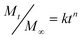DOI:
10.1039/C2RA21093B
(Paper)
RSC Adv., 2012,
2, 9555-9564
Synthesis and characterization of maleated cyclodextrin-grafted-silylated montmorillonite for the controlled release and colon specific delivery of tetracycline hydrochloride
Received
31st May 2012
, Accepted 25th July 2012
First published on 26th July 2012
Abstract
In order to exploit the potential of host–guest interaction of cyclodextrin molecules and the controlled release properties of sodium montmorillonite, we have developed a novel composite hydrogel (CH), namely maleated cyclodextrin-grafted-silylated montmorillonite (MACD-g-MPTMS/MMT) for colon specific tetracycline hydrochloride (TCH) drug delivery. The CH was characterized by using techniques such as FTIR, SEM and XRD. The effect of the pH on the encapsulation of TCH was studied and found to be maximum at pH 3.0. The study of the surface morphology of CH using SEM showed an irregular and rough surface morphology, which favors TCH drug loading. In TCH loaded CH, the shift of the FTIR bands of amide I and II in comparison to crystalline TCH suggested a strong interaction between the amide groups and the clay surfaces. The swelling behavior of CH was measured in different media and found to be a maximum at pH 7.4. The drug delivery results demonstrated that CH could successfully deliver TCH to the colon without losing the drug in the stomach, and could be a good candidate for an orally administered drug delivery system. Results showed that the prepared CH showed good biocompatibility in the range 10–75 μg ml−1, being also able to effectively stimulate cell proliferation.
1. Introduction
Targeted drug delivery into the colon is highly desirable for local treatment of a variety of bowel diseases such as ulcerative colitis, Crohn's disease, amebiosis, colonic cancer, local treatment of colonic pathologies, and systemic delivery of protein and peptide drugs.1 In fact, a selective release of bioactive substances in the colon allows not only a lowering of the dosage necessary to obtain the therapeutic effect, but also a reduction of the side effects that these drugs produce when released and absorbed by the upper gastrointestinal tract.2 Hydrogels are very attractive materials for application in colon specific drug delivery systems because of their hydrophilic character and potential to be biocompatible.3,4 An important parameter to consider in the utilization of a hydrogel as a drug delivery system is the degree of swelling. In the design of oral colon-specific drug delivery systems, pH-sensitive hydrogels have attracted increasing attention recently as useful tools for targeting colon-specific diseases in a stimuli-responsive manner based on pH changes in the human gastrointestinal tract.5 However, conventional hydrogels are weak and fragile because of the large proportion of water in the hydrogel and its randomly crosslinked network structure. Therefore, composite hydrogels (CH) have recently gained importance due to their enhanced mechanical properties.6,7 Clay mineral CH as known drug delivery systems show unique hybrid properties superior to the components and the capability to incorporate various drug substances.8,9 Montmorillonite (MMT) with net negatively charged layers has good swelling properties in the presence of water and, therefore, positively charged bioactive compounds can be intercalated into the interlayer spaces by electrostatic interaction under these conditions.10 Natural clay minerals are suitable to effectively modulate drug release. MMT itself has some attractive properties for oral application such as rheology, bioadhesion, chemical inertness and low or null toxicity etc., and these properties make MMT a potential future drug delivery carrier. In addition, it is highly mucoadhesive, which is a useful property for molecules to cross the gastrointestinal barrier.11 Bulky and organic molecules have a low possibility of approaching the interlayer reaction sites of MMT. So, modification of MMT is needed for increasing its adsorption capacity. Various types of modifications, for example incorporation of inorganic cationic species such as ammonium or phosphonium salts, or silylation reactions with silane coupling agents, have been done so far. Among these modifications, the silylation reaction has attracted great attention because the basal spacing of silylated clay is larger than for pure clay.12,13 This silylation reaction facilitates the penetration of bulky and polymeric chains inside the clay galleries.
β-Cyclodextrin (β-CD) based hydrogels are widely used in biomedical applications as drug delivery systems because they increase the aqueous solubility, stability and bioavailability of drugs. β-CDs are known to be rarely hydrolyzed and only a small fraction of them is absorbed in the passage through the stomach and small intestine. However, they are fermented into small saccharides by colonic microflora.14,15 Further, cyclodextrins are rapidly excreted in an intact form in the urine after intravenous administration: more than 95% of β-CDs were recovered from rat urine within 6 h.16 These biodegradable properties of β-CDs are useful for a colon-targeting carrier. In addition to all these properties, they can reduce gastrointestinal irritation, mitigate the unpleasant taste and smell of drugs, and prevent drug–drug or drug–additive interactions.17 However, β-CD has several adverse effects like nephrotoxicity and low aqueous solubility due to the relatively strong binding of the β-CD molecules in the crystal state.18 Therefore, modification of β-CD is of great importance. Maleated cyclodextrin (MACD) offers several advantages over the parent β-CD such as more water solubility, less toxicity and pH sensitivity.19,20 According to Mohamed et al.,21 the development of copolymer materials containing β-CD and suitable crosslinking agents will result in compounds that have interesting physicochemical properties, which will widen the range of application of native β-CD. In the present work, to obtain a new colon specific drug delivery system, we have combined the pH-responsiveness and enzymatic biodegradability of MACD in a single material.
Several antibiotics have been used for the treatment of colon related diseases. Some examples are vancomycin, ciprofloxacin, metronidazole, tetracycline hydrochloride etc. Among these, tetracycline hydrochloride is active against different types of bacterial diseases associated with the colon. Tetracycline has potent bacteriostatic activity and it affects anaerobic and facultative organisms, Gram positive and negative bacteria, and mycoplasm; but this antibiotic is poorly adsorbed by humans and animals.22 So, a controlled delivery of this drug is needed for its long bioavailability. Several attempts have been made to study the controlled release of tetracycline using various drug delivery systems.23,24 Several studies have demonstrated that tetracycline antibiotic strongly interacts with clay minerals and cyclodextrin molecules.25,26
Controlled release of tetracycline was performed with various drug delivery systems such as bioactive glassy compounds, auto-catalyzed poly(ortho esters), supramolecular gels based on amphiphilic 3,4,5-trihydroxybenzoic derivatives, semi-IPN hydrogels based on polyvinyl alcohol and poly(acrylamide-co-styrene).27 Compared with these existing controlled drug delivery systems, MACD-g-MPTMS/MMT composite hydrogel has some advantages as follows: various classes of compounds, including MMT and β-CD derivatives, are known to serve as host compounds having several types of inclusion spaces, such as three-dimensional cages, single or parallel channels and layers, which can accommodate therapeutic compounds between their layers and form a variety of intercalated adducts. The present drug delivery system consists of both clay and MACD. Compared to β-CD, modified MACD shows unique swelling properties in weak alkaline conditions due to the higher –COOH group content.32 It is known that some degradation reactions of TCH can take place in the stomach which leads to severe side-effects, but using a β-CD derivative as a carrier for TCH increases the stability.28 Earlier investigations in this direction also suggest that a synergistic effect exists between TCH and β-CD, whereby the efficacy of TCH is increased substantially through the bio-adhesive characteristics of β-CD. Moreover, the unique properties of the polymer layered silicate composites such as easy degradation, biocompatibility and tunable mechanical properties are essential for pharmaceutical applications.29 Considering all these premises, in the present work, we thought it worthy of interest to develop and investigate the feasibility of a new drug delivery system for TCH administration based on a combined strategy which exploits both MACD complexation, to enhance drug stability/absorption, pH-sensitivity, drug solubility and dissolution properties, and the intercalating properties of MMT, to improve controlled drug delivery. To the best of our knowledge, there is no report in the accessible literature regarding the use of this novel composite hydrogel by exploiting the advantages of both the clay and MACD simultaneously in a single drug delivery system, for the delivery of TCH into the colon.
