Exploration of the versatility of ring opening metathesis polymerization: an approach for gaining access to low density polymeric aerogels†
Sung
Ho Kim
*a,
Marcus A.
Worsley
a,
Carlos A.
Valdez
a,
Swanee J.
Shin
b,
Christoph
Dawedeit
b,
Tom
Braun
b,
Theodore F.
Baumann
a,
Stephan A.
Letts
b,
Sergei O.
Kucheyev
b,
Kuang
Jen J. Wu
b,
Juergen
Biener
b,
Joe H.
Satcher
Jr.
a and
Alex V.
Hamza
b
aChemical Sciences Division, Lawrence Livermore National Laboratory, P. O. Box 808, L-235, 7000 East Avenue, Livermore, CA 94550, USA. E-mail: kim61@llnl.gov; Tel: +1-925-423-2072
bNanoscale Synthesis and Characterization Laboratory, Lawrence Livermore National Laboratory, P. O. Box 808, L-235, 7000 East Avenue, Livermore, CA 94550, USA
First published on 24th July 2012
Abstract
We report the preparation of low density polymeric aerogels using the ring opening metathesis polymerization (ROMP) approach to copolymerize dicyclopentadiene (DCPD) with norbornene-based monomers (NB-R) employing a first generation Grubbs' ruthenium-based catalyst. The ROMP approach offers an attractive synthetic method that enables the fabrication of low-density (0.02–0.05 g cm−3), uniform thickness aerogel coatings on non-planar substrates. First, we explore the effect of crosslinking in the polymer backbone on the uniformity of the gel coatings formed under shear by either adding a multi-norbornene based crosslinker (NBn-R) to increase, or linear NB-R comonomers to decrease, the degree of crosslinking, respectively. We observed that adding linear monomers dramatically improved the uniformity of the gel films which we attribute to cross-linking induced changes in the rheological properties at the gel point. Second, the copolymerization of DCPD and NB-R with a different pendant group also causes a significant change in the morphology of the PDCPD-based aerogels by modifying the lengths of the strands in the fibrous polymer network. The effect of NB-R addition on the pore structure of the aerogels is discussed in the context of a molecular and interparticle crosslinking model. Finally, (bis)iodo-norbornene was synthesized to demonstrate the feasibility to fabricate functionalized aerogels by using our copolymerization approach. Our results highlight the potential of the ROMP-based copolymerization approach as a facile and versatile route to functionalized low density polymeric aerogels.
1. Introduction
Aerogel materials with large intrinsic surface areas and ultralow densities have become attractive candidates for a wide range of applications including supercapacitors and batteries, thermal/electrical insulators, advanced catalyst supports, absorbents and carriers, and hydrogen storage.1–4 Organic and polymeric aerogels were first reported as the counterparts of inorganic aerogels.5–9 The sol–gel synthesis of inorganic aerogels typically involves the transformation of molecular precursors into highly cross-linked inorganic gels that are subsequently dried using special techniques (i.e., supercritical drying, freeze drying, etc.) in order to preserve their tenuous solid network. In a similar fashion, the preparation of organic and polymeric aerogels has been achieved by either (i) physical phase inversion (or coagulation) of polymers from the liquid phase to solids by changing the solvent/non-solvent pairs or (ii) polymerization of multi-functional organic species into three-dimensional cross-linked polymer networks.5–9 While most of the previous work has focused on the utilization of bulk aerogels, we are currently exploring the fabrication of low-density, uniform thickness (from 10 μm to 100 μm) aerogel coatings on the inside of cylindrical and spherical substrates.10,11 Such porous polymer coatings open the door to many interesting new applications such as superhydrophobic surfaces, anti-reflection coatings, column chromatography, and porous polymer membranes.12 Our specific interest is in the fabrication of inertial confinement fusion (ICF) targets.11 These targets consist of a small spherical capsule, whose inside wall is coated with a thin layer of a low density foam that is used (i) to support and define the shape of the cryogenic liquid or solid hydrogen fuel for the fusion reaction and/or (ii) as a scaffold to support dopants for diagnostics. Briefly, our strategy to fabricate aerogel coatings consists of filling a prefabricated shell with the