5 rue Bommel, L-4940, Hautcharage, Luxembourg. E-mail: vincent.ball@tudor.lu; Fax: +352 42 59 91 555
bInstitut de Physique et Chimie des Matériaux de Strasbourg, Centre National de la Recherche Scientifique, Unité Mixte de Recherche 7504, Université de Strasbourg, 23 rue du Loess, BP 43, 67034 Strasbourg Cedex, France. E-mail: aziz.dinia@ipcms.u-strasbg.fr
cInstitut d'Électronique du Solide et des Systèmes-CNRS-ULP, 23 rue du Loess, BP 43, 67037 Strasbourg Cedex 2, France. E-mail: Dominique.Muller@iness.c-strasbourg.fr
First published on 22nd August 2012
Abstract
Plasma polymer films produced via dielectric barrier discharge under atmospheric conditions can simultaneously host charged segments and poly(dimethysiloxane)/silica like polymers. The former segments afford some anion exchange properties and the latter ones allow stabilization of the whole coating in the presence of water. The anion exchange capacity of the film can then be used to nucleate and to grow inorganic particles in the plasma polymer coating. In particular, we exploit the presence of allylamine oligomers in a plasma coating made from a mixture of allylamine and hexamethyldisiloxane to hydrolyse titanium(IV) (bisammonium lactato dihydroxyde) and to condense it in TiO2. As a second example, Prussian Blue is produced by the successive incubation of the coating in a solution of potassium hexacyanoferrate and iron(III) chloride. The distribution of TiO2 and of Prussian Blue across the film thickness is investigated, in a semi quantitative manner, by means of Rutherford backscattering. The functional properties of the hybrid coatings are then investigated and it is found that the TiO2 containing films display photoinduced hydrophilicity whereas the films with Prussian Blue display magnetic properties.
Introduction
The synthesis of nanocomposites is of major importance for the design of coatings having (i) lower permeability for a target gas, (ii) increased sensing abilities, i.e. higher sensitivity and selectivity for a given analyte, or (iii) increased dynamic response to an external stimuli. In the first case, for permeability control, the nanocomposite has to be dense with a low degree of tortuosity, have a high aspect ratio of the filler, and have controlled interfaces between the matrix and the filler.1 In the case of nanocomposites for sensing applications, the permeability of the coating has to be high in order for the analytes to reach the nanoparticles whose properties will change in their presence. The matrix will play the role of stabilizing the whole nanocomposite and to ensure optimal distribution of the nanoparticles. Finally, coatings able to respond to external stimuli or coatings displaying self healing properties should contain molecules or nanoparticles able to change their properties in a reversible manner in response to environmental modifications, such as temperature, pH, salt concentration, presence of oxidants, exposure to light, etc..2,3Various preparation methods to obtain such nanocomposite films are known, such as in situ polymerization, along with various solution processing methods such as razor blading of a mixture containing the polymer matrix and the nanoparticulate filler,4 the layer-by-layer deposition of both components,5 the diffusion of the nanofiller in the matrix film6 and finally the diffusion of precursors allowed to react and crystallise in the environment of the matrix.7–9 In this last preparation method, the template films used for the nucleation and subsequent crystal growth of nanoparticles were made either by using the layer-by-layer deposition method, thin gel films, or nanoporous materials.10,11
To the best of our knowledge, thin coatings prepared via atmospheric plasma polymerization in a dielectric barrier discharge (DBD) configuration have never been used for such a purpose. This film preparation method can be easily up-scaled for the preparation of films on large scale substrates,12 does not require the use of organic solvents and expensive pumping systems, and allows for the preparation of films with controllable thickness and porosity. In addition, it allows for a fine tuning of the film's chemical composition13 and porosity. Enzymes can be co-deposited with the active monomer, allowing the production of a polymer film that remains active with respect to the hydrolysis of target substrates.14 This finding shows that the energetic content of the reactive species in the plasma can remain low enough to keep the native conformation of enzymes, which is mandatory for their biological activity. In addition, this last investigation shows the possibility for films produced in DBD conditions to behave as active nanocomposite films.14 In the same framework, fluorescent lanthanide-based coordination polymers can be directly incorporated in the coatings during the nebulization and polymerization of the monomer yielding the plasma polymer film.15 In another investigation, cerium oxide nanoparticles were directly incorporated into a HMDSO/ethanol mixture and injected into a DBD discharge reactor to produce a composite having anti-corrosive properties.16