2. Materials and methods
2.1. Materials
The swelling clay MMT used to conduct this study was purchased from Fluka. 3-Methyl acryloxypropyl trimethoxysilane (MPTMS) with 98% purity, ethyleneglycoldimethacrylate (EGDMA), α,α-azobisisobutyronitrile (AIBN) and β-CD were obtained from Sigma-Aldrich, USA and used as received. Analytical grade maleic anhydride was purchased from SRL, Mumbai. The pH was adjusted by using phosphate and citrate buffer solutions. Distilled water with specific conductivity less than 1 μΩ cm−1 was used throughout the study. All other reagents used in this study were of analytical grade.
2.2. Methods
2.2.1. Preparation of Na-montmorillonite (Na-MMT).
The preparation of Na-MMT was achieved according to the procedure reported in our earlier work.30
2.2.2. Preparation of 3-methylacryloxypropyltrimethoxy silylated montmorillonite (MPTMS/MMT).
About 5.0 g of Na-MMT and 3.6 g of 3-methylacryloxypropyltrimethoxysilane were dispersed in 100 mL of ethanol separately and were stirred (60 °C, 30 min) in a N2 atmosphere.31 The resulting Na-MMT dispersion and silane were mixed and stirred for about 18 h. Thus, the grafting reaction was carried out. After cooling, the reaction product was recovered by centrifugation and sterilized (100 °C, 5 h) in a vacuum. Then the product was rinsed with distilled water to remove the excess of ethanol. The product was dried at 80 °C under vacuum and grained to powder. The reaction scheme is shown in Scheme 1.
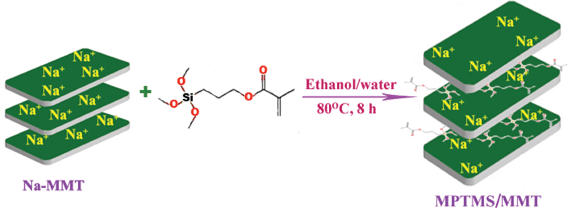 |
| | Scheme 1 Proposed reaction scheme for the preparation of MPTMS/MMT. | |
2.2.3. Preparation of maleated cyclodextrin (MACD).
The procedure (shown in Scheme 2) for preparation of MACD was in accordance with earlier reported work.32 Briefly, 4.90 g of maleic anhydride (0.05 M) were added to a solution of β-CD (5.682 g) in 30 mL DMF . The mixture was stirred well (80 °C, 10 h). Then the solution was allowed to cool and poured into chloroform (30 mL). The white precipitate obtained was filtered, washed with acetone and dried in air. The amount of final product was found to be 6.02 g (73.98% yield). So, the amount of maleic anhydride incorporated in MACD was calculated and found to be 0.2866 g g−1. Elemental analysis of the sample was also performed and the following results were obtained: specifically, for a molecular structure of C42H65O35[OCOCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOH]5, the amount of C and H obtained from instrumental analysis was C: 43.24% and H: 4.98% and the corresponding amount obtained from theoretical calculation was C: 43.54% and H: 5.01%.
CHCOOH]5, the amount of C and H obtained from instrumental analysis was C: 43.24% and H: 4.98% and the corresponding amount obtained from theoretical calculation was C: 43.54% and H: 5.01%.
 |
| | Scheme 2 Proposed reaction scheme for the preparation of MACD. | |
2.2.4. Preparation of MACD-grafted-silylated montmorillonite (MACD-g-MPTMS/MMT).
This was achieved by an intercalation method as follows. In a three-necked round bottomed flask equipped with a magnetic stirrer and a reflux condenser, MMT/MPTMS (2.5 g), MACD (5.0 g), EGDMA (0.5 g) and AIBN (0.02 g) were placed in 25 mL of methanol. The flask was purged by dry oxygen-free nitrogen for 15 min. The mixture was heated while being magnetically stirred at 70 °C for 4 h. The product was separated by filtration and washed well with distilled water and methanol to remove the untreated chemicals. The product (MACD-g-MPTMS/MMT) was then dried overnight and ground to a particle size in the range of 40–80 mesh. The reaction scheme is shown in Scheme 3.
 |
| | Scheme 3 Proposed reaction scheme for the preparation of MACD-g- MPTMS/MMT. | |
2.3. Instruments for characterization
SEM images of the material synthesized and TCH loaded material were taken with a JEOL JSM 6390 LA scanning electron microscope. FTIR spectra of materials were recorded between 4000–200 cm−1 using a Perkin-Elmer FTIR spectrophotometer. XRD analysis of the samples was done on Rigaku Dmax IC model (Japan). Elemental analysis was performed on a Carlo Erba 1106 vario EL Elementar. All pH measurements were made on a microprocessor Systronic pH meter (model 361). A temperature controlled water bath shaker (Lab line shaking incubator) with a temperature variation of ±1 °C was used for equilibrium studies. The absorbance measurements of TCH solutions were performed on a JASCO-530 UV-Visible Spectrophotometer at λmax = 276 nm (37 °C), using distilled water as reference. The equilibrium concentrations of TCH solutions were determined by means of pre-calibrated scales.
2.4. Measurements of equilibrium swelling capacity and swelling kinetics
0.05 g of sample were immersed in an excess of distilled water at room temperature for 4 h to achieve swelling equilibrium. The swollen composite hydrogels were then separated from the solution using a 100-mesh screen. After weighing the swollen composite hydrogels, the equilibrium swelling capacities (Qeq, g g−1) of the composite hydrogels were derived from the mass change before and after swelling, and calculated using the following equation (eqn (1)):| |  | (1) |
where Wd and Ws are the weights of the dry sample and the swollen composite hydrogel sample, respectively. Qeq was calculated as grams of water per gram of sample.
For swelling kinetics, at consecutive time intervals, the water absorption of the hydrogel was measured according to the above mentioned method. To determine the pH-sensitivity of the hydrogel, the swelling was measured in a range of pH buffer solutions instead of distilled water. Aqueous stock NaOH (pH 7.4) and HCl (pH 2.4) solutions were diluted with distilled water to reach the desired basic and acidic pHs, respectively.
2.5. Effect of pH on encapsulation efficiency (EE)
The optimum pH for the best encapsulation of TCH onto CH was investigated as follows. The TCH loading was carried out by dispersing 100 mg of MACD-g-MPTMS/MMT in aqueous TCH solution (drug concentration = 0.1 mg mL−1) containing 50 mL buffer (either 0.2 M acetate buffer at pH 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, or 0.2 M phosphate buffer at pH 6.0, 6.5, 7.0, 7.5 and 8.0) and stirred for 12 h. Then the TCH loaded MACD-g-MPTMS/MMT particles were centrifuged and washed with deionized water to remove the loosely attached TCH on the surface of CH. The supernatant was collected and stored at 4 °C, then subjected to UV analysis at λmax = 276 nm. The encapsulation efficiency was calculated using eqn (2). Each experiment was carried out in independent triplicate and the mean value was considered.| |  | (2) |
2.6.
