Filtered EGR – a step towards an improved NOX/soot trade-off for DPF regeneration
S.S.
Gill
a,
J.M.
Herreros
a,
A.
Tsolakis
*a,
D.M.
Turner
a,
E.
Miller
a and
A.P.E.
York
b
aSchool of Mechanical Engineering, University of Birmingham, Edgbaston, B15 2TT, UK. E-mail: a.tsolakis@bham.ac.uk; Fax: +44 (0) 121 414 7484; Tel: +44 (0) 121 414 4170
bJohnson Matthey Technology Centre, Blount's Court, Sonning Common, Reading RG4 9NH, UK
First published on 6th September 2012
Abstract
Exhaust gas recirculation (EGR) is currently widely used in commercial diesel engines to provide an effective solution in reducing the levels of nitrogen oxide (NOX) emissions. However, this currently comes at the expense of an exponential increase in particulate matter (PM) emissions resulting directly from the dilution effect (i.e. reduction in oxygen availability), as well as a further penalty arising from the recirculation of the exhaust emissions such as soot and hydrocarbons. In our earlier work it was observed that filtered EGR (FEGR) was able to play a significant role in controlling the soot recirculation penalty and thus improve the overall NOX/soot trade-off. In order to further our understanding of the effect of recirculated exhaust gases and in particular recirculated soot and hydrocarbon (HC), comparisons were made between standard EGR, FEGR and pure nitrogen (N2), a direct cleaner replacement of the exhaust gas. When implementing FEGR, a diesel oxidation catalyst (DOC) and diesel particulate filter (DPF) were introduced into the exhaust to not only filter the soot particulates but reduce the recirculation of HC which can play a role in particulate surface growth. It was observed that the recirculated HC species and soot particles (especially at high load and EGR ratios) play a role in promoting the production and growth of further particles within the combustion chamber. Similarly, by comparing at the same O2 intake concentration as that of FEGR and introducing N2 as the EGR replacement gas, it was possible to correlate the increase in engine-out mass of soot with EGR to the recirculation of soot particles, HC species as well as the presence of H2O and CO2.
1. Introduction
With ongoing improvements aimed at enhancing performance and reducing noise and emissions, the diesel engine has become an increasingly attractive option for passenger car applications. Over the past years, stringent emission legislations have been imposed to regulate emissions such as oxides of nitrogen (NO, NO2) and particulate matter (PM) emitted from automotive diesel engines worldwide. The emissions from a diesel engine are composed of gaseous pollutants and PM. The main constituents which make up the gaseous phase are the hydrocarbon (HC), carbon monoxide (CO), NOX and sulphur dioxide (SO2).1,2 The particulates are a combination of soot and other liquid or solid phase materials that are collected once exhaust gases are passed through a filter medium at 52 °C (EPA) or 47 °C (EC) and is responsible for the black smoke associated with diesel powered vehicles. Soot (referred to as the insoluble dry fraction), mostly clusters of solid carbon particles are formed from the unburned fuel, nucleating from the vapour to solid phases in the fuel-rich regions during combustion.3 Depending on the local conditions, the hydrocarbons may then condense on or be adsorbed by the soot forming soluble liquid or solid phase materials. The liquid HC (i.e. the heavier component of the HC emission) present in the PM are a combination of unburned diesel fuel and evaporated lubricating oil which appear as soluble (soluble organic fraction, SOF) or volatile organic compounds (volatile organic fractions, VOF) in the exhaust which tend to adsorb onto the dry carbon particles.4,5Although, the diesel engine is an attractive solution for carbon dioxide (CO2) reduction, there remains a challenge to control simultaneously NOX and PM emissions to a level required by prevailing regulations.6,7 Unfortunately, if the diesel combustion system is not well controlled, it can produce higher levels of PM and/or NOX. NOX emissions need to be controlled because of their contribution to the formation chemistry of low-level ozone, or smog, an environmental and human health hazard, while PM can have associated health issues. The control of NOX under lean conditions is much more difficult, especially as the fuel-efficient characteristics of diesel engines result in a much lower exhaust gas temperature. Currently the legislative NOX emissions of diesel vehicles have been met through engine control methods alone. However, with future legislation limits becoming more stringent, lean NOX control would be required. The control of PM emissions involves a combined strategy of engine modification (e.g. high pressure pumps, increased number of smaller injector nozzles and multiple injections) and the introduction of a diesel particulate filter to the exhaust system.8 Although this has been successfully introduced, controlled filter regeneration methods are still being developed to maintain their long-term operation. As a result, further development and optimisation of the aftertreatment system is necessary to comply with future stringent emission legislation. Technologies such as EGR, soot traps and exhaust gas aftertreatment are essential to cater to the challenges posed by increasingly stringent environmental emission legislations.