required amount of the sol precursor followed by the continuous rotation of the shell to form a smooth layer of uniform thickness during the gel formation.11 Cross-linking provides the required control over the viscosity of the precursor solution at the sol–gel transition.Here, we use the ring opening metathesis polymerization (ROMP) approach for the synthesis of polymeric aerogels. To our knowledge, there have only been a few previous examples of applying the ROMP approach to prepare bulk porous aerogel materials.13,14 Lee and Gould first demonstrated the feasibility of polydicyclopentadiene (PDCPD) aerogels as new insulation materials.13 Leventis et al. reported a novel low temperature synthetic route to extremely robust polyimide aerogels via ROMP of a crosslinkable norbornene end-capped diimide monomer.14a Although these works demonstrate the ability to produce monolithic aerogels, our goal of forming uniform aerogel coatings adds another layer of complexity. For example, the coating of non-planar surfaces with thin and homogeneous aerogel films requires continuous rotation of the object to be coated. Without proper control over viscosity and gel time, shear forces can damage the growing gel networks and prevent the formation of uniform coating.10 Using the example of polydicyclopentadiene-based gels, we demonstrate that both the moduli and viscosity at a gel point can be tuned by modifying the degree of cross-linking through the copolymerization of dicyclopentadiene (DCPD) with a linear norbornene comonomer. The effect of monomer addition on the morphology of the resulting aerogels was investigated by using substituted norbornene monomers (NB-R) with various pendant groups R. Finally, a (bis)iodo-norbornene monomer was added to demonstrate the fabrication of functionalized aerogels and to give further quantitative understanding on this copolymerization process. The goal of our study is to provide a simple and versatile synthetic route to tailor the gelation behavior, pore structure, and the morphology of the final polymer network expanding the applicability of low density polymeric aerogels.
2. Experimental
2.1 Materials and general characterization
Dicyclopentadiene (DCPD, Aldrich), norbornene (NB, 99%, Aldrich), 5-norbornene-2-methanol, (3, 98%, Aldrich), 5-norbornene-2-exo,3-exo-dimethanol (10, 97%, Aldrich) and a first generation Grubbs' catalyst, bis(tricyclohexylphosphine)benzylidene ruthenium dichloride (+97%, Aldrich), and other chemicals were used as received unless noted otherwise. As supplied, the DCPD and monomer 3 are a mixture of mostly endo and exo isomers. They were used without further purification. Toluene (anhydrous, 99.8%, Aldrich) was degassed by bubbling with high purity nitrogen prior to use.1H NMR spectra were obtained with a Bruker Avance 600 instrument at 600 MHz. Rheological properties of precursor solutions during the gelation were characterized with a Rheometrics Fluids Spectrometer RFS 8400 in a dynamic-time-sweep mode with a Couette geometry (a 17 mm radius cup and a 16 mm radius bob). Measurements were carried out at room temperature with a strain amplitude of 5% of the 1 mm gap between the cup and the bob and a frequency of 1 radian per second. To minimize solvent evaporation, the cup was covered with a custom built lid. The morphology of the monolithic aerogels was investigated with a Jeol JSM-7401F scanning electron microscope (SEM) at an acceleration voltage of 2–3 kV in a lower secondary electron image (LEI) mode with no sputter coating. The 4 MV ion accelerator (NEC, model 4UH) at Lawrence Livermore National Laboratory was used to perform Rutherford backscattering spectrometry (RBS) measurements with 1.5 MeV 4He+ ions incident normal to the sample surface and backscattered into a detector at 164° relative to the incident beam direction. During the ion-beam analysis, samples were neutralized by coirradiation with a 500 eV electron beam. Analysis of the RBS spectra was done with stopping powers and scattering cross sections from the RUMP code15 with the assumption of a specimen composition of C10H12Ix. BET surface areas, pore volumes and pore size distributions were measured by nitrogen absorption porosimetry with a Micromeritics Instrument ASAP 2000 after degassing at 70 °C for 12 h. Bulk densities were calculated from the weight and the physical dimensions of the samples.