In vitro drug release study
2.6.1. Drug loading.
0.1 g of powdered MACD-g-MPTMS/MMT were added in 100 mL of TCH drug solution (1.2 × 10−4 mol L−1) and stored in the dark for 2 days to complete drug loading. The amount of loaded drug was determined at specific time intervals by UV spectroscopy at 276 nm using a calibration curve constructed from a series of drug solutions with different known concentrations.
2.6.2. In vitro drug release and kinetic study.
To measure the release performances of TCH from MACD-g-MPTMS/MMT, 0.1 g of TCH loaded MACD-g-MPTMS/MMT were added to 250 mL of phosphate and citrate buffer solutions (pH 2.4. and pH 7.4, respectively) and were stirred at 37 °C. At specified time intervals, 5 mL of solution were removed and filtered through a 0.2 μm syringe filter. The amount of released TCH was determined by UV spectroscopy at 276 nm using a calibration curve constructed from a series of TCH solutions with standard concentrations. The tests were run in triplicates and the mean percentage cumulative of drug release was calculated (± standard deviation). The release profiles were plotted as the relative release percentages of TCH against time.
2.7.
In vitro biocompatibility test
The cytotoxicity of MACD-g-MPTMS/MMT, TCH-loaded-MACD-g-MPTMS/MMT and TCH was evaluated in L929 mouse fibroblast cells. Fibroblast cells are widely used in cytotoxicity studies of biomaterials, as they are the main cellular components of connective tissues. Cells were cultured in 96-well culture plates at a density of 1 × 104 cells per well in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum at 37 °C in a humidified environment of 5% CO2 for 24 h. The cells were then treated with 10, 25, 50, 75 and 100 μg mL−1 of MACD-g-MPTMS/MMT, TCH-loaded-MACD-g-MPTMS/MMT and TCH separately, extracted with the culture medium and incubated for another 24 h at 37 °C in the humidified environment of 5% CO2. The well with no sample was the control. After 24 h, the wells in one plate were replenished with 100 μL DMEM containing 10% fetal bovine serum. Then 50 μL of 1 mg mL−1 MTT (3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution were added to each well and incubated for another 2 h. The supernatant was carefully removed by centrifugation for 10 min, and 200 μL of DMSO were added in each well. The absorbance of the solution was measured using a microplate reader at 570 nm to determine the optical density (OD570) value. In order to investigate the time-dependent biocompatibility of MACD-g-MPTMS/MMT, TCH-loaded-MACD-g-MPTMS/MMT and TCH, a separate biocompatibility study was also performed using L929 cells with 50 μg mL−1 of MACD-g-MPTMS/MMT, TCH-loaded-MACD-g-MPTMS/MMT and TCH respectively. The cell viability was calculated as follows:| | 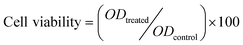 | (3) |
where ODtreated was obtained for the cells treated by the MACD-g-MPTMS/MMT, TCH-loaded-MACD-g-MPTMS/MMT and TCH after a 12, 24, 36, and 48 h incubation. ODcontrol was obtained for the cells untreated by the sample, and the other culture conditions were the same. The data were given as mean ± standard deviation (SD) based on three independent measurements.
3. Results and discussion
MACD-g-MPTMS/MMT composite hydrogel for the adsorption of TCH was synthesized by the grafting of MACD to MPTMS/MMT in the presence of EGDMA crosslinker. The functionalization of MMT by silylation occurs in two steps – hydrolysis of the silane molecule followed by silanol condensation. The hydroxyl groups present on the edges and the interlayer of MMT can act as active sites for the silylation process. The MPTMS is grafted to the interlayer of MMT by removing the adsorbed water from the interlayer. The presence of silane in the interlayer of the Na-MMT results in a prominent increase of the basal spacing of the clay.33,34 Na-MMT can accommodate TCH molecules in its interlayer through ion exchange with Na+ ions or it can accommodate TCH molecules on the surface through electrostatic attraction.35 According to De Sousa et al.,26 TCH molecules can form multiequilibria with two molecules of β-CD. One β-CD molecule interacts with the aromatic region and the second molecule of β-CD interacts with the opposite extreme of the TCH molecule. MACD contains an active carboxyl functional group, which facilitates encapsulation of the water soluble TCH molecule.
3.1. Characterization studies
Fig. 1a, 1b and 1c depict the SEM photographs of Na-MMT, MPTMS/MMT, β-CD, MACD, MACD-g-MPTMS/MMT and TCH loaded MACD-g-MPTMS/MMT taken at ×1500 magnification. The SEM image of Na-MMT indicates that its surface morphology has an aggregated and foliated structure. Compared to Na-MMT, the surface of MPTMS/MMT appeared as an individual aggregate of particles with modified Na-MMT.36 The aggregation phenomenon is driven by the nature of the inorganic particles, which consist of polar lamellae (from pristine MMT) and hydrophobic units (from the silylating agent on the Na-MMT edges) with different polarity and reactivity. The SEM image of β-CD shows an irregularly shaped crystal-like structure37 while the surface of MACD indicates that particles are very small, and these particles possess a compact and homogeneous structure. In the case of MACD-g-MPTMS/MMT, the surface morphology itself indicates that it is a combination of MPTMS/MMT and MACD with a rugged surface. The irregular and rough morphology of CH facilitates TCH drug loading. Compared with MACD-g-MPTMS/MMT, TCH-loaded-MACD-g-MPTMS/MMT shows a smooth surface and the entire rugged surface was saturated with TCH; which clearly indicates an extensive encapsulation of TCH into MACD-g-MPTMS/MMT has taken place.