EGR has been a key strategy in controlling NOX formation, influencing the thermodynamic properties and the oxygen concentration of the cylinder charge whilst keeping minimum degradations in power and efficiency. Apart from the reduction in oxygen (O2) availability, the effect of adding CO2, water vapour (H2O) and N2 through the use of EGR reduces the flame temperature while increasing the overall heat capacity of the cylinder charge.9,10 In the work of Ladommatos et al.11 and Zhao et al.,12 it was concluded that the majority of the NOX reduction from CO2 was primarily the result of the dilution effect (i.e. a reduction in oxygen mass fraction) with a minor contribution due to a chemical and thermal effect. tables
Table 1 gives a general overview of the dilution, chemical and thermal effects of EGR.13 Thermal throttling is also known to have a direct influence on the emissions of NOX and PM; however this has been effectively controlled through the implementation of cool EGR, reducing the inlet charge temperature and thus resulting in an increased volumetric efficiency. In addition, the work of Anderlohr et al.14 describes the influence of CO, CO2 and H2O on the thermal and chemical effects during EGR with a direct comparison to pure nitrogen on the post-oxidation of hydrocarbons. It was concluded that the thermal effect of CO2 and H2O resulted in the reduction in engine-out NOX emissions as a consequence of a lower local gas temperature. However, the chemical or kinetic effect of CO2 and H2O on EGR enhanced the rate of hydrocarbon oxidation through third body reactions. Thus, CO2 and H2O kinetically accelerated the dissociation of H2O2 which is a key step in further hydrocarbon oxidation. The work of Westbrook et al.15 shows the dissociation of H2O2 and the production of the OH radical which is a well known oxidant of hydrocarbons.
| EGR Effect | Species | Result of…. | Effect on NOX | Effect on PM |
|---|---|---|---|---|
| a Dependant on engine mode. b At constant charge mass flow rate and charge composition. c Proportional to inlet charge temperature. | ||||
| Dilution | O2 | Reduction in O2 availability | Decrease 80–90% | Increase 80–90% |
| Chemical | CO2 | Thermal dissociation and its products participate in combustion | Decrease 5–10%a | Decrease 5–10%a |
| H2O | Increase 5–10% a | |||
| Thermal | CO2 | Higher specific heat capacity than O2 and N2 | Decrease <5% a | Minor/Increase |
| H2O | ||||
| Inlet Temperatureb (Hot EGR) | — | Increased inlet charge temperature and reduced volumetric efficiency | Increasec | Increasec |
Our preliminary study demonstrated the potential of filtering the EGR loop with engine-out soot reductions reaching 50% when 20%Vol.EGR was implemented.16 However, in a practical system the EGR take-off would be downstream the after-treatment system (e.g. representing a low pressure loop system) which will be the focus of this particular study. This involves understanding the role of the soot penalty when particles are reintroduced to the combustion chamber through EGR and the effect this can have on the overall NOX/soot trade-off. It has been shown that as the level of EGR increases so does the number of particles per agglomerate.17 In addition, the hydrocarbons present in the exhaust gas can also condense or adsorb onto the soot particulates when subjected to low temperatures, thus leading to heavier and larger soot particles re-entering the combustion chamber. These agglomerated soot particles are also very difficult to oxidise and require large amounts of energy to break down, especially those produced under higher engine loads where the transition between amorphous to graphitic structures occur.18,19
The main research objective is to develop further information on the soot and HC recirculation penalty when using EGR and how this can help to improve the NOx/PM trade-off. This study will endeavour to provide a further understanding of the impacts of this penalty by implementing FEGR into a full scale EGR system. This involves recirculation of exhaust gas into the combustion chamber downstream a combined DPF and DOC aftertreatment system. The main difference with respect to our earlier study is that the whole exhaust flow passes through the aftertreatment system rather than only the EGR loop being treated. Comparisons were made between standard EGR, FEGR (with and without DOC) and pure N2 as a replacement gas, highlighting the potential benefits this can have on future systems. The second objective of this work is to see whether the implementation of FEGR can have any benefit towards passive DPF regeneration. This will also extend to understanding the influence of H2 over the diesel oxidation catalyst for the improvement of the NO2/NOX ratio to further facilitate low temperature regeneration. It has previously been reported that H2 has a positive effect on CO and hydrocarbon oxidation as well as NO2 formation over diesel oxidation catalysts. However, there is still uncertainty whether this is due to the temperature rise caused by H2 oxidation or if there is an influence in the reaction kinetics. Therefore, in this study the effect of varying the hydrogen concentration over a platinum loaded diesel oxidation catalyst will also be studied.