It is our aim herein to show that plasma polymer films, produced in DBD conditions from a gaseous precursor containing a blend of hexamethyldisiloxane (HMDSO) and allylamine (Aam), can be used as templates to produce inorganic materials as diverse as TiO2 and Prussian Blue (PB), [MFeIII{FeII(CN)6}], where M+ is a cation such as Li+, Na+ or K+. Both types of composite films will display some specific properties, namely some photo-induced wettability and some magnetic behavior as expected for TiO2 and PB17 containing films, respectively. The reason why we chose a mixture of HMDSO and Aam to produce a plasma polymer film comes from the expected role of each polymer, namely poly(allylamine) and a tunable mixture of poly(dimethyl siloxane) (PDMS) and silica.18,13 Poly(allylamine), among other polyamines, is known to play an important role in activating the hydrolysis and polycondensation of aqueous soluble precursors of oxides, namely silicic acid19,20 or Ti(IV) (ammonium bis(lactato) dihydroxide) (TiBisLac).21 Hence plasma polymer films produced from Aam should be ideal candidates to play the role of a template for the deposition of composite films containing silica or TiO2. However, such Aam coatings suffer from their inherent instability in aqueous solution when prepared in DBD conditions. Hence, plasma polymer allylamine films (pp-Aam) put in contact with a TiBisLac containing solution undergo more than 95% material erosion during hydrolysis/polycondensation leading to TiO2.22 This is obviously a major drawback of poly(allylamine) films produced through plasma polymerization in DBD conditions. On the other hand, films prepared through the atmospheric plasma polymerization of HMDSO can be tuned from a composition close to PDMS or close to silica by a change of the plasma power for a given flux of polymers.13 Hence, we expect that a mixture of HMDSO and Aam as precursors for the plasma polymer would allow the production of a template film displaying all the requirements to produce a nano-reactor able to activate the polycondensation of TiO2 from a [Ti(IV)Lac2OH2]2− precursor, namely the presence of available amino groups and a sufficient silica framework to ensure the mechanical stability of the composite in an aqueous medium. The general principle of the concept which underlines this work is depicted in Scheme 1.
![Use of plasma polymer template films made from HMDSO and Aam for the production of composite films containing TiO2 after reaction of the free amino groups with [Ti(iv)Lac2OH2]2−. The same concept will be used to produce PB crystals.](/image/article/2012/RA/c2ra21028b/c2ra21028b-s1.gif) | ||
| Scheme 1 Use of plasma polymer template films made from HMDSO and Aam for the production of composite films containing TiO2 after reaction of the free amino groups with [Ti(IV)Lac2OH2]2−. The same concept will be used to produce PB crystals. | ||
In addition, we expect that the presence of accessible amino groups will allow the HMDSO–Aam films to behave as an anion exchange membrane and, hence, to concentrate anions allowing for a subsequent in situ controlled precipitation reaction.
As a prototypal example, we will show that the HMDSO–Aam films are able to concentrate hexacyanoferrate anions which will react subsequently with Fe3+ ions provided in solution to yield PB.
Results and discussion
The XPS survey spectrum of the plasma polymer films made from a precursor containing HMDSO and Aam (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) is given in Fig. 1 and shows the presence of all elements expected for a film made from the two used monomers. In particular the surface composition of the coating shows the presence of Si (from HMDSO) and N (from Aam).
1 molar ratio) is given in Fig. 1 and shows the presence of all elements expected for a film made from the two used monomers. In particular the surface composition of the coating shows the presence of Si (from HMDSO) and N (from Aam).
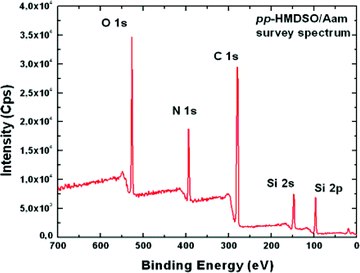 | ||
Fig. 1 XPS survey spectrum of a plasma polymer coating made from a mixture of HMDMSO and Aam (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio). 1 molar ratio). | ||
The atomic composition of the extreme surface of the different coatings is given in Table 1. These coatings were about 200 nm thick as inferred by cross-sectional analysis of scanning electron micrographs (ESI, Fig. S1†), which is significantly thicker than the analysis depth of the X-ray beam in the sample (about 10 nm).