 |
| | Fig. 1 1a. SEM images of Na-MMT and MPTMS/MMT; 1b. SEM images β-CD and MACD; 1c. SEM images of MACD-g-MPTMS/MMT and TCH-loaded-MACD-g-MPTMS/MMT. | |
The FTIR spectra of Na-MMT, β-CD, MACD, MPTMS/MMT, MACD-g-MPTMS/MMT and TCH loaded MACD-g-MPTMS/MMT are given in Fig. 2. In the case of Na-MMT, the intense peak at 3600 cm−1 was attributed to the stretching of the hydroxyl group bonded to Al and Mg in Al–Mg(OH) or Al–Al(OH)38,39 and a bending vibration of Al–Al(OH) at 920 cm−1 was also identified. The band at 1033 cm−1 is characteristic of the Si–O stretching absorption in MMT.40 After silylation, the clay mineral shows an absorption band between 3050–2700 cm−1 due to the vibrations of the –CH bonds introduced by the organic modifier, indicating that the silane group is not only covalently bonded to the clay mineral, but also intercalated in the hydrolyzed form, interacting with the water molecules present in the clay mineral through hydrogen bonding. Vibration frequency at 1030–1082 cm−1 was observed in both the spectra of β-CD and MACD, which is characteristic of a C–O–C vibration.41 It could be seen that an intense absorption band at 1727 cm−1 appeared in the spectrum of MACD but was absent in β-CD, which was due to the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching vibration of the ester groups and carboxylic acid groups in MACD. Actually, the band at 1628 cm−1 in the β-CD spectrum was due to the first overtone of O–H bending, which was overlapped by the band due to the C
O) stretching vibration of the ester groups and carboxylic acid groups in MACD. Actually, the band at 1628 cm−1 in the β-CD spectrum was due to the first overtone of O–H bending, which was overlapped by the band due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration at 1644 cm−1 in MACD. Besides, another new band at 1216 cm−1, corresponding to the stretching vibration of C(O)–O in C
C stretching vibration at 1644 cm−1 in MACD. Besides, another new band at 1216 cm−1, corresponding to the stretching vibration of C(O)–O in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C(O)–O, appeared in MACD but was absent in β-CD. All these indicated that the esterification reaction successfully occurred between β-CD and MACD.42 The decrease in the intensity of the O–H band at 3400 cm−1 in MACD is due to deprotonation of some of the hydroxyl groups suggesting a covalent bonding between maleic anhydride and β-CD.43 In the FTIR spectrum of MPTMS/MMT, absorption bands appeared at 3010 and 3053 cm−1 that can be attributed to the stretching of the vinylic C–H bonds.44 Compared to the FTIR spectrum of MPTMS/MMT, the peaks corresponding to a C
C–C(O)–O, appeared in MACD but was absent in β-CD. All these indicated that the esterification reaction successfully occurred between β-CD and MACD.42 The decrease in the intensity of the O–H band at 3400 cm−1 in MACD is due to deprotonation of some of the hydroxyl groups suggesting a covalent bonding between maleic anhydride and β-CD.43 In the FTIR spectrum of MPTMS/MMT, absorption bands appeared at 3010 and 3053 cm−1 that can be attributed to the stretching of the vinylic C–H bonds.44 Compared to the FTIR spectrum of MPTMS/MMT, the peaks corresponding to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration are absent in MACD-g-MPTMS/MMT indicating a polymerization reaction between the vinyl group of MACD and that of MPTMS/MMT. In addition to this, some peaks are overlapped and some peaks are low in intensity in the spectrum of CH. These results suggest that the hydrogel is formed according to our expectations and is able to encapsulate the TCH drug molecule. In the pure IR spectrum of tetracycline, the peak at 1745 cm−1 corresponds to a C
C stretching vibration are absent in MACD-g-MPTMS/MMT indicating a polymerization reaction between the vinyl group of MACD and that of MPTMS/MMT. In addition to this, some peaks are overlapped and some peaks are low in intensity in the spectrum of CH. These results suggest that the hydrogel is formed according to our expectations and is able to encapsulate the TCH drug molecule. In the pure IR spectrum of tetracycline, the peak at 1745 cm−1 corresponds to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration band, with the peak at 1650–1600 cm−1 corresponding to the C
O vibration band, with the peak at 1650–1600 cm−1 corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching band of the aromatic ring present in TCH. The peak at 1460–1310 cm−1 is related to the δ(OH), δ(C–C) and ν(C–C) stretching vibrations. The bands at 1250–1200 cm−1 correspond to δ(N–H) and ν(C–N) vibrations.45 In the case of TCH loaded composite hydrogel, the ν(C–O–C) band of β-CD at 1030–1082 cm−1 is strongly reduced in intensity which is evidence of host–guest interactions between TCH and β-CD (Scheme 4 shows a proposed model). In TCH loaded CH, the shift of FTIR bands of amide I and II in comparison to crystalline TCH suggested a strong interaction between the amide groups and the clay surfaces. The band at 1445 cm−1 remained the same after TCH intercalation into composite hydrogel, suggesting that complexation was not a dominant mechanism for TCH uptake. Cation exchange with Na+ ions present in the interlayer is the major mechanism of TCH intercalation into these CHs under acidic pH conditions.46
C stretching band of the aromatic ring present in TCH. The peak at 1460–1310 cm−1 is related to the δ(OH), δ(C–C) and ν(C–C) stretching vibrations. The bands at 1250–1200 cm−1 correspond to δ(N–H) and ν(C–N) vibrations.45 In the case of TCH loaded composite hydrogel, the ν(C–O–C) band of β-CD at 1030–1082 cm−1 is strongly reduced in intensity which is evidence of host–guest interactions between TCH and β-CD (Scheme 4 shows a proposed model). In TCH loaded CH, the shift of FTIR bands of amide I and II in comparison to crystalline TCH suggested a strong interaction between the amide groups and the clay surfaces. The band at 1445 cm−1 remained the same after TCH intercalation into composite hydrogel, suggesting that complexation was not a dominant mechanism for TCH uptake. Cation exchange with Na+ ions present in the interlayer is the major mechanism of TCH intercalation into these CHs under acidic pH conditions.46
 |
| | Fig. 2 FTIR spectra of Na-MMT, β-CD, MACD, MPTMS/MMT, MACD-g- MPTMS/MMT, TCH and TCH-loaded- MACD-g- MPTMS/MMT. | |
 |
| | Scheme 4 Proposed scheme for multi-equilibrium of one molecule of TCH with two molecules of β-CD. | |
Fig. 3 shows the XRD spectra for Na-MMT, MPTMS/MMT, MACD, MACD-g-MPTMS/MMT and TCH loaded composite hydrogel. The peak at 2θ = 7.5°, corresponding to a d-spacing value of 1.26 nm, is the characteristic peak of the monolayer hydrate form of Na-MMT. The peak at 2θ = 22.5° indicates that the interior surface of MMT consists of hydrophobic regions associated with the isomorphic substitutions in the clay layer itself (largely Mg2+ for Al3+). Compared to the XRD spectrum of Na-MMT, the basal spacing of MPTMS/MMT is increased from 1.26 nm to 2.29 nm and a new peak appeared at 2θ = 8.0° suggesting the successful intercalation between MMT and MPTMS.47 In the XRD pattern of MACD, the most intense peak at 2θ = 12.5° (d = 0.71 nm) corresponds to the depth of the β-CD cavity, which is also present in MACD-g-MPTMS/MMT indicating that the structure of the β-CD cavity does not change after the formation of the hydrogel. The peak corresponding to MPTMS/MMT at 2θ = 29.5° is also retained in the CH. The XRD pattern of MACD-g-MPTMS/MMT is just a superimposition of the XRD spectra of MACD and MPTMS/MMT, with the loss of some of the peaks. But in the case of TCH loaded-MACD-g-MPTMS/MMT, the peak at 2θ = 12.5° is absent showing the inclusion complexation between TCH and CH. Some peaks disappeared and some peaks were broadened showing the reaction between the drug and CH, destroying the crystalline structure of CH.48,49
 |
| | Fig. 3 XRD patterns of the Na-MMT, MACD, MPTMS/MMT, MACD-g-MPTMS/MMT and TCH-loaded-MACD-g-MPTMS/MMT. | |
3.2. Effect of the pH on the encapsulation of TCH
To establish the optimum pH value for the encapsulation of TCH into MACD-g-MPTMS/MMT, the pH of the encapsulation medium was changed from 1.5 to 8.5. As seen in Fig. 4, the amount of TCH loaded was remarkably affected by the medium pH ranging from 1.5 to 8.5. The maximum TCH encapsulation occurred at pH 3.0. At pH values higher than 3.0, TCH encapsulation decreased appreciably. At pH 3.0, the TCH drug encapsulation efficiency ranges from 99.00 to 94.00% for 75.0 and 50.0 mg L−1 of drug loading respectively. The TCH molecules have the ability to undergo protonation and deprotonation reactions depending on the pH of the medium and hence TCH will exist in four different forms. At low pH values (pH < 3.2) it exists as the fully protonated species, TCH3+. At higher pH values (between pH = 3.4 and 7.7), it appears as TCH2 (as the zwitterion) and at still higher pH values (between pH 7.7 and 9.5), the species present is TCH−. The fourth species present at a pH value above 9.5 is TC2−.35,50 The encapsulation of TCH was found to be high at low pH, i.e. between pH 2.0–4.0, and decreases with the increase of the pH. This type of pH effects were reported for the adsorption of TCH on several clay minerals.51 The predominant species of TCH in the pH range 2–4 was TCH3+ and this species could interact with the CH. The affinity of TCH species for MMT decreases in the order TCH3+ > TCH2 > TCH−. At higher pH values, in order to avoid repulsion between the negatively charged species of TCH and CH, the positive charged group present in the molecule of TCH is oriented near to the surface of CH and the negatively charged group is oriented far away from it, thus avoiding repulsion and this fact explains the adsorption of TCH at higher values of pH, although adsorption is low. MACD is able to entrap a suitably shaped TCH molecule in its hydrophobic tubular cavity. This type of inclusion complexes don't involve the formation of bonds but there is hydrophobic interaction between MACD and organic species and this is known as host–guest interaction.26 Since the adsorption of TCH is highest in the pH range 2–4, the positively charged species of TCH is aligned near to the Na-MMT surface and the other end of TCH species enters into the hydrophobic cavity of MACD of CH. From the obtained experimental data, it was also seen that, on increasing the drug loading, the drug encapsulation efficiency was increased. This trend is attributed to the increase of drug molecule diffusion into the interlayer of MACD-g-MPTMS/MMT with increase in drug loading, which results in a high encapsulation rate of TCH.