2. Experimental
The experimental apparatus is detailed and illustrated in Fig. 1. The investigation was carried out using an experimental single cylinder direct injection diesel engine. The main engine specifications are given in Table 2. In this work all tests were examined under steady state conditions, at a constant engine speed of 1500 rpm with an engine load of 5 bar Indicated Mean Effective Pressure (IMEP) reflecting ∼70% of full load.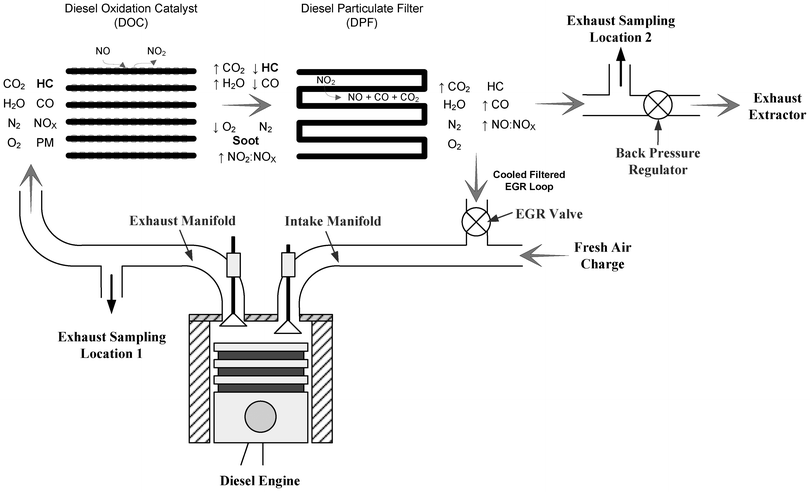 | ||
| Fig. 1 Schematic of experimental setup. | ||
| Engine Specification | Data |
|---|---|
| Number of cylinders | 1 |
| Bore/stroke | 98.4 mm/101.6 mm |
| Connecting rod length | 165 mm |
| Displacement volume | 733 cm3 |
| Compression ratio | 15.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Rated power (kW) | 8.6@2500 rpm |
| Peak torque (Nm) | 39.2@1800 rpm |
| Injection system | Three holes, pump-line-nozzle |
| Injection Timing (°bTDC) | 22 |
| Engine piston | Bowl-in-piston |
The DOC and DPF used during the FEGR tests were supplied by Johnson Matthey Plc. The DOC (Ø = 115 mm, L = 75 mm with a channel cell density of 600 cpsi) is coated with a platinum catalyst designed to promote the oxidation of hydrocarbons and carbon monoxide. The space velocity (SV) was 60 kh−1 based on the DOC volume and exhaust flow rate. The ceramic honeycomb DPF (Ø = 58 mm, L = 153 mm with a channel cell density of 300 cpsi) was positioned downstream the DOC and both secured into place using an intumescent mat.
The main differences between EGR and FEGR are the levels of soot and HC species being reintroduced to the combustion chamber. The system allows for passive oxidation of the HC and CO whilst filtering soot. Filtration efficiencies are known to reach as high as 95–98%, therefore, by knowing the oxygen concentrations present in the combustion chamber during this process the soot recirculation effect can be isolated within FEGR. It is also important to note that during each individual test the filter was preloaded to ensure maximum performance and filtration efficiency. The EGR ratio of 0, 10, 20 and 30%Vol. were determined volumetrically as a percentage reduction in volume flow rate of air at a fixed engine operating point, with the results presented in terms of intake mass O2 availability. However, during the addition of pure N2 the DPF and DOC were removed and the EGR modified allowing the connection of a pure N2 gas bottle. The amount of N2 addition was determined based on the intake O2 availability, ensuring this was consistent with that of the EGR tests. In order to obtain accurate and comparable results the exhaust back pressure was continuously monitored and adjusted by an electronic valve located in the main exhaust downstream the catalysts. This is especially important in the case of pure nitrogen where there is no aftertreatment present in the exhaust and as a result the exhaust back pressure would be substantially different.
The fuel used in this study was Ultra Low Sulphur Diesel (ULSD) provided by Shell Global Solutions UK. The fuel specifications and properties are listed in Table 3. The data acquisition and combustion analysis were carried out using in-house developed LabVIEW software. Output from the analysis of engine cycles includes the in-cylinder pressure, IMEP, percentage coefficient of variation (% COV) of IMEP and combustion characteristic information. The COV of IMEP was used as a criteria for combustion stability (cyclic variability) which remained consistent and well below the acceptable limit throughout this study.20 In this study the emissions have been calculated on a specific basis using the indicated power.
| Fuel Analysis | Method | Diesel (ULSD) |
|---|---|---|
| Cetane Number | ASTM D613 | 53.9 |
| Density at 15 °C (kg m−3) | ASTM D4052 | 827.1 |
| Viscosity at 40 °C (cSt) | ASTM D445 | 2.467 |
| 50% Distillation (°C) | ASTM D86 | 264 |
| 90% Distillation (°C) | ASTM D86 | 329 |
| LCV (MJ kg−1) | 43.3 | |
| Sulphur (mg kg1) | ASTM D2622 | 46 |
| Aromatics (% wt) | 24.4 | |
| O (% wt) | — |
A Horiba MEXA 7100 DEGR gas analyser was used to take measurements of CO2 and CO by Non-Dispersive Infrared (NDIR), O2 by a magnetopneumatic method, NOX by chemiluminescence detection (CLD) and HC by flame ionisation detection (FID). A MultiGas 2030, FTIR spectrometry based analyser was also used for the measurement of multiple gas species including H2O. A Horiba Mexa 1230 PM was used to measure soot by a diffusion charging (DC) method and soluble organic material (SOM) by a dual flame ionisation detection (FID) method equipped with a 47 mm diameter Teflon (PTFE)-coated (PALLFLEX) glass fibre filter. Hot dilution was considered to prevent nuclei mode, with the dilution ratio of the soot diluter set to approximately 40. The soluble organic fraction (SOF) can be expressed in the following way:
| SOF = SOM/(Soot + SOM) |
The total particulate mass and number concentration measurements were carried out using a TSI Scanning Mobility Particle Sizer (SMPS). This is comprised of an electrostatic classifier series 3080, a 3081 Differential Mobility Analyser (DMA) and a model 3775 Condensation Particle Counter (CPC). The sample and sheath flow rates were set such that the measurement (diameter) range was nominally 12–437 nm, with the dilution ratio set to 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using a rotating disc. A temperature controlled dilution process was carried out (150 °C) to minimise any HC condensation and nucleation which can occur during sampling.
