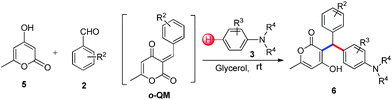
A catalyst-free C–H hydroarylation of coumarin derived ortho-quinone methide (o-QM) with electron rich arenes in glycerol†
Atul
Kumar
*,
Mukesh
Kumar
,
Maneesh Kumar
Gupta
and
Lalit Prakash
Gupta
Medicinal and Process Chemistry Division, Central Drug Research Institute, Lucknow, CSIR, Lucknow India. E-mail: dratulsax@gmail.com; Fax: 91-522-26234051; Tel: 91-522-2612411
First published on 31st July 2012
Abstract
An efficient, catalyst-free approach for C–H hydroarylation of in situ generated ortho-quinone methide (o-QM) with electron rich arenes (tertiary aryl amines) in glycerol has been developed. Several C-3-benzylated 4-hydroxycoumarin and 4-hydroxypyrone have been synthesized in good to excellent yields under mild conditions.
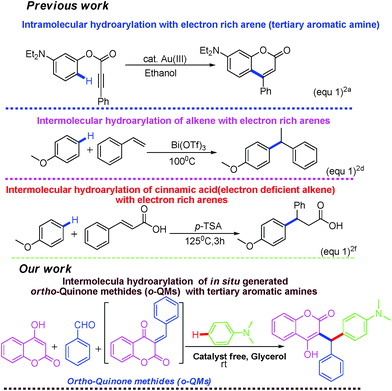
Intermolecular hydroarylation of alkenes is an efficient, atom-economic and powerful approach for the construction of new C–C bonds.1 Among the various techniques, hydroarylation of alkenes constitutes a useful tool for the functionalization of arenes. In recent years, several transition-metal and Lewis acid catalysts have been used for the intermolecular hydroarylation of alkenes in different organic solvents (Fig. 1).2 Such reactions have many disadvantages such as the need for an expensive metal-catalyst, extreme reaction conditions, e.g. high temperature and strong acid, poor regioselectivity and the use of organic solvents. As a result of this, huge amounts of waste products are generated, which are harmful to the environment. Therefore, there is urgent need for the development of catalyst-free protocols for the intermolecular hydroarylation of alkenes with electron rich arenes. Herein, we wish to report a catalyst-free and green protocol for the hydroarylation of in situ generated o-quinone methides with electron rich arenes in glycerol at room temperature.
Quinone methides are short-lived, highly reactive intermediates3 used for the synthesis of complex natural products,4 modern materials, fine chemicals and pharmaceuticals.5 In particular, o-quinone methides (o-QMs) are versatile intermediates, which are mainly involved in 1,4 Michael type additions as well as aza-Michael reactions with various nucleophiles. Ortho-quinone methides also give Diels–Alder and hetero-Diels–Alder cycloaddition products.6o-Quinone methides especially derived from 4-hydroxycoumarin undergo [4 + 2] cycloaddition reaction with pentafulvenes to afford pyranocoumarin and pyranopyrone.7
To the best of our knowledge, the utilization of coumarin derived o-quinone methides in hydroarylation with electron rich arenes has not been reported.
Recently, glycerol has become an alternative green solvent, since it is easily available, cheap, non-toxic, non-flammable, biodegradable, highly polar, recyclable and environmentally friendly. Several organic reactions have been performed in glycerol such as Heck and Suzuki cross-couplings,8 ring closing metathesis of diolefins,9 Michael addition of thiophenol,10 ring-opening of styrine oxide with p-anisidine,11 and asymmetrical reductions.
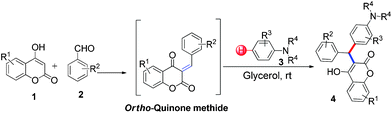
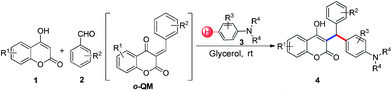
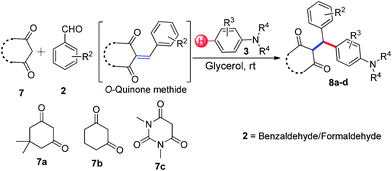
3-Alkylated-4-hydroxycoumarin and 4-hydroxy 2-pyrone are “privileged” oxygen containing heterocycles present in many natural products and bioactive compounds. These molecules exhibit promising biological properties such as anticoagulant, anti-HIV, analgesic, anti-fungal, anti-arthritis, anti-inflammatory, anti-pyretic, anti-bacterial, anti-viral, and anti-cancer capabilities.12 Considering the importance of 3-subsitituted 4-hydroxycoumarin and 4-hydroxy 2-pyrone in several biological process and in drugs, we became interested in developing a mild and efficient method for the synthesis of 3-subsitituted coumarin and pyrone that would be helpful in drug research. As part of our ongoing efforts devoted to the synthesis of biologically active heterocycles,13 herein we set out to explore a cost-effective, atom-economical and catalyst-free synthesis of 3-substituted coumarin and pyrone derivatives via the direct hydroarylation of in situ generated ortho-quinone methides with electron rich arenes in glycerol at room temperature (Scheme 1).