 |
| | Fig. 4 Effect of encapsulation pH on TCH encapsulation efficiency. | |
3.3. Swelling kinetics
Fig. 5 represents the swelling capacity of the hydrogel in distilled water at consecutive time intervals. Initially, the rate of water uptake sharply increases and then begins to level off. The equilibrium swelling was achieved after 30–45 min approximately. A power-law behavior is obvious from Fig. 5. The data may be well fitted with a Voigt profile according to the following equation:52| |  | (4) |
where St (g g−1) is the swelling at time t, Se is the equilibrium swelling (power parameter, g g−1), t is the time (min) for swelling, and τ (min) stands for the “rate parameter”. For calculating the “rate parameter”, by using the above formula (after a little modification), one can plot  versus time (t). The slope of the fitted straight line (
versus time (t). The slope of the fitted straight line ( ) gives the rate parameter. According to Fig. 5 and using eqn (4), the rate parameter for the swelling of the composite hydrogel at pH 2.4 and 7.4 is 34 and 5 min, respectively. This decrease in rate parameter of composite hydrogel with increase in pH indicates that at alkaline pH composite hydrogels become more fast-swelling than at acidic pH. Since the τ value is a measure of the swelling rate (i.e., the lower the τ value, the higher the rate of swelling), it can be used for comparative evaluation of the rate of water absorption of composite hydrogels at different pH values. The maximum swelling obtained at pH 2.4 and 7.4 was 41.98 and 188.96 g g−1, respectively. Under acidic conditions (pH 2.4), most of the carboxylate anions are protonated, so the main anion–anion repulsive forces are eliminated and consequently swelling values are decreased. Furthermore, some additional attractive interactions, like —OH− hydrogen bonding, lead to more decreased absorptions. At pH 7.4, some of the carboxylate groups are ionized and the electrostatic repulsion between —COO− groups causes an enhancement of the swelling capacity. The composite hydrogel showed a reproducible On–Off switching/Pulsatile drug release behavior when the environmental pH of the sample was alternated between 2.4 and 7.4 (Fig. 6). This responsiveness and fast-swelling behavior may be of significant importance in colonic controlled delivery of drugs in an alkaline medium.
) gives the rate parameter. According to Fig. 5 and using eqn (4), the rate parameter for the swelling of the composite hydrogel at pH 2.4 and 7.4 is 34 and 5 min, respectively. This decrease in rate parameter of composite hydrogel with increase in pH indicates that at alkaline pH composite hydrogels become more fast-swelling than at acidic pH. Since the τ value is a measure of the swelling rate (i.e., the lower the τ value, the higher the rate of swelling), it can be used for comparative evaluation of the rate of water absorption of composite hydrogels at different pH values. The maximum swelling obtained at pH 2.4 and 7.4 was 41.98 and 188.96 g g−1, respectively. Under acidic conditions (pH 2.4), most of the carboxylate anions are protonated, so the main anion–anion repulsive forces are eliminated and consequently swelling values are decreased. Furthermore, some additional attractive interactions, like —OH− hydrogen bonding, lead to more decreased absorptions. At pH 7.4, some of the carboxylate groups are ionized and the electrostatic repulsion between —COO− groups causes an enhancement of the swelling capacity. The composite hydrogel showed a reproducible On–Off switching/Pulsatile drug release behavior when the environmental pH of the sample was alternated between 2.4 and 7.4 (Fig. 6). This responsiveness and fast-swelling behavior may be of significant importance in colonic controlled delivery of drugs in an alkaline medium.
 |
| | Fig. 5 Swelling kinetics of the MACD-g-MPTMS/MMT composite hydrogel at pH 7.4 and pH 2.4. | |
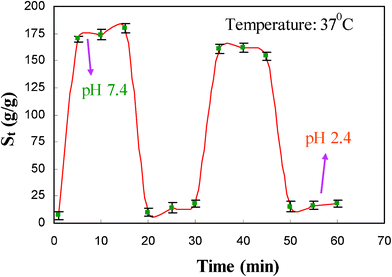 |
| | Fig. 6 On–Off switching behavior of the MACD-g-MPTMS/MMT composite hydrogel in buffered solutions with pH 7.4 and pH 2.4. | |
3.4. Controlled release properties of MACD-g-MPTMS/MMT composite hydrogels
The chemical structure of the hydrogel can affect the drug release behavior by two mechanisms.23 First, the fractional release is directly proportional to the swelling ratio of the hydrogels. The swelling ratio is directly related to the functional groups types which exist in the hydrogel structure. The higher swelling ratios of the hydrogel create a larger surface area for the diffusion of the drug from inside the hydrogel to the environment. In other words, the release of water-soluble drugs from hydrogels occurs only after penetration of water into the polymer networks to swell and dissolve the drug, followed by drug diffusion through the aqueous pathways to the surface of the device. The second mechanism involves the interaction between functional groups of the hydrogel and drug. The strong interactions favor the drug loading into the hydrogel, and decrease the release of the drug from it. These interactions can be controlled by adjusting the pH of the environment. The release profile of TCH from the drug loaded MACD-g-MPTMS/MMT composite hydrogel in different pH buffers is shown in Fig. 7. It can be observed from the release profile that the amount of drug released from the composite hydrogel is higher in pH 7.4 buffer solution than in pH 2.4 buffer solution. At lower pH values, the –COOH groups of the composite hydrogel do not ionize and keep the polymeric network in its collapsed state. At higher pH values, these are partially ionized, and the charged –COO− groups repel each other, leading to a higher swelling of the polymer and resulting in an increase in drug diffusion from the MACD-g-MPTMS/MMT composite hydrogel. In addition to this, in basic solutions (pH 7.4), the electrostatic repulsion between the phenoxide groups of TCH and the carboxylate anions of the hydrogel accelerates the release of TCH from the hydrogel.