1 using a rotating disc. A temperature controlled dilution process was carried out (150 °C) to minimise any HC condensation and nucleation which can occur during sampling.
Pure H2 was mixed with the exhaust gas before passing it over the DOC at different hydrogen concentrations (1000, 2500 and 5000 ppm). Additionally, H2 concentrations were measured by sampling the gas-stream through a Hewlett-Packard (HP) gas chromatograph (GC) equipped with a thermal conductivity detector (TCD). Higher TCD sensitivity to H2 was achieved by using argon as the carrier-gas flowing through two separation columns. In order to determine the concentration of H2 present in the gas sample, the equipment was first calibrated using a certified gas mixture composed of 30% H2 balance N2. Several samples were taken and averaged before the final value of H2 was used.
3.1 Results and discussion
3.1. The implementation of a DOC to a FEGR system
In our earlier work it was concluded that there was a soot recirculation penalty with EGR, and this could effectively be controlled by using a DPF in a FEGR configuration.16 However, it is believed that there may be some additional penalties, for example, from the recirculation of HC species which may also participate in particulate growth and hence put a limit to the amount of EGR that can be introduced to a diesel engine before we reach smoke limited conditions. In order to further assess the FEGR technique, a system consisting of a DOC and DPF was implemented to not only filter the soot particles but also oxidise the gas phase HC species from re-entering the combustion chamber through EGR (Table 4). Particular focus with the filtered system was placed on the exhaust gas back-pressure, ensuring this remained consistent between the FEGR and EGR conditions to allow for a direct comparison.| Component | FEGR without DOC | FEGR with DOC |
|---|---|---|
| CO (ppm) | 183 | 17 |
| THC (ppm) | 918 | 168 |
| CO2 (%) | 6.20 | 6.31 |
| H2O (%) | 6.68 | 6.82 |
The DOC is effective in controlling the CO and gaseous HC emissions from the exhaust stream, with the possibility of soot oxidation and deposition on the surface of the DOC.21Fig. 2 shows the direct influence of temperature on the oxidation ability of PM over the catalyst. It is important to note that during this particular test the engine mode (1500 rpm, 5 bar IMEP) was kept constant (i.e. keeping the gaseous hourly space velocity and exhaust composition over the DOC constant), while the catalyst inlet temperature was varied using an integrated furnace. Although there is a reduction in soot, the level is consistent over the whole temperature range, thus increasing the catalyst temperature has no direct influence in the removal of the soot particles. This suggests the reduction in soot in our system and under these conditions was simply the result of deposition on the surface of the catalyst rather than reactions with solid particulates, which would require a catalyst support/substrate which exhibits some PM holding capacity or a longer residence time within the catalyst.
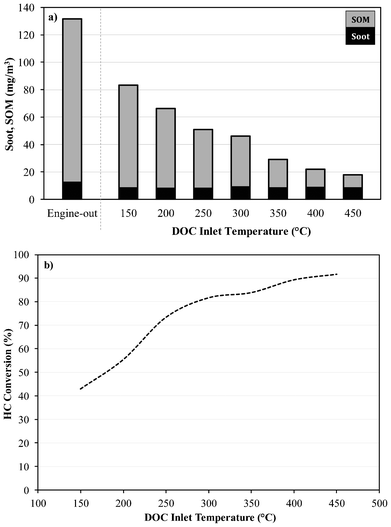 | ||
| Fig. 2 Influence of Temperature on the Oxidation Ability of, a) Particulate Matter and b) Gas Phase Hydrocarbon. | ||
It can also be observed in Fig. 2 there is a good correlation between the reduction in SOM and gas phase HC conversion (i.e. oxidation), which are both directly influenced by the inlet catalyst temperature. The reduction in SOM is suggested to be the result of the oxidation of the gas phase HC (which will eventually form SOM during the dilution process, rather than the oxidation of the adsorbed or condensed HC already present on the particles upstream the DOC. As the sample needs to be cooled and diluted according to the definition of PM, there will be condensation or adsorption of the vapour compounds (i.e. adsorption of HC onto the soot cores) resulting in the growth of the PM and shifting the particulate size distribution to larger particulate sizes (an increase in emitted particle mass). The higher the catalyst temperature, the greater the oxidation rate of gaseous HC until the catalyst reaches its optimum achievable performance, reducing the amount of HC that can be adsorbed onto the particulates. Klein et al.22 also suggested that the reduction in SOM was likely to be a reduction in the gas phase HC rather than the oxidation of adsorbed HC. Thus, the DOC had no influence on the primary particulates (soot cores) as well as the aggregates of primary particles. Therefore, using a DOC it is difficult to oxidise the SOM already adsorbed or condensed onto the soot particles, however it is possible to control the recirculation of the gaseous HC which may form SOM during the cooler parts of the exhaust during FEGR. It is also suggested that the control of the gaseous HC emissions through the use of a DOC will also minimise the influence they can have on particulate growth and formation of new particles when reintroduced into the combustion chamber during EGR. To observe the benefit of controlling the soot and HC recirculation penalties, comparisons between unfiltered EGR, FEGR (with and without a DOC) and the addition of pure N2 were made.