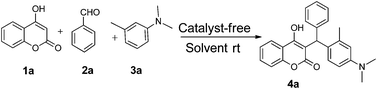
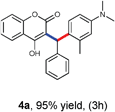
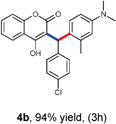
Our initial investigation focused on the model reaction of 4-hydroxycoumarin 1a, benzaldehyde 2a, and m-methyl N,N-dimethylaniline 3a in different solvents under catalyst-free conditions at room temperature (Table 1). When the model reaction was carried out in dichloromethane in the absence of catalysts at room temperature, unfortunately no product was detected (Table 1, entry 1). In the next experiment, we performed the same reaction in chloroform, toluene and tetrahydrofuran which also resulted in no products (Table 1, entries 2, 3 and 4). The desired product 4a was obtained in very trace amount using dioxane and acetonitrile as solvents at room temperature for 8 h (Table 1, entries 5 and 6). However, when the reaction was carried out in polar aprotic solvents like DMF and DMSO, it gave poor yields of 4a (Table 1, entries 7 and 8). No improvement was detected when the reaction was carried out in polar protic solvents like methanol and ethanol at room temperature (Table 1, entries 9 and 10). To our delight, the desired product 4a was isolated and obtained in 40% yield when the reaction was carried out in water at room temperature (Table 1, entry 12). However, no improvement in the yield of 4a was achieved when reaction was performed at reflux (Table 1, entry 13). In light of this result, we decided to perform the same reaction in glycerol as solvent. Fortunately we afforded excellent 95% yield of the desired product 4a at room temperature in 3 h (Table 1, entry 14).
|
|
||||
|---|---|---|---|---|
| Entry | Solvent | Condition | Time/h | Yield (%)b |
| a Reaction conditions: 4-hydroxycoumarin 1a (1 mmol), benzaldehyde 2a (1 mmol), m-methyl N,N-dimethylaniline 3a (1 mmol), in 3 mL of solvent. b Isolated yield. | ||||
| 1 | CH2Cl2 | rt | 8 | 0 |
| 2 | CHCl3 | rt | 8 | 0 |
| 3 | Toluene | rt | 8 | 0 |
| 4 | THF | rt | 8 | 0 |
| 5 | Dioxane | rt | 8 | Trace |
| 6 | CH3CN | rt | 8 | Trace |
| 7 | DMF | rt | 7 | < 6 |
| 8 | DMSO | rt | 7 | < 6 |
| 9 | MeOH | rt | 7 | 15 |
| 10 | EtOH | rt | 7 | 20 |
| 11 | EtOH | reflux | 6 | < 15 |
| 12 | H2O | rt | 5 | 40 |
| 13 | H2O | reflux | 4.3 | 42 |
| 14 | Glycerol | rt | 3 | 95 |
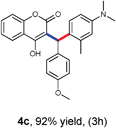
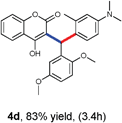
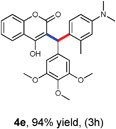
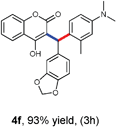
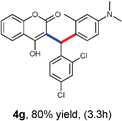
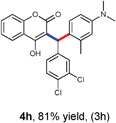
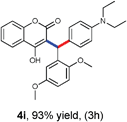
With optimized reaction conditions in hand, we explored the aldehyde substrate scope with 4-hydroxycoumarin and m-methyl N,N-dimethylaniline for C–H hydroarylation of in situ generated o-QM. The results are shown in Table 2.