2.2 Synthesis of norbornene-based cyclic olefin monomers
Scheme 1 summarizes the synthesis of the norbornene-based cyclic olefin crosslinker and comonomers used in this study. First, the norbornene-based crosslinker, 1,4-di(exo-bicyclo[2.2.1]hept-5-en-2-yl)benzene (2), was prepared as previously reported (Scheme 1a).16 Purification was carried out by column chromatography (ethyl acetate/hexanes) followed by re-crystallization in ethyl acetate. Next, several norbornene monomers with different lengths of the alkyl groups were prepared similarly to the synthesis of 5-methoxymethylbicyclo[2.2.1]hept-2-ene (4) (Scheme 1b).17,18 Other alkyl substituted norbornene-based monomers 5–7 were synthesized by reacting 5-norbornene-2-methanol (3) with corresponding alkyl halides such as hexyl bromide, dodecyl bromide, and benzyl bromide in the presence of sodium hydride. The purification of the monomers was carried out by extraction followed by silica gel flash column chromatography. Finally, iodo-norbornene monomer 9 was prepared and used as a monomer with a strong electronic interaction. The synthesis of the iodo-norbornene monomers was accomplished by initially tosylating the corresponding norbornene alcohol 3 in the presence of 4-(dimethylamino)pyridine (DMAP) followed by tosyl displacement using sodium iodide (Scheme 1c).19–21 As a practical way to double the number of chemical functionalities in one monomer, 5-norbornene-2,3-dimethanol 10 was used to prepare the bis-tosylated norbornene monomer 11 and bisiodo-norbornene monomer 12 as a typical example of functional monomers for the monitoring of gel formation. Full details of the synthesis and characterization of the norbornene-based monomers are described in the ESI.†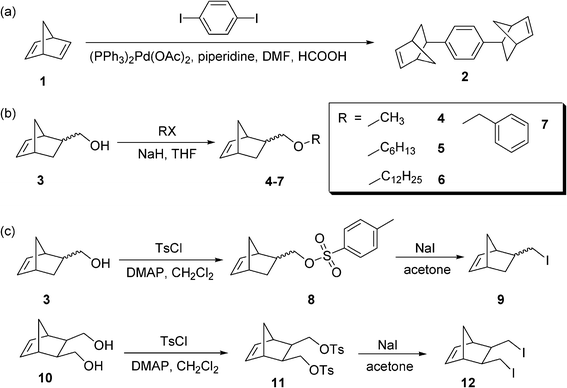 | ||
| Scheme 1 The synthesis of cyclic olefin monomers. | ||
2.3 Preparation of poly(dicyclopentadiene-random-substituted norbornene) P(DCPD-r-NB-R) aerogels
Low-density (0.02–0.05 g cc−1) P(DCPD-r-NB-R) gels were prepared from a ring opening metathesis polymerization (ROMP) of DCPD and NB-R in toluene employing a first generation Grubbs' catalyst as the catalyst/initiator (Scheme 2). Here, NB-R is a generic term for norbornene (NB) and other NB derivatives. Different P(DCPD-r-NB-R) (100/x) (wt/wt) copolymer wet-gels were obtained by controlling the feed ratios of DCPD and NB-R by weight. For example, to fabricate 50 mg cc−1 P(DCPD-r-NB-R) (100/20) (wt/wt) aerogels, separate 50 mg cc−1solutions of DCPD and NB-R monomers in toluene were prepared and then mixed as needed (0.83 mL of the DCPD and 0.17 mL of the NB-R solution for a 100/20 wt/wt aerogel). After adding an aliquot of the catalyst (0.1 mg) dissolved in toluene, the mixture was gelled in a sealed vial under ambient conditions.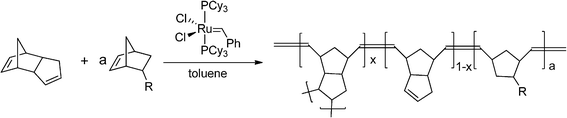 | ||
| Scheme 2 Ring opening metathesis polymerization of DCPD and NB-R. | ||
2.4 Supercritical CO2 drying
Wet gels were first placed into an agitated acetone bath for 2–3 days and then transferred to a Polaron critical point dryer. Acetone-filled gels were exchanged with a continuous flow of liquid CO2 at 850 psi. Once the solvent was completely removed, the temperature and pressure in the dryer were increased to the supercritical regime (50 °C and ∼1500 psi) and kept for 2–4 h. The pressure was then allowed to slowly decrease to atmospheric pressure while keeping the temperature constant. Dry aerogels were subsequently recovered by slowly cooling down to room temperature.3. Results and discussion
Considering that pure PDCPD polymers have a partially cross-linked structure,22–29 our approach was to control the degree of crosslinking in the polymer backbone through either (i) the use of a more reactive crosslinker to increase the crosslinking density or (ii) the copolymerization of DCPD with a linear monomer to decrease the degree of crosslinking. The norbornene-based crosslinker 2 was first used as a control to overcome the inherent limitation in the crosslinking of DCPD molecules. Scheme 1a shows the synthesis of the norbornene-based crosslinker 2 from a carbon–carbon coupling reaction of norbornadiene 1 with diiodobenzene, as previously reported.16 Second, various cyclic-olefin monomers, NB-R, were prepared from 5-norbornene-2-methanol (3) and 5-norbornene-2,3-dimethanol (10) in order to investigate the effect of a pendant group on the