3.2. The effectiveness of combining a DOC and DPF for FEGR
To observe the effects of EGR and FEGR (with and without the presence of the DOC), comparisons were made to pure N2 as a benchmark. The results of engine-out NOX and soot emissions have been collated and presented in Fig. 3 and 4. The primary focus switches to the dilution effect and its impact on diesel particulate formation and NOX emissions. By directly isolating the dilution effect (i.e. comparing at the same O2 intake concentration) which is the main effect according to Ladommatos et al.,11 it was be possible to distinguish a relationship with regards to the recirculation of the soot, gaseous HC and different CO2 and H2O concentrations with an increasing EGR ratio. Results are given at 5 bar IMEP, mainly as the concentration of soot in the exhaust is large enough to show a soot recirculation penalty as observed in our earlier study.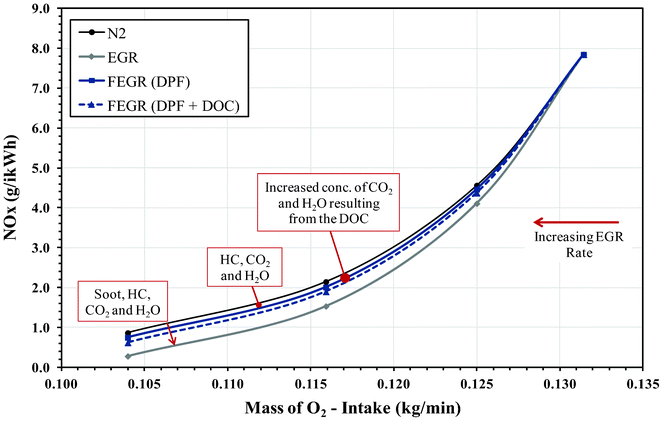 | ||
| Fig. 3 Effect of NOX emissions during EGR and FEGR compared to pure N2 EGR. | ||
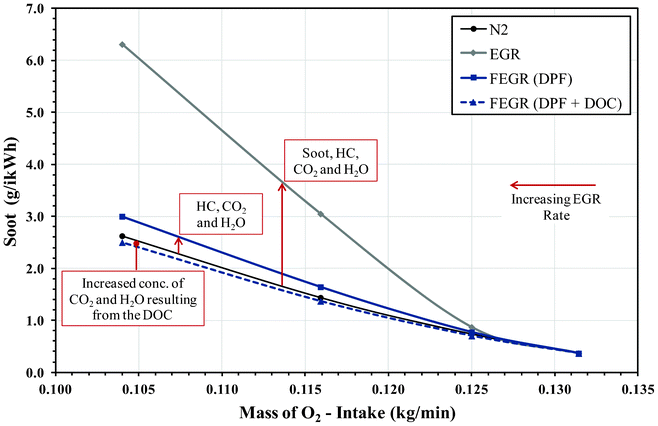 | ||
| Fig. 4 Effect of soot emissions during EGR and FEGR compared to pure N2 EGR. | ||
The difference between introducing pure N2 to unfiltered EGR (for the same mass of intake O2) lies primarily on the chemical and thermal effects of CO2 and H2O. However, additional effects such as the mass of soot present in the EGR loop and the presence of HC can also play a role in the thermal and chemical effects during combustion and should also be taken into account. Introducing N2 provides an example of clean EGR with no recirculated soot and gaseous HC occurring from the earlier cycle and thus reducing particulate growth. However, if the dilution was the main effect of EGR, the emissions of soot and NOX for both unfiltered EGR and N2 should be comparable for a given intake oxygen concentration. It was observed that the removal of these specific emissions (mainly HC, soot, CO2 and H2O) during the addition of N2 resulted in a substantial reduction in engine-out mass of soot, although there was an increase in engine-out NOX emissions. This effect is more visible as the EGR ratio is increased and shows the influence CO2, H2O and soot (which tends to play a role in absorbing heat, thus reducing the combustion temperature) have on the reduction of NOX.