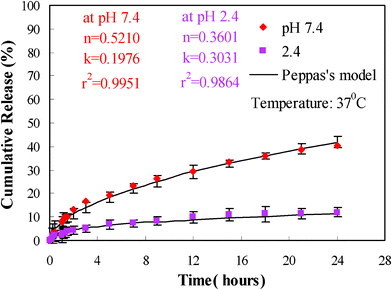 |
| | Fig. 7 Releasing profiles of TCH from the MACD-g- MPTMS/MMT at different pH. The lines are based on the fitting of Peppas's empirical model. | |
To further understand the release mechanism, the results were analyzed on the basis of Peppas's empirical model:53
| | 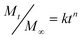 | (5) |
where
Mt and
M∞ are the absolute cumulative amounts of TCH released at time
t and infinite time, respectively,
k is a constant incorporating structural and geometric characteristics of the sample, and
n is the release kinetics exponent. The
n value can be used to identify the release mechanisms. When
n = 0.5, Fickian diffusion is dominant and when
n > 0.5, an anomalous, non-Fickian
drug diffusion occurs. Values of
n between 0.43 and 0.85 are an indication of both diffusion and swelling controlled release of
drug.
54 The
n values presented in
Fig. 7 are obtained from the best fitting of our experimental curves. The
n value obtained in a pH 7.4 medium indicates a swelling controlled, non-Fickian type mechanism while at pH 2.4 a diffusion controlled, Fickian type mechanism occurs. The swelling of the composite hydrogels has been observed to increase at a higher pH value corresponding to the pH of the colon and hence a colon specific delivery of therapeutic agents can be expected.
3.5.
In vitro biocompatibility test
The viability of cells (%) was evaluated by the MTT method. This method is based on the ability of mitochondrial dehydrogenases in viable cells to convert the MTT salt into formazan, which can be quantitatively detected by spectrophotometry at a λmax of 570 nm. The obtained absorbance is, thus, related to the number of viable cells. It is noteworthy that MACD-g-MPTMS/MMT, TCH-loaded- MACD-g-MPTMS/MMT and TCH-mediated reduction of cell viability were dose and time dependent (Fig. 8a and 8b). Exposure of cells with 50 μg mL−1 of MACD-g-MPTMS/MMT for 12, 24, 36 and 48 h leads to a significantly lower decrease in cell viability (97.5, 93.5, 90.5 and 86.6%, respectively). Cell viability remained in the range 96.5–82.5% at concentrations ranging from 10 to 75 μg mL−1 of MACD-g-MPTMS/MMT after 48 h. From the results of these MTT assays, it appears that the MACD-g-MPTMS/MMT composite hydrogel is sufficiently biocompatible. There was no significant change in L929 cell viability at up to 25 μg mL−1 concentration of free TCH. However, when the TCH concentration was increased from 25 to100 μg mL−1, a significant decrease in cell viability was observed in a medium containing free TCH, but the cell viability was unchanged in a medium containing the same concentration of TCH bound to MACD-g-MPTMS/MMT. This is due to the slow release of TCH from the MACD-g-MPTMS/MMT composite hydrogel.
 |
| | Fig. 8 8a. Dose-dependant biocompatibility profile MACD-g-MPTMS/MMT, TCH and TCH-loaded- MACD-g-MPTMS/MMT in L929 cells; 8b. Time-dependant biocompatibility profile MACD-g-MPTMS/MMT, TCH and TCH-loaded- MACD-g-MPTMS/MMT in L929 cells. | |
4. Conclusion
After silylation, the interlayer spacing of Na-MMT is increased, which is evident from the X-ray diffraction pattern of the two samples. The d-spacing value for Na-MMT is 1.26 nm and that for MPTMS/MMT is 1.7 nm, indicating the intercalation of silane molecules. After MACD grafting in the presence of a crosslinker, EGDMA, polymerization occurs between MPTMS/MMT and MACD. The obtained results allowed us to conclude that the composite hydrogel MACD-g-MPTMS/MMT had several different reaction sites for the encapsulation of the drug, including the interlayer region, the external negatively charged surface of modified montmorillonite and the hydrophobic cavity of the cyclodextrin moiety. The investigation of pH effects on encapsulation efficiency showed that TCH adsorption is strongly pH dependant. As the pH increases, encapsulation of TCH onto the adsorbent decreases considerably. The pH at which maximum adsorption takes place was found to be 3.0. At low pH values, the prominent species of TCH is TCH3+ which is adsorbed on to the CH through an ion exchange reaction with MPTMS/MMT. In vitro swelling behavior and release studies showed the usefulness of CH for TCH colonic delivery, showing that they are able to release the drug preferentially and almost completely at colonic pH (pH = 7.4). Biocompatibility assessment revealed the lack of toxicity of CH.
References
- A. K. Philip, S. Dabas and K. Pathak, J. Drug Targeting, 2009, 17, 235–241 CrossRef CAS.
- M. Ashford and J. T. Fell, J. Drug Targeting, 1994, 2, 241–257 CrossRef CAS.
- A. Saima, R. Saeid and K. Kanchan, Scientific Research and Essay., 2009, 3, 1175–1183 Search PubMed.
- T. Houwei, Q. Yi, H. Xiaohua, Y. Yihua, Z. Hua, X. Peihu and X. Fuliang, Carbohydr. Polym., 2010, 82, 440–445 CrossRef.
- H. Radi and A. Mansoor, J. Controlled Release, 2003, 89, 151–165 CrossRef.
- X. H. Xia, J. Yih, N. A. D'Souza and Z. B. Hu, Polymer, 2003, 44, 3389–3393 CrossRef CAS.
- W. F. Lee and Y. C. Chen, J. Appl. Polym. Sci., 2004, 91, 2934–2941 CrossRef CAS.
- R. S. Byrne and P. B. Deasy, J. Microencapsulation, 2005, 22, 423–437 CrossRef CAS.
- C. Aguzzi, C. Viseras, P. Cerezo, S. Rossi, F. Ferrari, A. Lopez-Galindo and C. Caramella, Appl. Clay Sci., 2005, 30, 79–86 CrossRef CAS.
- A. A. Fudala, I. I. Palinko and I. Kiricsi, Inorg. Chem., 1999, 38, 4653–4658 CrossRef CAS.
-
D. Dobrozsi, Oral liquid mucoadhesive compositions. US Patent US 6, 2003, 638, 521B2..
- A. C. Kathleen, Appl. Clay Sci., 2000, 17, 1–23 CrossRef.
- S. Pavlidou and C. D. Papaspyrides, Prog. Polym. Sci., 2008, 33, 1119–1198 CrossRef CAS.
- B. Flourie, C. Molis, L. Achour, H. Dupas, C. Hatat and J. C. Rambaud, J. Nutr., 1993, 123, 676–680 CAS.
- K. Udo, K. Hokonohara, K. Motoyama, H. Arima, F. Hirayama and K. Uekama, Int. J. Pharm., 2010, 388, 95–100 CrossRef CAS.