|
|
||
|---|---|---|
| a Reaction conditions: 4-hydroxycoumarin 1a (1 mmol), benzaldehyde 2a (1 mmol) and tertiary aromatic amines 3a (1 mmol) in 3 mL glycerol at room temperature. b Isolated yield of purified products. | ||
|
|
|
|
|
|
|
|
|
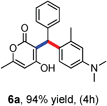
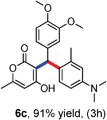
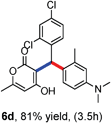
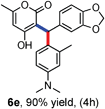
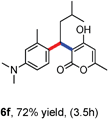
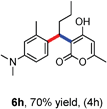
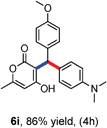
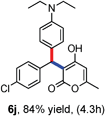
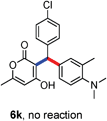
All the reactions proceeded very nicely and furnished the desired products under optimized reaction conditions. A wide range of aromatic aldehyde containing electron-neutral, electron-withdrawing and electron-donating groups could be employed as coupling partner with 4-hydroxycoumarin 1a (for o-QM) which were smoothly converted to the corresponding 3-hydroarylated coumarin with m-methyl N,N-dimethylaniline 3a in good to excellent yields (Table 2, entries 4a–4i). Furthermore, sterically demanding ortho-substituted aldehydes were well tolerated, giving the corresponding products in 83–81% yield (Table 2, entry 4d and 4g). Next we also examined the scope of tertiary aromatic arenes with aldehyde and 4-hydroxypyrone under the optimized conditions. The results were summarized in Table 3. When m-methyl N,N-dimethylaniline 3a was reacted with intermediate (o-QM) of 4-hydroxypyrone and arylaldehyde it afforded the desired products in good to moderate yields (Table 3, entry 6a–6h). Unsubstituted tertiary aromatic amines, like N,N-dimethylaniline and N,N-diethylaniline also reacted with benzaldehyde and 4-hydroxypyrone to afford suitable results (Table 3, entry 6i and 6j). The ortho-substituted N,N-dimethylaniline failed to react with o-QM (Table 3, entry 6k and 6l). The lack of reactivity of ortho-substituted N,N-dimethylaniline has been observed before.14
|
|
||
|---|---|---|
| a Reaction conditions: 4-hydroxypyrone 5a (1.0 mmol), benzaldehyde 2a (1.0 mmol) and tert-aryl amines 3a (1.0 mmol) in 3 mL glycerol at room temperature. b Isolated yields of purified products. | ||
|

|
|
|
|
|

|
|
|
|
|

|
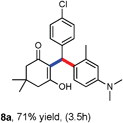
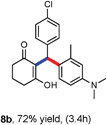
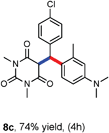
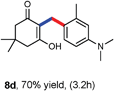
Various aliphatic aldehydes like isovaraldehyde, isobutyraldehyde and n-butyraldehyde coupled with 4-hydroxypyrone and tertiary aromatic amines, affording yields of 72, 74 and 70% for the corresponding products (Table 3, entries 6f, 6g and 6h). A variety of 1,3 diketo compounds were also used to explore the scope of reaction. In all the cases, the corresponding hydroarylated products were obtained in 70–74% yields (Table 4, entries 8a, 8b, 8c and 8d).
|
|
||
|---|---|---|
| a Reaction conditions: 1,3 diketone 7a–c (1.0 mmol), benzaldehyde/formaldehyde 2a (1.0 mmol) and tert-aryl amines 3a (1.0 mmol) in 3 mL glycerol at room temperature. b Isolated yields. | ||
|
|
|
|
||
Conclusion
In conclusion we have developed a catalyst-free and convenient green protocol of C-alkylation of 4-hydroxycoumarin and 4-hydroxypyrone via in situ generated o-quinone methides with electron rich arenes (tertiary aromatic amines) in glycerol at room temperature. A number of 3-substituted 4-hydroxycoumarin and 4-hydroxypyrone derivatives were obtained with good to excellent yields. This protocol has several valuable advantages, such as its simple procedure, mild reaction condition, avoidance of metal-catalyst, easily available low-cast starting materials and reusable solvents. These features make this protocol potentially useful for industrial applications.Acknowledgements
Authors (MK, MKG and LPG) are thankful to CSIR UGC New Delhi for the award of a senior research fellowship. We also gratefully acknowledge SAIF, CDRI for providing analytical facilities. The CDRI communication no. is 8296.References
- (a) S. Murai, F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda and N. Chatani, Nature, 1993, 366, 529 CrossRef CAS; (b) G. Dyker, Angew. Chem., Int. Ed., 1999, 38, 1698 CrossRef; (c) V. Ritleng, C. Sirlin and M. Pfeffer, Chem. Rev., 2002, 102, 17319 CrossRef.