Comparing N2 to FEGR (without DOC) there was a small reduction in engine-out NOX emissions, which can be attributed to the presence of CO2 and H2O within the EGR gas agreeing with Ladommatos et al.11 and Anderlohr et al.14 However, the NOX emissions of FEGR are higher than that of unfiltered EGR and can be directly linked to the absence of soot, which again suggests soot exhibits some radiative heat absorption capacity. With less heat being radiated away from the diffusion flame region as a result of lower soot particle concentration, it is believed that there will be a higher flame temperature which will result in further NOX formation. In Fig. 4 it can also be observed that there is an increase in engine-out soot emissions with respect to N2, resulting directly from the recirculation of HC even though the presence of H2O and CO2 effectively could have an opposing influence. Comparing to unfiltered EGR, the removal of soot plays a substantial role in reducing the engine-out soot emissions, again indicating its potential towards particulate growth. The effectiveness of the FEGR system can be further improved with the addition of a DOC upstream the DPF. The DOC inlet temperature ranged between 330–340 °C, resulting in 99% oxidation of CO and 80% oxidation of gas phase HC emissions present in the exhaust (see Fig. 2b). The oxidation of HC and CO to H2O and CO2 (Table 4) gives a further benefit in terms of its thermal and chemical abilities towards NOX and soot control; with the removal of HC also limiting soot formation and surface growth. Radicals such as OH are also known to be effective for soot oxidation and it is suggested that CO2 and H2O kinetically enhance soot oxidation in a similar way to hydrocarbon oxidation through third body reactions. As a conclusion and combining the works of Ladommatos et al.11 and Anderlohr et al.,14 the thermal effect of CO2 and H2O results in a reduction in NOX emissions and slight increase in net soot, while the kinetic effect results in a decrease in NOX and an enhancement in the rate of hydrocarbon and soot oxidation.
Overall it can be observed that the presence of H2O and CO2 have a positive impact on the reduction of both engine-out NOX and soot emissions through the chemical and thermal effects as previously illustrated in Table 1 and mentioned in the introduction section of this paper. In terms of soot emissions, it is suggested that there is more than one benefit that can be gained from having species such as CO2 and H2O present in the EGR gas stream. Firstly, the chemical effect of CO2 and H2O through third body reactions, which increases the production of the OH radical (Anderlohr et al.14), enhances soot oxidation. Secondly, the increase in OH radicals also enhances hydrocarbon oxidation (Anderlohr et al.14), restricting soot formation and surface growth through the hydrocarbons recirculation penalty. However, the greatest impact comes from the soot recirculation penalty significantly favouring particulate growth, which is also supported by the recirculation of HC emissions. It is suggested that these recirculated HC emissions condense onto the soot when the exhaust gas and the fresh inlet charge meet within the intake manifold, resulting in an increased surface mass. Therefore, in order to obtain the best possible NOX/soot trade-off, soot recirculation must be controlled while promoting the oxidation of CO and HC emissions to promote the presence of H2O and CO2.
3.3. Assessing the potential of FEGR towards DPF regeneration
The main benefit of FEGR (with DOC) is that there are fewer gaseous HC's and particles with which agglomerates can form and undergo surface growth during their time in the primary reaction zone and cooler parts of the combustion chamber and exhaust. This can be observed in Fig. 5 where the engine-out particle mass concentration during FEGR is reduced considerably for particles with diameters over 100 nm suggesting particle growth has been reduced in comparison to unfiltered EGR. However, it is important to note that there is no significant influence or increase in the nanoparticles (<50 nm) during FEGR, although when the EGR rate is increased the particulate mass concentration increases for every particle size diameter. The results of the particulate mass distributions have a good correlation with the results of the soot presented earlier, further showing the benefit of FEGR on the overall NOX/soot trade-off. Table 5 shows a similar analysis to that implemented in our earlier investigation.16 Comparisons with N2 and FEGR have been made based on the same intake O2 availability. This again shows the mass per cycle of soot recirculated through EGR and the global effect of combustion on both the newly and recirculated soot particles. This includes