morphology of gels (Scheme 1b and 1c). The alcohol functionalized norbornene monomers can be used to form derivatives having various functional groups, as previously reported.17,18 Norbornene-based monomers were selected for their mild polymerization conditions and ability to form statistical copolymers with dicyclopentadiene rings. In this study, we prepared alkyl norbornene monomers (4–7) by protecting a pendant alcohol group with various alkyl halides including methyl iodide, hexyl bromide, dodecyl bromide, and benzyl bromide, respectively (Scheme 1b). Based on the difference in the polarity of reactant 3 and the protected adducts 4–7, thin layer chromatography (TLC) and column chromatography were used for the analysis and purification of the crude product. 1H NMR spectra before and after the protection reaction show the characteristic shift of the peaks assigned to the –C(2)H–, –C(2)H–CH2O–R, and –C(5)H![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(6)H– bonds. Finally, a norbornene monomer 8 with a good leaving group was prepared from the reaction of 3 with p-toluenesulfonyl chloride (TsCl) in the presence of a base such as triethylamine (TEA), pyridine, or 4-(dimethylamino)pyridine (DMAP) (Scheme 1c). The use of DMAP or pyridine was found to be suitable to promote the reaction and led to a quantitative tosylation, while TEA resulted only in a partial conversion. This reaction was monitored by 1H NMR spectroscopy, in which the resonances at 5.90 ppm, 3.75–3.30 ppm and 2.30–1.60 ppm, corresponding to –C(5)H
C(6)H– bonds. Finally, a norbornene monomer 8 with a good leaving group was prepared from the reaction of 3 with p-toluenesulfonyl chloride (TsCl) in the presence of a base such as triethylamine (TEA), pyridine, or 4-(dimethylamino)pyridine (DMAP) (Scheme 1c). The use of DMAP or pyridine was found to be suitable to promote the reaction and led to a quantitative tosylation, while TEA resulted only in a partial conversion. This reaction was monitored by 1H NMR spectroscopy, in which the resonances at 5.90 ppm, 3.75–3.30 ppm and 2.30–1.60 ppm, corresponding to –C(5)H![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(6)H–, –C(2)H–CH2OH, and –C(2)H– in 3, respectively, clearly shifted to 5.69 ppm, 4.08–3.57 ppm, and 2.40–1.73 ppm without overlapping those of the starting material. Norbornene activated with a good leaving group provides us with a useful platform for the introduction of additional chemical functionalities in the polymer. For example, iodo-norbornene monomer 9 was prepared by reacting monomer 8 with sodium iodide. This reaction was confirmed from the change in the chemical shift and the disappearance of –OTs at 7.78–7.34 ppm. Norbornene-diol monomer 10 was also selected as a starting material to double the number of functionalities in one monomer. Direct reaction of monomer 10 with TsCl and subsequent precipitation of the mixture in diethyl ether gave bis-tosylated norbornene 11 as a white powder. Further purification of the residual solution by column chromatography isolated mono-substituted tosyl norbornene and cyclic ether norbornene byproducts, as identified by 1H NMR. Then monomer 11 was reacted with excess NaI and transformed into bisiodo-norbornene monomer 12 in quantitative yield.
C(6)H–, –C(2)H–CH2OH, and –C(2)H– in 3, respectively, clearly shifted to 5.69 ppm, 4.08–3.57 ppm, and 2.40–1.73 ppm without overlapping those of the starting material. Norbornene activated with a good leaving group provides us with a useful platform for the introduction of additional chemical functionalities in the polymer. For example, iodo-norbornene monomer 9 was prepared by reacting monomer 8 with sodium iodide. This reaction was confirmed from the change in the chemical shift and the disappearance of –OTs at 7.78–7.34 ppm. Norbornene-diol monomer 10 was also selected as a starting material to double the number of functionalities in one monomer. Direct reaction of monomer 10 with TsCl and subsequent precipitation of the mixture in diethyl ether gave bis-tosylated norbornene 11 as a white powder. Further purification of the residual solution by column chromatography isolated mono-substituted tosyl norbornene and cyclic ether norbornene byproducts, as identified by 1H NMR. Then monomer 11 was reacted with excess NaI and transformed into bisiodo-norbornene monomer 12 in quantitative yield.