| Quantification (at. %) | ||||
|---|---|---|---|---|
| Si | C | O | N | |
| pp-Aam | — | 68.2 | 6.7 | 25.1 |
| pp-HMDSO/Aam as deposited | 11.7 | 53.7 | 20.7 | 13.9 |
| pp-HMDSO/Aam after dipping in H2O | 11.2 | 52.5 | 24.3 | 12.0 |
The fraction of N and Si in the plasma polymer films decreases and increases respectively in the composite HMDSO–Aam films with respect to the films prepared only from the Aam precursor. More interestingly, the surface composition of the film made from the pp-HMDSO/Aam blend is almost unaffected after immersion of the film in water for 1 h with subsequent drying before XPS characterization. In addition, the film thickness, measured by SEM in cross-sectional analysis, is not affected upon immersion in water and subsequent drying. This shows that the films made from the blend are stable in contrast to the film prepared from Aam as the only precursor. Hence, the presence of PDMS/silica in the film ensures the stability of functional groups against decomposition in water,23 a common phenomenon for pp-Aam films produced under the same conditions.
The presence of Aam at the surface of the films made from the HMDSO–Aam blend is also indirectly reflected in its zeta potential versus pH titration curve. The zeta potentials of the glass plates undergo a shift in their isoelectric points by about one pH unit after functionalization with a plasma polymer film made from the HMDSO and Aam blend (ESI, Fig. S2†). It has to be noted that the coatings made from such blends are homogeneous films when deposited on hydrophilic substrates as shown by cross-sectional SEM analysis (ESI, Fig. S1†). Hence, such coatings meet all the necessary requirements, in terms of chemical stability and chemical composition, to be used as nano-reactors for the synthesis of Ti–O– containing moieties from an aqueous precursor, [Ti(IV)Lac2OH2]2−. UV-Vis spectroscopy showed that after only a few minutes of incubation, the coatings contain a material that strongly absorbs photons with an energy higher than about 3.3 eV, which is consistent with the presence of titanium oxides in the films (Fig. 2A). A detailed analysis of the Ti2p region of the XPS spectrum shows that Ti is present in the film in different oxidation states, with Ti(IV) being predominant (Fig. 2B).This suggests that the plasma polymer films contain TiO2 as well as other Ti–O– containing species. Control experiments were performed with plasma polymer films made from HMDSO only, and no incorporation of Ti was found after incubation in the [Ti(IV)Lac2OH2]2− containing solution. This shows that allylamine segments in the film are required to interact with the Ti(IV) complex. To get an idea of the average Ti content over the entire film thickness, Rutherford backscaterring experiments have been performed as will be explained later on.
![A: UV-Vis spectra of the HMDSO–Aam films put in contact with a Tris buffer solution containing 5 mM of [Ti(iv)Lac2OH2]2− anions after different reaction times. The quartz substrates were rinsed with water and dried under a stream of nitrogen before spectral acquisition. B: Analysis of the Ti2p region of the XPS spectrum of a plasma polymer coating put in contact with a 5 mM [Ti(iv)Lac2OH2]2− containing solution for 1 h.](/image/article/2012/RA/c2ra21028b/c2ra21028b-f2.gif) | ||
| Fig. 2 A: UV-Vis spectra of the HMDSO–Aam films put in contact with a Tris buffer solution containing 5 mM of [Ti(IV)Lac2OH2]2− anions after different reaction times. The quartz substrates were rinsed with water and dried under a stream of nitrogen before spectral acquisition. B: Analysis of the Ti2p region of the XPS spectrum of a plasma polymer coating put in contact with a 5 mM [Ti(IV)Lac2OH2]2− containing solution for 1 h. | ||
The obtained particles are distributed in the form of μm sized clusters made of a large distribution of smaller aggregates (ESI, Fig. S3†). We also performed a cross-sectional analysis of the films by TEM after 1 h of reaction with a 5 mM containing TiBisLac solution (data not shown). Unfortunately a clear identification of the distribution of the Ti–O containing species across the section of the film was not possible owing to rapid damage under the electron beam. These images nevertheless show that the film thickness does not change significantly with respect to the pristine film after reaction with TiBisLac. The films incubated in a [Ti(IV)Lac2OH2]2− containing solution display the expected light induced hydrophilicity for coatings containing TiO2 or Ti–O– containing species at the film/air interface.24 The static water contact angle of the as prepared films was 85° ± 1.5, which decreased down to 45° ± 3 after incubation in the [Ti(IV)Lac2OH2]2− solution for 1 h and finally down to 11.5° ± 2.5 after exposure to UV light. The cycle of storage in the dark followed by laser illumination could be repeated two times with stable values for the contact angles before film degradation occurring after a higher number of storage-illumination cycles. Such a degradation might well be due to the influence of the reactive oxygen species produced when water is in contact with TiO2 having undergone a light induced charge separation process.25 The wavelength used to produce such a charge separation (266 nm) corresponds to the spectral domain where the film strongly absorbs light and where some electrons should be promoted from the valence band to the conduction band of the semi-conducting titanium dioxide or Ti–O– containing particles.