the growth and re-oxidation of the recirculated particles and the formation, growth and oxidation of the newly formed particles. However, the analysis now compares unfiltered EGR with N2 as well as FEGR. As observed in our previous investigation, the effect of particulate growth through combustion (taking into consideration the re-oxidation of recirculated soot) on the engine-out mass per cycle of soot is greater than the mass per cycle of soot recirculated through EGR. Although a benefit can be gained from the obvious removal of the soot particles, it is also evident from Fig. 4 that the gas phase HC emissions also play an important role. The removal of both soot and HC emissions therefore cannot participate in further soot formation and growth in the combustion chamber. It is also known that the main difference between the addition of N2 and FEGR is the presence of H2O and CO2, with some recirculated HC emissions. Therefore, if the entire HC emission could be oxidised upstream the EGR loop during FEGR, the benefit of this system would be greater.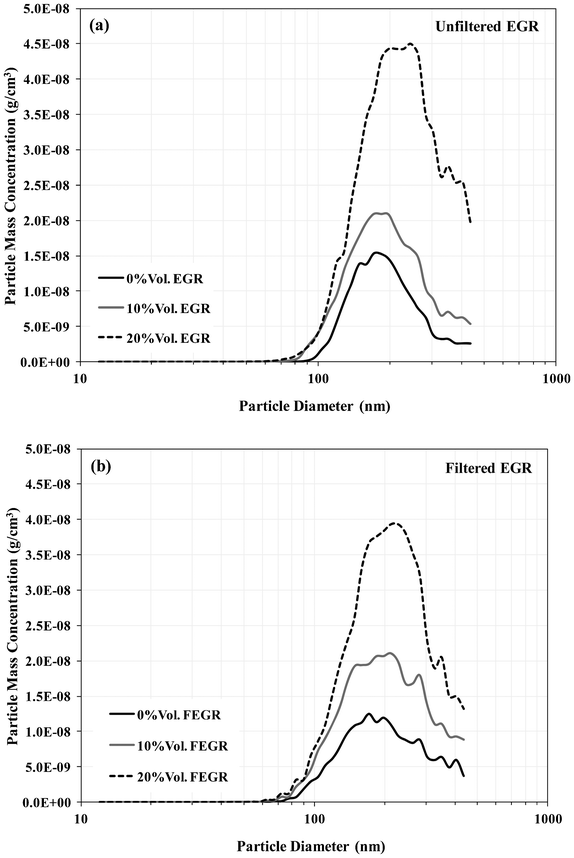 | ||
| Fig. 5 Particulate mass distribution. | ||
| Unfiltered EGR | Pure N2 EGR | FEGR (DOC + DPF) | ||
|---|---|---|---|---|
| EGR | mg/cycle | 0.0385 | 0 | 0.0009 |
| Exhaust | mg/cycle | 0.3096 | 0.1498 | 0.1372 |
| Net Combustion | mg/cycle | 0.2711 | 0.1498 | 0.1363 |
Comparing between filtering the whole exhaust with respect to only filtering the EGR loop as previous reported in our earlier investigation,16 there is a greater mass flow rate of exhaust being filtrated by the DPF. However, calculations have shown that this is partially outweighed by a lower engine-out soot concentration brought about from controlling the soot and HC recirculation penalties. By calculating the mass flow rates of soot (corresponding to 20%Vol.EGR), it was observed that there was approximately 50% less mass of soot present in the exhaust when the whole exhaust was filtered compared to our earlier investigation. It is important to note that this takes into consideration the greater exhaust gas flow being filtered through the DPF. Therefore, this shows that the resulting reduction in engine-out soot emissions through the control of the soot and HC recirculation penalty is far more beneficial compared to when only the EGR loop is filtered. Thus, the final result would be a lower rate of DPF soot loading and hence a reduced level of active regeneration intervals. It also seems that by controlling the HC recirculation we have gained a further benefit compared to our previous investigation which was focused primarily on soot emissions.
In addition to the engine-out soot reduction, there also seems to be an overall improvement in the NOX/soot ratio with FEGR compared to unfiltered EGR, which can be beneficial towards passive DPF regeneration. In Fig. 6 it is shown that the NO to NO2 oxidation over the DOC is temperature dependant, with a limit is placed on the NO–NO2 oxidation over the DOC at high temperatures. After this limit the NO2 concentration reduces similar to the rate at which it increased. In previous studies it was suggested that the NO2/NOX ratio can be further improved through the addition of small quantities of H2, introduced with the engine exhaust gas before directed through to the DOC.23 The results presented here show that the H2 promotes the rate of NO–NO2 oxidation by improving the catalyst light off performance, thus greater NO2 can be obtained under lower exhaust gas temperatures. However, using a high H2 concentration does not further enhance the NO–NO2 oxidation suggesting that the oxidation of H2 may be more predominant than the oxidation of NO. Therefore, the benefit of H2 addition for NO2 production is a combined effect of H2 oxidation resulting in an increased local temperature, due to this being exothermic, and an increase of the reaction rates thus reducing the catalyst light-off temperature so further NO oxidation can occur at low exhaust gas temperatures.21 However, this is dependent on the H2 concentration, with smaller concentrations being more advantageous at higher catalyst inlet temperatures, while larger H2 concentrations being more beneficial at lower catalyst inlet temperatures as shown in Fig. 6.
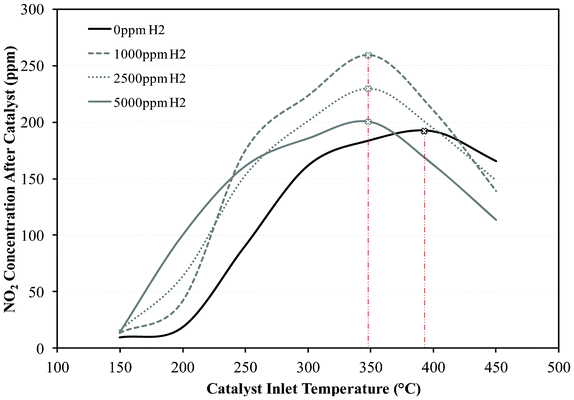 | ||
| Fig. 6 The influence of H2 addition on NO2 formation over the DOC. | ||
The results presented in this study have given an insight into how a cooled EGR system can be enhanced, allowing further control of the engine-out soot emissions with an improved NOX/soot trade-off. By implementing H2 it is suggested that the overall NO2/soot ratio can also be promoted and along with a reduced rate of soot loading there will be a benefit for low temperature passive DPF regeneration.