Scheme 2 shows the synthetic scheme of P(DCPD-r-NB-R) copolymer gels from the ROMP reaction of a mixture of DCPD and NB-R in toluene. In proposing the chemical structures of the copolymers, it is assumed that the strained norbornene-derived double bond in the DCPD monomer is much more reactive than the cyclopentene-derived double bond, and the olefin addition contributes to the formation of crosslinked PDCPD.27 Modified PDCPD-based aerogels were prepared by controlling the composition of the mixture of cyclic-olefin monomers including DCPD and NB-R. The gelation behavior of the DCPD–NB-R mixture showed a dependency on the ratio of NB-R to DCPD as well as the concentration of the olefin monomers and the catalyst, consistent with a previous study of pure PDCPD aerogels.10,13 Bulk PDCPD and P(DCPD-r-NB-R) copolymer wet-gels were stable even after a few weeks, and pure NB solutions in the same conditions did not form gels. In the present study, the ratio of the catalyst to monomers and the total monomer concentration were fixed as 0.002 (wt/wt) and 50 mg cc−1, respectively, unless otherwise noted. Both pure DCPD and mixtures of DCPD and NB-R were gelled at room temperature, and the catalyst did not appear to have any acute air or moisture sensitivity. It is worthy to mention that the use of a first generation Grubbs' catalyst over a second generation Grubbs' catalyst is due to its shorter gelation time in our typical experimental conditions (not in a glove box). Typically, the addition of NB-R monomers increases the gelation time from ∼10 min for pure DCPD to a few hours up to a few days. The addition of the NB-based crosslinker 2, on the other hand, reduces the gelation time to ∼1 min (see Table S1 in the ESI†). During the gelation of pure DCPD or 2, there was a phase transition from a transparent solution to an opaque gel due to the light scattering by polymerized wet-gels. However, wet-gels prepared from a higher loading of the NB-R monomer remained transparent after gelation. All the wet-gels were supercritically dried to form white monolithic aerogels.
The ability to form low-density thin film aerogel coatings with a thickness of ∼10 μm to 100 μm is crucial to our intended application. Recently, we reported on the effect of NB addition on the uniformity of PDCPD films.10 Here, we extend our study to higher NB concentrations and to other NB-based monomers, NB-R. For comparative purposes, we include our previous results in the following discussion. The effect of NB-R additions was first evaluated by studying the formation of thin films in rotating horizontal glass vials (∼20 mL) containing a precursor solution (1 mL) on a compact roller system, commonly used in cell culture studies (Fig. 1). Fig. 1b through 1d show the results of gel coating experiments with pure PDCPD in conjunction with a norbornene based crosslinker and P(DCPD-r-NB-R) copolymer gels. These figures reveal that pure PDCPD gels do not form monolithic thin films under these conditions, instead, the vial was coated with irregularly-shaped small lumps of the gel and contained a significant amount of fluid suggesting that the lumps consisted of a higher density gel. The norbornene based crosslinker 2 does not show the superfluous fluid, but does not form a film probably due to its extremely fast gelation behavior. In sharp contrast, a uniform coating of a transparent wet-gel layer on the inside wall of the glass vial was observed from P(DCPD-r-NB-R) (100/20) at 50 mg cc−1, regardless of the structure of the –R substituents. In order to understand this unique change in gelation behavior under rotation and shear, we investigate the effect of norbornene addition on the rheological properties of P(DCPD-r-NB) gels, as shown in Fig. 1e through 1g (Fig. S1 in the