- C. Creminon, F. Djedaini-Pilard, R. Vienet, C. Pean, J. M. Grognet, J. Grassi, B. Perly and P. Pradelles, Pharm. Res., 1999, 16, 1407–1411 CrossRef CAS.
- T. Loftsson and M. E. Brewster, J. Pharm. Sci., 1996, 85, 1017–1025 CrossRef CAS.
- M. E. Brewster and T. Loftson, Adv. Drug Delivery Rev., 2007, 59, 645–666 CrossRef CAS.
- M. Prabaharan and R. Jayakumar, Int. J. Biol. Macromol., 2009, 44, 320–325 CrossRef CAS.
- S. Y. Park, O. Muhammad and H. Sajjad, Polymer, 2011, 52, 91–97 CrossRef.
- M. H. Mohamed, L. D. Wilson, D. Y. Pratt, R. Guo, C. Wu and J. V. Headly, Carbohydr. Polym., 2012, 87, 1241–1248 CrossRef CAS.
- A. K. Sarmah, M. T. Meyer and A. B. A. Boxall, Chemosphere, 2006, 65, 725–759 CrossRef CAS.
- G. R. Bardajee, A. Pourjavadi and R. Soleyman, Colloids Surf., A, 2011, 392, 16–24 CrossRef CAS.
- S. Wu, W. Jiang, X. Zhang, H. Sun, W. Zhang, J. Dai, L. Liu, X. Chen and F. Li, J. Magn. Magn. Mater., 2012, 324, 124–127 CrossRef CAS.
- P. H. Chang, Z. Li, W. T. Jiang and J. S. Jean, Appl. Clay Sci., 2009, 46, 27–36 CrossRef CAS.
- F. B. De Sousa, M. F. Oliveira, I. S. Lula, M. T. C. Sansiviero, M. E. Cortes and R. D. Sinisterra, Vib. Spectrosc., 2008, 46, 57–62 CrossRef CAS.
- G. R. Bardajee, A. Pourjavadi and R. Soleyman, Colloids Surf., A, 2011, 392, 16–24 CrossRef CAS.
- A. L. Pataro, C. F. Franco, V. R. Santos, M. E. Cort!es and R. D. Sinisterra, Biomaterials, 2003, 24, 1075–1080 CrossRef CAS.
- T. Pongjanyakul, Int. J. Pharm., 2009, 365, 100–108 CrossRef CAS.
- T. S. Anirudhan and S. Sandeep, New J. Chem., 2011, 35, 2869–2876 RSC.
- N. N. Herrera, L. Jean-Marie, P. Jean-Luc, L. David and E. Bourgeat-Lami, Langmuir, 2004, 20, 1564–1571 CrossRef CAS.
- O. Muhammad, H. Sajjad and P. Soo-Young, Polymer, 2011, 52, 91–97 CrossRef.
- A. Shimojima, D. Mochizuki and K. Kuroda, Chem. Mater., 2001, 13, 3603–3609 CrossRef CAS.
- K. W. Park, S. Y. Jeong and O. Y. Kwon, Appl. Clay Sci., 2004, 27, 21–27 CrossRef CAS.
- M. E. Parolo, M. C. Savini, J. M. Vallés, M. T. Baschini and M. G. Avena, Appl. Clay Sci., 2008, 40, 179–186 CrossRef CAS.
- R. Ianchis, L. O. Cinteza, D. Donescu, C. Petcu, M. C. Corobea, R. Somoghi, M. Ghiurea and C. Spataru, Appl. Clay Sci., 2011, 52, 96–103 CrossRef CAS.
- C. Franco, L. Schwingel, I. Lula, R. D. Sinisterrab, L. S. Koester and V. L. Bassani, Int. J. Pharm., 2009, 369, 5–11 CrossRef CAS.
- T. S. Anirudhan and S. Sandeep, J. Mater. Chem., 2012, 22, 12888–12899 RSC.
- A. D. Gianni, A. D. E. Amerio, O. Monticelli and R. Bongiovanni, Appl. Clay Sci., 2008, 42, 116–124 CrossRef.
- Z. Q. Peng, Y. J. Zhu, H. He, D. Liu and S. Yang, Appl. Clay Sci., 2010, 50, 546–553 CrossRef.
- M. E. Cortes, R. D. Sinisterra, M. J. A. Capos and N. Tortamano, J. Inclusion Phenom. Macrocyclic Chem., 2001, 40, 297–302 CrossRef CAS.
- Y. Y. Liu and X. D. Fan, Colloids Surf., A, 2006, 289, 193–199 CrossRef.
- L. A. C. Cruz, C. A. M. Perez, H. A. M. Romero and P. E. G. Casillas, J. Alloys Compd., 2008, 466, 330–334 CrossRef.
- Y. Mansoori, S. V. Atghia, M. R. Zamanloo, G. h. Imanzadeh and M. Sirousazar, Eur. Polym. J., 2010, 46, 1844–1853 CrossRef CAS.
- C. F. Leypold, M. Peiher, G. Brehm, M. O. Schmitt, S. Schneider, P. Matousek and M. Towrie, Phys. Chem. Chem. Phys., 2003, 5, 1149–1157 RSC.
- A. M. L. Denadai, M. M. Santoro, M. T. P. Lopes, A. Chenna, F. B. Sousa, G. M. Avelar, M. R. T. Gomes, F. Guzman, C. E. Salas and R. D. Sinisterra, BioDrugs, 2006, 20, 283–291 CrossRef CAS.
- A. A. Silva, K. Dahmouche and B. G. Soares, Appl. Clay Sci., 2011, 54, 151–158 CrossRef CAS.
- J. Jingou, H. Shilei, L. Weiqi, W. Danjun, W. Tengfei and X. Yi, Colloids Surf., B, 2011, 83, 103–107 CrossRef.
- L. Kong, T. Sun, F. Xin, W. Zhao, H. Zhang, Z. Li, Y. Li, H. Y. S. Li and A. Hao, Colloids Surf., A, 2011, 392, 156–162 CrossRef CAS.
- L. J. Leeson, J. E. Krueger and R. A. Nash, Tetrahedron Lett., 1963, 18, 1155–1160 CrossRef.
- J. E. Browne, J. R. Feldkamp, J. L. White and S. L. Hem, J. Pharm. Sci., 1980, 69, 816–823 CrossRef CAS.
- H. Omidian, S. A. Hashemi, P. G. Sammes and I. Meldrum, Polymer, 1998, 39, 6697–6704 CrossRef CAS.
- N. Peppas and J. Siepmann, Adv. Drug Delivery Rev., 2001, 48, 139–157 CrossRef.
- T.S. Anirudhan and S. Sandeep, Polym. Chem., 2011, 2, 2052–2061 RSC.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOH]5, the amount of C and H obtained from instrumental analysis was C: 43.24% and H: 4.98% and the corresponding amount obtained from theoretical calculation was C: 43.54% and H: 5.01%.
CHCOOH]5, the amount of C and H obtained from instrumental analysis was C: 43.24% and H: 4.98% and the corresponding amount obtained from theoretical calculation was C: 43.54% and H: 5.01%.