X-Ray diffraction analysis revealed that no diffraction peaks are present in the powder obtained from the composite film, probably due a low cristallinity, an insufficient content of crystalline titanium oxide, or to its large size distribution in the hybrid material. To elucidate this point, TEM analyses have been performed to determine the size and the crystalline structure of the inorganic material present in the plasma polymer template (Fig. 3). At high magnification, Titania or Ti–O containing nanoparticles have been detected with an average size of 5 nm in the analysed region. In addition, atomic planes characteristic of nanocrystalline TiO2 are found. Two sets of planes are evidenced which correspond to d101 = 0.364 nm and d200 = 0.188 nm. These interplanar distances are characteristic of a polycrystalline anatase structure.
In order to generalize the concept that plasma polymer films made from HMDMSO and Aam can act as active templates for the deposition of inorganic material from solutions containing precursor ions, we put the freshly prepared films in the presence of a 1 mM K4Fe(CN)6 solution followed by a 10 mM FeCl3 solution. Indeed, it is well known that hexacyanoferrate anions interact with Fe3+ cations to yield PB crystals17 which can display interesting electrochromic properties in thin films. After only a few minutes of successive incubation of the plasma polymer films in both solutions, their surface was homogeneously covered (a large scale SEM topography is provided in the ESI, Fig. S4†) with cubic crystals of about 1 μm in size (Fig. 4A). This crystal habit is expected for PB. The presence of PB in the films was confirmed by means of X-ray diffraction (Fig. 4B) and IR spectra which show the presence of –CN elongation bands due to FeII–CN–FeIII species at 2074 cm−1 Such a band in the IR spectrum is expected for cyano groups in the environment of PB.26 This is further supported by both XPS, which confirms the presence of iron in the coating (ESI, Fig. S5†) and the magnetization hysteresis loop taken at 2 K (Fig. 5). The magnetization curve shows also a ferromagnetic behaviour as expected for BP with a Curie temperature of 5.5 K as deduced from the thermal variation of the magnetization which is shown in the inset of Fig. 5.
![TEM (A) and HRTEM (B) micrographs of the plasma polymer coatings put in contact with a 5 mM [Ti(iv)Lac2OH2]2− containing solution for 1 h.](/image/article/2012/RA/c2ra21028b/c2ra21028b-f3.gif) | ||
| Fig. 3 TEM (A) and HRTEM (B) micrographs of the plasma polymer coatings put in contact with a 5 mM [Ti(IV)Lac2OH2]2− containing solution for 1 h. | ||
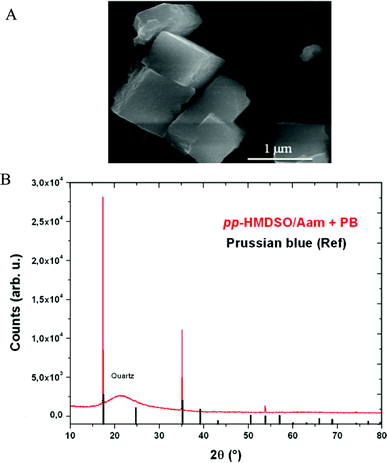 | ||
| Fig. 4 A: SEM micrograph of a HMDSO–Aam film put in contact with a 1 mM potassium hexacyanoferrate solution followed by a 10 mM FeCl3 solution. The film was rinsed with distilled water and blown dry with nitrogen before observation. B: X-Ray diffractogram of the same film. The broad background signal corresponds to the quartz substrate as indicated. | ||
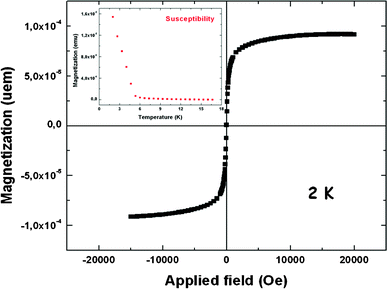 | ||
| Fig. 5 An example of the magnetization curve vs. the applied magnetic field of the material issued from the plasma polymer film in which PB has been subsequently synthesized as described in the main text. The inset is a magnetization versus temperature curve for the PB synthesized in the presence of the HMDMSO–Aam plasma film. | ||
It has to be noted that the film incubated in the hexacyanoferrate containing solution could be rinsed intensively with water without impeding the possibility of producing PB, meaning that the interactions of the film with hexacyanoferrate anions are pretty strong. These interactions are due to the presence of the amino groups from the poly(allylamine) polymer, indeed when a plasma polymer film made from HMDSO only (in the same conditions as for the blend) is put in the presence of hexacyanoferrate anions and Fe3+ cations, no observable coloration can be seen either by the naked eye or UV-visible spectroscopy.