ESI†). Isothermal dynamic time tests at a given frequency and strain show that both shear moduli and complex viscosity increase over time. A sudden increase in moduli and viscosity in a short period of time is a typical change seen in gelation. In this study, we determined the gel point, or the initial formation of an infinite network, to be the time when the storage shear modulus, G′, exceeds the loss shear modulus, G′′,24 although several different protocols have been reported in the literature.30 Our rheological measurements reveal that the time at which G′ and G′′ cross over drastically increases with increasing NB concentration up to 10 wt % and then levels off even after further NB addition. Both moduli and viscosity at the crossover point increase almost linearly up to 40 wt %, as summarized in Table 1. The higher viscosity of P(DCPD-r-NB) near the gelation point reduces the shear experienced by the growing polymer network and makes it possible to fabricate a uniformly coated wet-gel layer on specific substrates, as reported in our previous work.10 The BET surface areas and bulk densities of the aerogel samples are also summarized in Table 1. It is worthy to mention that norbornene has a much higher ROMP reactivity (kobs of 0.42 s−1) than endo-DCPD (kobs of 0.019 s−1) under the same conditions.22 Surprisingly, the gel time for PDCPD (491 s) is shorter than for P(DCPD-NB) gels (∼2100 s). Although PDCPD aerogels show no volume change during the gelation and supercritical CO2 drying process, we observe that the measured density (∼37 mg cm−3) is lower than the target density (∼50 mg cm−3). This suggests the incomplete conversion of monomers to gels, for example due to the early precipitation of highly crosslinked PDCPD networks caused by their limited solubility in toluene. Some amounts of shrinkage of the gels during the acetone exchange process and the CO2 drying process were observed in the P(DCPD-r-NB) samples with more than 20 wt% NB, causing a sudden decrease in the BET surface areas and a deviation from the target bulk density (see Fig. S2 in the ESI†). Preventing volume shrinkage at higher NB concentrations is the subject of current work that will be described in a future publication.
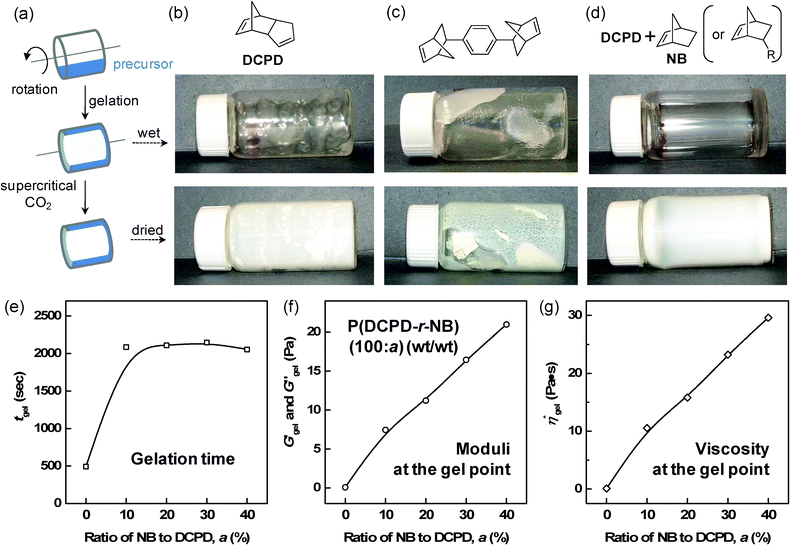 | ||
| Fig. 1 (a) Schematic representation of the coating experiment of the precursor solution. (b–d) Typical examples of the formation of wet-gels and supercritical CO2 dried aerogel films prepared from pure DCPD, a multi-norbornene (nNB)-based crosslinker, and a mixture of DCPD and NB (or NB-R). (e–g) The influence of NB addition on the formation of P(DCPD-r-NB) copolymer gels under shear and the rheological properties of the shear moduli and complex viscosity at the gel point. | ||
| Sample | Feed ratio of DCPD to NBa | t gel (s) | G′gelb (Pa) | η*gelb (Pa s) | Surface areac (m2 g−1) | Total pore volumec (m2 g−1) | Bulk densityd (mg cm−3) | |
|---|---|---|---|---|---|---|---|---|
| by weight | by moles | |||||||
| a DCPD and NB monomers were separately dissolved to have a concentrations of 50 mg cc−1 in toluene and mixed to a final composition. b The gel point of the system was determined from the crossover of the storage shear modulus, G′, and the loss shear modulus, G′′, measured by rheometry. c The surface area and total pore volume of the aerogels measured by BET. d Bulk densities were calculated from the weight and physical dimensions and averaged with the multiple sample measurement | ||||||||
| P(DCPD-r-NB) | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
491 | 0.07 | 0.08 | 103 | 0.31 | 37 ± 4 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
2086 | 7.45 | 10.53 | 114 | 0.21 | 48 ± 8 | |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
2110 | 11.22 | 15.81 | 102 | 0.19 | 82 ± 13 | |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
2148 | 16.45 | 23.23 | 57 | 0.07 | 102 ± 37 | |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56 56 |
2055 | 20.97 | 29.59 | 22 | 0.02 | 244 ± 48 | |
In addition to modifying the rheological behavior, the copolymerization also impacted on the morphologies of the PDCPD-based aerogels. Fig. 2a through 2c are SEM images of dried PDCPD aerogels with different DCPD concentrations of 50, 30, and 20 mg cc−1. At a concentration lower than 10 mg cc−1, the reaction was too slow to form gels within 7 days. Regardless of the target concentrations, pure PDCPD aerogels have a similar three-dimensional porous network of randomly oriented fibrous-shape structures with a non-uniform irregular pore structure.13 Copolymerization with norbornene (NB) decreases the crosslinking density of pure PDCPD-based gels. Fig. 2d through 2f show the effect of the NB concentration (10 to 40 wt %) on the morphologies of dried 50 mg cc−1 P(DCPD-r-NB). P(DCPD-r-NB) copolymer aerogels have a fibrous three-dimensional porous network similar to that of pure PDCPD. However, the length of the individual fiber strands decreases from a few μm in the case of pure PDCPD to hundreds of nm in P(DCPD-r-NB) gels with higher concentrations of the NB monomer. This is consistent with the observation that NB addition results in the formation of clear wet-gels.
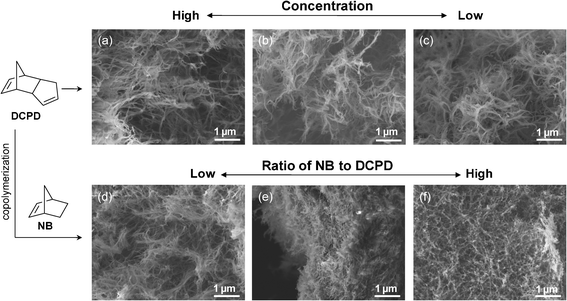 | ||
| Fig. 2 Effect of the composition of precursor solutions on aerogel morphologies. (a–c) Pure PDCPD aerogels prepared from (a) 50, (b) 30, and (c) 20 mg cc−1 solutions and (d–f) P(DCPD-r-NB) copolymer aerogels prepared from mixtures with different DCPD–NB ratios of (d) (100/10), (e) (100/20), and (f) (100/40) (wt/wt) at 50 mg cc−1. | ||
The effect of monomer addition on the morphologies of aerogels was further explored by using various norbornene monomers (NB-R) containing substituted groups with different steric and electronic structures. Fig. 3a through 3c are typical SEM images of aerogels prepared from mixtures of DCPD and NB-R with different lengths of alkyl groups derived from methyl iodide, hexyl bromide, and dodecyl bromide. P(DCPD-r-NB-R) aerogels prepared using monomer 4 (R = methyl) show the characteristic fibrous network morphology observed in pure PDCPD and P(DCPD-r-NB) at low NB concentrations, while monomers 5 and 6, which contain a longer alkyl chain group, created aerogels with a shorter and more entangled network. Pure PDCPD formed a number of clusters of long linear ligaments, whose entanglement led to the development of large, irregular shaped pores. However, the addition of comonomers such as NB and monomers 4–6 appears to have either reduced the length of the ligaments or caused more entanglement between them, the result of which is drastically different pore shape and size. As shown in Fig. 3d to 3f, more drastic changes in not only the pore size but also the pore morphology of the aerogels were observed from the addition of comonomers 7–9 that contain strong hydrophobic or electronic interactions. Monomers 7 and 8 with benzyl and tosyl groups resulted in spherical pores with sizes of ∼50 nm, while the addition of iodo-monomer 9 produced extended long fibers reminiscent of pure PDCPD (see Fig. S3 in the ESI†). It is worthy to mention that the norbornene-based crosslinker 2 with a similar reactivity of two norbornene rings does not accompany the unique fiber-like morphologies of PDCPD-based aerogels. It formed a fine particulate morphology, often observed in silica aerogels (see Fig. S4 in the ESI†).13 The effect of NB-R (or NB) monomer addition on the morphologies is schematically represented in Fig. 3g. The distinct features in P(DCPD-r-NB-R) copolymer networks are changes in the