4. Conclusion
As the initial study primarily focused on controlling the soot emissions, further work was carried out to also control the recirculation of organic and polyaromatic unburned hydrocarbons (PM precursors) back into the combustion chamber during EGR. This involved using a combined DPF and DOC system, paying significant attention to the exhaust back pressure. The results again showed the recirculated soot particles (especially at high load and high EGR ratios) playing an important role in promoting the growth of further particles within the combustion chamber. However, by isolating the dilution effect and introducing N2 as the exhaust gas it was possible to correlate the increase in soot mass with EGR to the recirculation of these particles as well as the presence of unburned HC emissions, CO2 and H2O. The findings from this report particularly highlight the possibility of extending the EGR tolerance at high engine loads giving the potential to further reduce NOX before reaching smoke limited conditions in order to meet future regulations that are likely to dominate future engine designs. The key advantages of this system is that FEGR can be implemented in current EGR systems to reduce engine-out soot emissions, thus reducing the DPF loading and regeneration intervals, or used as an approach to extend the current EGR limits for further NOX control. In both cases this technique will also result in an improved NOX/soot ratio which can help promote passive DPF regeneration.Acknowledgements
The School of Mechanical Engineering at the University of Birmingham (UK) is gratefully acknowledged for the PhD scholarship to Mr. S.S Gill. Dr D. Turner thanks Engineering and Physical Science Research Council – EPSRC projects (EP/G038139/1) for supporting the research work and his post doctoral research at Birmingham. Dr J. M. Herreros thanks Johnson Matthey Plc for supporting his post doctoral research at the University of Birmingham. Johnson Matthey Plc is also recognised for supporting this work. Thanks to Advantage West Midlands and the European Regional Development Fund as part of the Science City Research Alliance Energy Efficiency project.References
- R. J. Farrauto and K. E. Voss, Appl. Catal., B, 1996, 10, 29–51 Search PubMed.
- K. Vaaraslahti, J. Ristimäki, A. Virtanen, J. Keskinen, B. Giechaskiel and A. Solla, Environ. Sci. Technol., 2006, 40, 4776–4781 Search PubMed.
- V. Ganesan, Internal combustion engines, McGraw-Hill Education (India) Pvt Ltd, 2008 Search PubMed.
- D. R. Tree and K. I. Svensson, Prog. Energy Combust. Sci., 2007, 33, 272–309 CrossRef CAS.
- I. M. Kennedy, Prog. Energy Combust. Sci., 1997, 23, 95–132 CrossRef CAS.
- K. Kitano, I. Sakata and R. Clark, SAE Paper, 2005, 2005-01-3763 Search PubMed.
- A. Abu-Jrai, A. Tsolakis, K. Theinnoi, R. Cracknell, A. Megaritis, M. L. Wyszynski and S. E. Golunski, Energy Fuels, 2006, 20, 2377–2384 Search PubMed.
- M. V. Twigg, Appl. Catal., B, 2007, 70, 2–15 CrossRef CAS.
- M. Zheng, G. T. Reader and J. G. Hawley, Energy Convers. Manage., 2004, 45, 883–900 CrossRef CAS.
- G. H. Abd-Alla, Energy Convers. Manage., 2002, 43, 1027–1042 CrossRef CAS.
- N. Ladommatos, S. M. Abdelhalim, H. Zhao and Z. Hu, SAE Paper, 1996a, 961165 Search PubMed.
- H. Zhao, J. Hu and N. Ladommatos, Proc. Inst. Mech. Eng., Part D, 2000, 214, 405–419 Search PubMed.
- N. Ladommatos, S. Abdelhalim and H. Zhao, Int. J. Engine Res., 2000, 1, 107–126 Search PubMed.
- J. M. Anderlohr, A. Pires da Cruz, R. Bounaceur and F. Battin-Leclerc, Combust. Sci. Technol., 2010, 182, 39–59 Search PubMed.
- C. K. Westbrook, Proc. Combust. Inst., 2000, 28, 1563–1577 CrossRef CAS.
- S. S. Gill, D. Turner, A. Tsolakis and A. P. E. York, Environ. Sci. Technol., 2012, 46, 4215–4222 Search PubMed.
- S. C. Choi, H. G. Roh, K. S. Lee and C. S. Lee, Energy Fuels, 2010, 24, 2875–2882 Search PubMed.
- J. Zhu, K. O. Lee, A. Yozgatligil and M. Y. Choi, Proc. Combust. Inst., 2005, 30, 2781–2789 Search PubMed.
- S. Rothenbacher, A. Messerer and G. Kasper, Part. Fibre Toxicol., 2008, 5, 9 Search PubMed.
- J. B. Heywood, Internal combustion engine fundamentals, Mc Graw-Hill, 1988 Search PubMed.
- S. R. Katare and P. M. Laing, SAE Paper, 2009, 2009-01-1268 Search PubMed.
- H. Klein, E. Lox, T. Kreuzer, M. Kawanami, T. Ried and K. Bächmann, SAE Paper, 1998, 980196 Search PubMed.
- K. Theinnoi, S. S. Gill, A. Tsolakis, A. P. E. York, A. Megaritis and R. M. Harrison, Energy & Fuels, 2012, 26, 1192–1201 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |