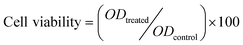

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching vibration of the ester groups and carboxylic acid groups in MACD. Actually, the band at 1628 cm−1 in the β-CD spectrum was due to the first overtone of O–H bending, which was overlapped by the band due to the C
O) stretching vibration of the ester groups and carboxylic acid groups in MACD. Actually, the band at 1628 cm−1 in the β-CD spectrum was due to the first overtone of O–H bending, which was overlapped by the band due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration at 1644 cm−1 in MACD. Besides, another new band at 1216 cm−1, corresponding to the stretching vibration of C(O)–O in C
C stretching vibration at 1644 cm−1 in MACD. Besides, another new band at 1216 cm−1, corresponding to the stretching vibration of C(O)–O in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C(O)–O, appeared in MACD but was absent in β-CD. All these indicated that the esterification reaction successfully occurred between β-CD and MACD.42 The decrease in the intensity of the O–H band at 3400 cm−1 in MACD is due to deprotonation of some of the hydroxyl groups suggesting a covalent bonding between maleic anhydride and β-CD.43 In the FTIR spectrum of MPTMS/MMT, absorption bands appeared at 3010 and 3053 cm−1 that can be attributed to the stretching of the vinylic C–H bonds.44 Compared to the FTIR spectrum of MPTMS/MMT, the peaks corresponding to a C
C–C(O)–O, appeared in MACD but was absent in β-CD. All these indicated that the esterification reaction successfully occurred between β-CD and MACD.42 The decrease in the intensity of the O–H band at 3400 cm−1 in MACD is due to deprotonation of some of the hydroxyl groups suggesting a covalent bonding between maleic anhydride and β-CD.43 In the FTIR spectrum of MPTMS/MMT, absorption bands appeared at 3010 and 3053 cm−1 that can be attributed to the stretching of the vinylic C–H bonds.44 Compared to the FTIR spectrum of MPTMS/MMT, the peaks corresponding to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration are absent in MACD-g-MPTMS/MMT indicating a polymerization reaction between the vinyl group of MACD and that of MPTMS/MMT. In addition to this, some peaks are overlapped and some peaks are low in intensity in the spectrum of CH. These results suggest that the hydrogel is formed according to our expectations and is able to encapsulate the TCH drug molecule. In the pure IR spectrum of tetracycline, the peak at 1745 cm−1 corresponds to a C
C stretching vibration are absent in MACD-g-MPTMS/MMT indicating a polymerization reaction between the vinyl group of MACD and that of MPTMS/MMT. In addition to this, some peaks are overlapped and some peaks are low in intensity in the spectrum of CH. These results suggest that the hydrogel is formed according to our expectations and is able to encapsulate the TCH drug molecule. In the pure IR spectrum of tetracycline, the peak at 1745 cm−1 corresponds to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration band, with the peak at 1650–1600 cm−1 corresponding to the C
O vibration band, with the peak at 1650–1600 cm−1 corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching band of the aromatic ring present in TCH. The peak at 1460–1310 cm−1 is related to the δ(OH), δ(C–C) and ν(C–C) stretching vibrations. The bands at 1250–1200 cm−1 correspond to δ(N–H) and ν(C–N) vibrations.45 In the case of TCH loaded composite hydrogel, the ν(C–O–C) band of β-CD at 1030–1082 cm−1 is strongly reduced in intensity which is evidence of host–guest interactions between TCH and β-CD (Scheme 4 shows a proposed model). In TCH loaded CH, the shift of FTIR bands of amide I and II in comparison to crystalline TCH suggested a strong interaction between the amide groups and the clay surfaces. The band at 1445 cm−1 remained the same after TCH intercalation into composite hydrogel, suggesting that complexation was not a dominant mechanism for TCH uptake. Cation exchange with Na+ ions present in the interlayer is the major mechanism of TCH intercalation into these CHs under acidic pH conditions.46
C stretching band of the aromatic ring present in TCH. The peak at 1460–1310 cm−1 is related to the δ(OH), δ(C–C) and ν(C–C) stretching vibrations. The bands at 1250–1200 cm−1 correspond to δ(N–H) and ν(C–N) vibrations.45 In the case of TCH loaded composite hydrogel, the ν(C–O–C) band of β-CD at 1030–1082 cm−1 is strongly reduced in intensity which is evidence of host–guest interactions between TCH and β-CD (Scheme 4 shows a proposed model). In TCH loaded CH, the shift of FTIR bands of amide I and II in comparison to crystalline TCH suggested a strong interaction between the amide groups and the clay surfaces. The band at 1445 cm−1 remained the same after TCH intercalation into composite hydrogel, suggesting that complexation was not a dominant mechanism for TCH uptake. Cation exchange with Na+ ions present in the interlayer is the major mechanism of TCH intercalation into these CHs under acidic pH conditions.46




 versus time (t). The slope of the fitted straight line (
versus time (t). The slope of the fitted straight line ( ) gives the rate parameter. According to Fig. 5 and using eqn (4), the rate parameter for the swelling of the composite hydrogel at pH 2.4 and 7.4 is 34 and 5 min, respectively. This decrease in rate parameter of composite hydrogel with increase in pH indicates that at alkaline pH composite hydrogels become more fast-swelling than at acidic pH. Since the τ value is a measure of the swelling rate (i.e., the lower the τ value, the higher the rate of swelling), it can be used for comparative evaluation of the rate of water absorption of composite hydrogels at different pH values. The maximum swelling obtained at pH 2.4 and 7.4 was 41.98 and 188.96 g g−1, respectively. Under acidic conditions (pH 2.4), most of the carboxylate anions are protonated, so the main anion–anion repulsive forces are eliminated and consequently swelling values are decreased. Furthermore, some additional attractive interactions, like —OH− hydrogen bonding, lead to more decreased absorptions. At pH 7.4, some of the carboxylate groups are ionized and the electrostatic repulsion between —COO− groups causes an enhancement of the swelling capacity. The composite hydrogel showed a reproducible On–Off switching/Pulsatile drug release behavior when the environmental pH of the sample was alternated between 2.4 and 7.4 (Fig. 6). This responsiveness and fast-swelling behavior may be of significant importance in colonic controlled delivery of drugs in an alkaline medium.
) gives the rate parameter. According to Fig. 5 and using eqn (4), the rate parameter for the swelling of the composite hydrogel at pH 2.4 and 7.4 is 34 and 5 min, respectively. This decrease in rate parameter of composite hydrogel with increase in pH indicates that at alkaline pH composite hydrogels become more fast-swelling than at acidic pH. Since the τ value is a measure of the swelling rate (i.e., the lower the τ value, the higher the rate of swelling), it can be used for comparative evaluation of the rate of water absorption of composite hydrogels at different pH values. The maximum swelling obtained at pH 2.4 and 7.4 was 41.98 and 188.96 g g−1, respectively. Under acidic conditions (pH 2.4), most of the carboxylate anions are protonated, so the main anion–anion repulsive forces are eliminated and consequently swelling values are decreased. Furthermore, some additional attractive interactions, like —OH− hydrogen bonding, lead to more decreased absorptions. At pH 7.4, some of the carboxylate groups are ionized and the electrostatic repulsion between —COO− groups causes an enhancement of the swelling capacity. The composite hydrogel showed a reproducible On–Off switching/Pulsatile drug release behavior when the environmental pH of the sample was alternated between 2.4 and 7.4 (Fig. 6). This responsiveness and fast-swelling behavior may be of significant importance in colonic controlled delivery of drugs in an alkaline medium.