- (a) J. H. Do, H. N. Kim, J. Yoon, J. S. Kim and H.-J. Kim, Org. Lett., 2010, 12, 932 CrossRef CAS; (b) M. Niggemann and N. Bisek, Chem.–Eur. J., 2010, 16, 11246 CrossRef CAS; (c) R. Jana and J. A. Tunge, Org. Lett., 2009, 11, 971 CrossRef CAS; (d) M. Rueping, B. J. Nachtshei and T. Scheidt, Org. Lett., 2006, 8, 3717 CrossRef CAS; (e) C.-M. Chu, W.-J. Huang, J.-T. Liu and G.-F. Yao, Tetrahedron Lett., 2007, 48, 6881 CrossRef CAS; (f) A. R. Jagdale and A. Sudalai, Tetrahedron Lett., 2007, 48, 4895 CrossRef CAS; (g) Z.-M. Sun, J. Zhang, R. S. Manan and P. Zhao, J. Am. Chem. Soc., 2010, 132, 6935 CrossRef CAS.
- For a comprehensive review on o-QM see: (a) S. E. Rokita, Quinone Methides Wiley Series of Reactive Intermediates in chemistry and biology; John Wiley & Sons, Inc. Hoboken, NJ., 2009; Vol. 1 Search PubMed.
- (a) P. E. Harrington, I. A. Stergiades, J. Erickson, A. Makriyannis and M. A. Tius, J. Org. Chem., 2000, 65, 6576 CrossRef CAS; (b) R. Rodriguez, et al. , Org. Lett., 2004, 6, 3617–3619 CrossRef CAS; (c) J. C. Green, S. Jiminez-Alonso, E. R. Brown and T. R. R. Pettus, Org. Lett., 2011, 13, 5500 CrossRef CAS.
- S. E. Wolkenberg and D. L. Boger, Chem. Rev., 2002, 102, 2477 CrossRef CAS.
- (a) A. E. Mattson and K. A. Scheidt, J. Am. Chem. Soc., 2007, 129, 4508 CrossRef CAS; (b) P. Wang, Y. Wang, W. Hu and X. Liang, Eur. J. Org. Chem., 2009, 13, 2055 CrossRef.
- V. Nair, C. N. Jayan, K.V. Radhakrishnan, G. Anilkumar and N. P. Rath, Tetrahedron, 2001, 57, 5807 CrossRef CAS.
- (a) A. Wolfson, C. Dlugy and Y. Shotland, Environ. Chem. Lett., 2007, 5, 67 CrossRef CAS; (b) A. Wolfson and C. Dlugy, Chem. Pap., 2007, 61, 228 CrossRef CAS.
- N. Bakhrou, F. Lamaty, J. Martinez and E. Colacino, Tetrahedron Lett., 2010, 51, 3935 CrossRef CAS.
- E.-J. Lenardao, D. O. Trecha, P. C. Ferreira, R. G. Jacob and G. Perin, J. Braz. Chem. Soc., 2009, 20, 93 CrossRef CAS.
- Y. Gu, J. Barrault and F. Jerome, Adv. Synth. Catal., 2008, 350, 2007 CrossRef CAS.
- (a) J. Kischel, K. Mertins, D. Michalik, A. Zapf and M. Beller, Adv. Synth. Catal., 2007, 349, 865 CrossRef CAS; (b) Y. S. Lee, Y. S. Lee, J. Y. Lee, S. N. Kim, C.-K. Lee, H. Park and R. A., Bioorg. Med. Chem. Lett., 2000, 10, 6225 Search PubMed; (c) G. Melagraki, et.al., Eur. J. Med. Chem., 2009, 44, 3020 CrossRef CAS; (d) S. Thaisrivongs, et.al, J. Med. Chem., 1996, 39, 2400 CrossRef CAS.
- (a) A. Kumar, G. Gupta and S. Srivastava, Org. Lett., 2011, 13, 6366 CrossRef CAS; (b) A. Kumar, V. D. Tripathi and P. Kumar, Green Chem., 2011, 13, 51 RSC; (c) A. Kumar, M. K. Gupta and M. Kumar, Green Chem., 2012, 14, 490 RSC.
- (a) B. B. P. Tice, I. Lee and F. H. Kendall, J. Am. Chem. Soc., 1963, 85, 329 CrossRef; (b) W. G.Brown, et al. , J. Am. Chem. Soc., 1939, 61, 2597 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21056h/ |
| This journal is © The Royal Society of Chemistry 2012 |