An important question is to know whether the TiO2 and PB materials are only present in the uppermost part of the coatings or homogeneously distributed through the whole film thickness, even if it is not the major aim of the present article. To answer to this question, Rutherford backscattering spectroscopy has been performed on the sample containing TiO2 and BP (ESI, Fig. S6†). The spectra show, as expected, the contributions of carbon, nitrogen, oxygen (between 400 and 800 keV) and silicon. In addition, Ti and Fe RBS lines are clearly observed at 1450 and 1500 keV (ESI, inset of Fig. S6†), for the samples containing TiO2 and BP, respectively. The shape of these lines, compared with simulated patterns, strongly suggests that both Ti and Fe are almost homogeneously distributed across the entire thickness of the films. The simulation of these RBS lines gives rise to the following average concentration: 2% for Ti and 0.7% for Fe.
In forthcoming studies, we aim to investigate the possibility of simultaneously incorporating titanium oxides and PB into HMDMSO–Aam plasma films produced in similar conditions. Indeed, the combination of both materials in a common host matrix is of the highest interest for the design of photomagnetic composites.27
Conclusions
Coatings made from an equimolar mixture of allylamine and hexamethylsiloxane via atmospheric polymerization in dielectric barrier discharge conditions are sufficiently robust in an aqueous environment and contain enough amino functions to be able to activate the hydrolysis of [Ti(IV)Lac2OH2]2−anions and their subsequent polycondensation to yield titanium oxides, with poorly crystalline TiO2 being the predominant species. Similarly, the protonated amino groups of the HMDSO–Aam coatings are able to interact with Fe(CN)64− anions which allow the production of PB crystals after further exposure to a solution containing Fe3+ cations. The coatings containing titanium oxides and PB display the expected properties of those compounds. Rutherford backscattering experiments strongly suggest that iron and titanium are homogeneously distributed through the entire thickness of the coatings. In forthcoming investigations we will investigate the possibility of simultaneously producing titanium oxides and PB in plasma coatings which are easy to produce and are stable in water. The close proximity of TiO2 and PB crystals could allow for the production of photomagnetic thin coatings.Experimental
Synthesis of nanocomposite plasma polymer coatings
The plasma polymer template films were prepared from a blend of hexamethydisiloxane (HMDSO) and allylamine (Aam) (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 wt%) precursors purchased from Sigma Aldrich and used without further modification . The precursor was atomized in a TSI 3076 device at a flow rate of 1.7 slm (standard liter per minute). Nitrogen plasma gas was used with a gas flow of 30 slm. The gas mixture containing the precursor aerosol was injected in the form of nano-droplets into the open air plasma chamber through a slit between the two top electrodes. During this work, a semi-dynamic dielectric barrier discharge (DBD) reactor was used at atmospheric pressure, as described completely in a previous paper.28 The DBD discharge was generated between an earthed bottom aluminium plate and two high-voltage aluminium top plates with a surface area of 300 cm2. The gap between the bottom and top electrodes was set at 2 mm. The silicon wafers (Siltronics, Archamps, France) and glass substrates were positioned at the bottom electrode. These substrates have been previously cleaned in a piranha solution (4
50 wt%) precursors purchased from Sigma Aldrich and used without further modification . The precursor was atomized in a TSI 3076 device at a flow rate of 1.7 slm (standard liter per minute). Nitrogen plasma gas was used with a gas flow of 30 slm. The gas mixture containing the precursor aerosol was injected in the form of nano-droplets into the open air plasma chamber through a slit between the two top electrodes. During this work, a semi-dynamic dielectric barrier discharge (DBD) reactor was used at atmospheric pressure, as described completely in a previous paper.28 The DBD discharge was generated between an earthed bottom aluminium plate and two high-voltage aluminium top plates with a surface area of 300 cm2. The gap between the bottom and top electrodes was set at 2 mm. The silicon wafers (Siltronics, Archamps, France) and glass substrates were positioned at the bottom electrode. These substrates have been previously cleaned in a piranha solution (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2SO4/30%H2O2) for 10 min then rinsed with MilliQ water (Millipore Simplicity system, ρ = 18 MΩ cm). Experiments were performed in a dynamic mode in which the top electrode block moved alternatively over the sample at a constant speed of 4 m min−1. For each sample, the plasma coating was obtained after several passes of the top electrode above the sample. For the coating process, the plasma discharges were generated by an AC power supply. The frequency was set to 6 kHz and the voltage was a sinusoidal function of time. The amplitude of the potential difference was 24 kV, corresponding to a power density over the electrodes of 1.3 W cm−2, which can be assimilated to soft plasma conditions. The deposition rate was evaluated at 35 nm min−1. After deposition, the plasma polymerized aminosiloxane coatings were directly used for the preparation of composite films containing either TiO2, PB, or a mixture thereof.
1 H2SO4/30%H2O2) for 10 min then rinsed with MilliQ water (Millipore Simplicity system, ρ = 18 MΩ cm). Experiments were performed in a dynamic mode in which the top electrode block moved alternatively over the sample at a constant speed of 4 m min−1. For each sample, the plasma coating was obtained after several passes of the top electrode above the sample. For the coating process, the plasma discharges were generated by an AC power supply. The frequency was set to 6 kHz and the voltage was a sinusoidal function of time. The amplitude of the potential difference was 24 kV, corresponding to a power density over the electrodes of 1.3 W cm−2, which can be assimilated to soft plasma conditions. The deposition rate was evaluated at 35 nm min−1. After deposition, the plasma polymerized aminosiloxane coatings were directly used for the preparation of composite films containing either TiO2, PB, or a mixture thereof.
For the preparation of the TiO2 nanocomposite film, a titanium(IV) bis(ammonium lactate) dihydroxide (ref. 388165 Sigma Aldrich) solution at 5 mM was prepared in the presence of a Tris (Tris(hydroxymethyl)aminomethane from Gibco BRL) buffer solution at 50 mM whose pH was adjusted to 7.5 with hydrochloric acid. Plasma polymer samples were exposed to this solution for a duration ranging from 5 min to 24 h and then rinsed in distilled water.
For the synthesis of PB, the plasma polymer films were immersed in Potassium hexacyanoferrate (II) trihydrate (C6FeK4N6·3H2O, ref. 9387 Sigma Aldrich) at 1 mM in the 50 mM Tris buffer solution at pH 7.5. Afterwards, the film was immersed in an iron(III) chloride (FeCl3 purchased from Sigma Aldrich) solution at 10 mM and pH 7.5. After 1 h, the films were removed, rinsed with distilled water and dried with a nitrogen stream at ambient temperature.
Characterization methods
FT-IR spectroscopy measurements have been used to evaluate the chemical composition of the coatings with a Bruker Optics Tensor 27 spectrometer. Films have been analyzed in transmission mode at a resolution of 4 cm−1 accumulating 56 scans between 400 and 4000 cm−1.The nanocomposite plasma polymer films deposited on quartz substrates were analyzed in the transmission mode with a Perkin Elmer Lambda 35 UV-Visible spectrophotometer. The spectra were acquired between 200 and 700 nm with a spectral resolution of 2 nm.
The crystalline structure of the particles synthesized in the plasma polymer films was investigated by means of X-ray diffraction using a Siemens D5000 apparatus with a monochromatic Cu-Kα1 (λ = 1.54056 Å) radiation. The X-ray diffraction peaks were indexed using the JCPDS files 00-052-1907 for PB and 00-002-0406 for TiO2, respectively.
Further structural information was obtained by TEM using a TOPCON 002B microscope equipped with a LaB6 gun and operating at 200 kV. The resolution by point is 1.8 Å.
The distribution of the chemical elements (C, N, O, Si, Ti, Fe) along the growth direction was investigated by Rutherford backscattering spectroscopy (RBS). The RBS technique is based on elastic scattering collisions between high energy (2 MeV) He ions and the stationary atoms located in the sample. This technique allowed us to investigate the atomic distribution (i.e. the stochiometry) as a function of depth in the film.
The magnetic properties of the PB containing films were investigated using a superconducting quantum interference device (SQUID) with the external magnetic field applied along the film plane. The magnetization hysteresis loop was taken at 2 K and the thermal variation of the magnetization was obtained under a magnetic field of 5 × 10−3 T.
The morphology of the plasma polymer coatings was evaluated with a quanta FEG environmental scanning electron microscope (ESEM) from FEI.
XPS analysis was carried out (Hemispherical Energy Analyzer SPECS, PHOIBOS 150) using monochromatic Al Kα radiation operating at 200 W with an anode voltage of 16 kV. The pressure in the analysis chamber was set at 10−9 mbar. The XPS spectra were referenced with respect to the C1s peak at 284.6 eV originating from carbon contamination. Core peaks were analyzed using a nonlinear Shirley-type background and fitted using 70% Gaussian and 30% Lorentzian lineshapes.
Static contact angles were measured using a Dataphysics instrument. Wettability was evaluated by the water sessile drop method at room temperature and constant relative humidity. Contact angle values correspond to averages over four measurements. The superhydrophilicity measurements were made after illumination thanks to a pulsed Nd:YAG laser (Surelite Continuum) fixed at 266 nm—a wavelength shorter than the band edge of TiO2 (387.5 nm).
Zeta potential measurements were made by measuring the streaming potential of plasma polymer coatings deposited on two identical and cleaned glass slides. The measurements were made with a Zeta Cad device (CAD Instrumentation, Les Essarts le Roi, France) in the presence of 5 mM NaCl electrolyte whose pH was adjusted with concentrated HCl and NaOH solutions. Each measurement at a given pH value was separated from the next one at a higher pH value by intensive rinsing with NaCl solution at the desired pH value. The zeta potential was calculated from the measured streaming potential using the Smoluchowski relationship.29
Acknowledgements
This work was financially supported by the Fonds National de la Recherche (FNR) of Luxembourg. We also thank the Collège Doctoral Européen of Strasbourg for partial support of our work. Christian Ringwald (INSERM UMR 977) is acknowledged for the streaming potential measurements.References
- T. V. Duncan, J. Colloid Interface Sci., 2011, 363, 1–24 CrossRef CAS.
- I. Tokarev, M. Motornov and S. Minko, J. Mater. Chem., 2009, 19, 6932–6948 RSC.
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Muller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef.
- A. Walther, I. Bjurhager, J.-M. Malho, J. Pere, J. Ruokolainen, L. A. Berglund and O. Ikkala, Nano Lett., 2010, 10, 2742 CrossRef CAS.
- S. Srivastava and N. A. Kotov, Acc. Chem. Res., 2008, 41, 1831–1841 CrossRef CAS.
- S. Srivastava, V. Ball, P. Podsiadlo, J. Lee, P. Ho and N. A. Kotov, J. Am. Chem. Soc., 2008, 130, 3748–3749 CrossRef CAS.
- S. Joly, R. Kane, L. Radzilowski, T. Wang, A. Wu, R. E. Cohen, E. L. Thomas and M. F. Rubner, Langmuir, 2000, 16, 1354–1359 CrossRef CAS.
- N. Laugel, F. Boulmedais, A. E. El Haitami, P. Rabu, G. Rogez, J.-C. Voegel, P. Schaaf and V. Ball, Langmuir, 2009, 25, 14030–14036 CrossRef CAS.
- R. Zouari, M. Michel, J. Di Martino and V. Ball, Mater. Sci. Eng., C, 2010, 30, 1291–1297 CrossRef CAS.
- S. Chang, Z. A. Combs, M. K. Gupta, R. Davis and V. V. Tsukruk, ACS Appl. Mater. Interfaces, 2011, 2, 3333–3339 Search PubMed.
- T. C. Wang, M. F. Rubner and R. E. Cohen, Langmuir, 2002, 18, 3370–3375 CrossRef CAS.
- P. A. Premkumar, S. A. Starostin, M. Creatore, H. de Vries, R. M. J. Paffen, P. M. Koenraad and M. C. M. van de Sanden, Plasma Processes Polym., 2010, 7, 635–639 CrossRef CAS.
- J. Petersen, R. Bechara, J. Bardon, T. Fouquet, F. Ziarelli, L. Daheron, V. Ball, V. Toniazzo, M. Michel, A. Dinia and D. Ruch, Plasma Processes Polym., 2011, 8, 895–903 CrossRef CAS.
- P. Heyse, A. Van Hoeck, M. B. J. Roeffaers, J.-P. Raffin, A. Steinbüchel, T. Stöveken, J. Lammertyn, P. Verboven, P. A. Jacobs, J. Hofkens, S. Paulussen and B. F. Sels, Plasma Processes Polym., 2011, 8, 965–974 CrossRef CAS.
- N. D. Boscher, P. Choquet, D. Duday, N. Kerbellec, J. C. Lambrechts and R. Maurau, J. Mater. Chem., 2011, 21, 18959–18961 RSC.
- J. Bardon, J. Bour, D. Del Frari, C. Arnoult and D. Ruch, Plasma Processes Polym., 2009, 6, 5655–5659 CrossRef.
- M. Verdaguer, G. Girolami, Magnetic Prussian Blue Analogs, in Magnetism: Molecules to Materials, eds. J. S. Miller and M. Drillon, Wiley: Weinheim, 2005, Vol. V, pp 283–346 Search PubMed.
- J. Petersen, T. Fouquet, M. Michel, V. Toniazzo, A. Dinia, D. Ruch and J. A. S. Bomfim, ACS Appl. Mater. Interfaces, 2012, 4, 1072–1079 CAS.
- T. Mizutani, H. Nagase, N. Fujiwara and H. Ogoshi, Bull. Chem. Soc. Jpn., 1998, 71, 2017–2022 CrossRef CAS.
- T. Coradin, O. Durupthy and J. Livage, Langmuir, 2002, 18, 2331–2336 CrossRef CAS.
- J. L. Sumerel, W. Yang, D. Kisailus, J. C. Weaver, J. H. Choi and D. E. Morse, Chem. Mater., 2003, 15, 4804–4809 CrossRef CAS.
- K. D. Anderson, K. Marczewski, S. Singamaneni, J. M. Slocik, R. Jakubiak, R. R. Naik, T. J. Bunning and V. V. Tsukruk, ACS Appl. Mater. Interfaces, 2010, 2, 2269–2281 CAS.
- M. Thomas, M. von Hausen, C. P. Klages and P. Baumhof, Plasma Processes Polym., 2007, 4, 475–481 CrossRef.
- N. Laugel, J. Hemmerlé, Y. Arntz, E. Gonthier, N. Ladhari, Y. Haikel, J.-C. Voegel, P. Schaaf and V. Ball, J. Colloid Interface Sci., 2008, 324, 127–133 CrossRef CAS.
- M. Grätzel, J. Photochem. Photobiol., A, 2004, 164, 3–14 CrossRef.
- A. Jaiswal, J. Collins, B. Agricole and S. Ravaine, J. Colloid Interface Sci., 2003, 261, 330–335 CrossRef CAS.
- T. Yamamoto, N. Saso, Y. Umemura and Y. Einaga, J. Am. Chem. Soc., 2009, 131, 13196–13197 CrossRef CAS.
- J. Bour, L. Charles, J. Petersen, M. Michel, J. Bardon and D. Ruch, Plasma Processes Polym., 2010, 7, 687–694 CrossRef CAS.
- R.J. Hunter, Zeta Potential in Colloid Sciences: Principles and Applications, Academic Press, London, 1981, p. 66 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: Cross-sectional SEM analysis of a plasma polymer coating, Zeta potential titration of the glass wafer and of the glass wafer coated with a plasma polymer film made from an HMDMSO-Aam blend, SEM image of an inorganic cluster grown on the surface of a plasma polymer film deposited from a mixture of HMDMSO and Aam after exposure to a solution containing 5 mM of [Ti(IV)Lac2OH2]2− anions, SEM micrograph of a plasma polymer film made from a blend of HMDMSO and Aam and subsequently put in contact with 1 mM Fe(CN)64− and 10 mM Fe3+ containing solution, Fe2p region of the XPS spectrum of a HMDMSO-Aam plasma coating put in the successive presence of 1 mM potassium hexacyanoferaate and 10 mM iron(III) chloride, RBS spectra of a HMDMSO–Aam plasma coating after in situ formation of TiO2 and BP. See DOI: 10.1039/c2ra21028b |
| This journal is © The Royal Society of Chemistry 2012 |