length of the fiber strands, and pore sizes and morphologies surrounded by the strands. Leventis and coworkers31–33 explained the formation of organic (polymer) aerogels by (i) crosslinking at the molecular level resulting in early phase separation of small surface-reactive particles and (ii) subsequent inter-particle crosslinking that improves the mechanical properties of the materials. Based on those principles, long PDCPD strands with lengths of hundreds of nm up to a few μm would be the combination of primary and secondary particles like in polyurea aerogels.32 In this study, the incorporation of NB-R with a different substituent groups might distort further growth of the primary PDCPD fibrils by either (i) decreasing the number of reactive sites (i.e., the crosslinking) at the surface or (ii) sterically hindering inter-particle crosslinking, as has been proposed for polyimide aerogels from anhydride and isocyanates.33 The formation of many shorter fiber strands with good solubility and/or better chain mobility makes it possible to form more entangled morphologies, and it could explain the transformation in the pore size and morphologies as well as the higher moduli and viscosity at the gel point in copolymer gel systems. Considering the effect of different crosslinkers, the unique fibril structure of primary particles seems to be attributed to the molecular structure of PDCPD and its inter-molecular interaction. In this regard, the effect of different DCPD stereo-isomers on morphologies is currently being investigated and will be a subject of further release. This result indicates that additional tuning over pore morphologies of polymeric aerogels could be gained by the copolymerization approach in conjunction with a careful design of comonomers and/or cross-linkers. Full details on the morphologies of P(DCPD-r-NB-R) aerogels with different compositions are included in the ESI†.
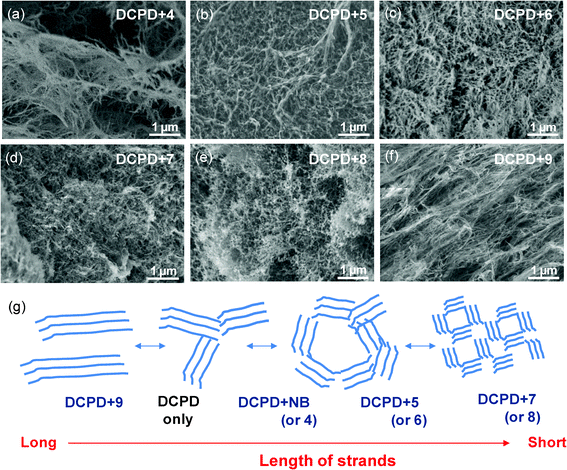 | ||
| Fig. 3 (a–f) Characteristic morphologies of P(DCPD-r-NB-R) aerogels prepared from a mixture of DCPD with a different NB-R comonomer: (a) DCPD+4, (b) DCPD+5, (c) DCPD+6, (d) DCPD+7, (e) DCPD+8, and (f) DCPD+9. (g) The proposed model for representing the effect of NB-R monomers on the formation of P(DCPD-r-NB-R) aerogels with different morphologies. | ||
The versatility of the copolymer networks derived from ring opening metathesis polymerization (ROMP) allows one to introduce chemical functionality by taking advantage of the tunability of the NB-R comonomer. For our specific application, a millimetre-sized spherical shell with a thin aerogel layer on the inside of the shell, the availability of a non-destructive imaging technique such as X-ray radiography is essential to judge the success of the coating experiment. This, however, requires increasing the weak X-ray absorption contrast of the CH-based foam by introducing a high-Z element. This motivated our effort to synthesize the bisiodo-norbornene monomer 12 (Fig. 4a). Fig. 4b shows a photo of P(DCPD-r-12) aerogels with different wt percentages, a, of monomer 12. Monolithic aerogels were formed from the supercritical CO2 drying process of P(DCPD-r-12). They did not show any serious shrinkage during the polymerization and drying process and retain the original shapes of PDCPD although a slight shrinkage occurred for high concentrations of monomer 12. A typical SEM image of the dried aerogels indicates that they have a fibrous-shape morphology, similar to other P(DCPD-r-NB-R) aerogels (Fig. 4c). The incorporation of iodine was verified with energy-dispersive X-ray spectroscopy (EDS) and the result is shown in Fig. 4d. Characteristic carbon and iodine EDS mapping images in Fig. 4e and 4f reveal a uniform distribution of iodine through the aerogels.
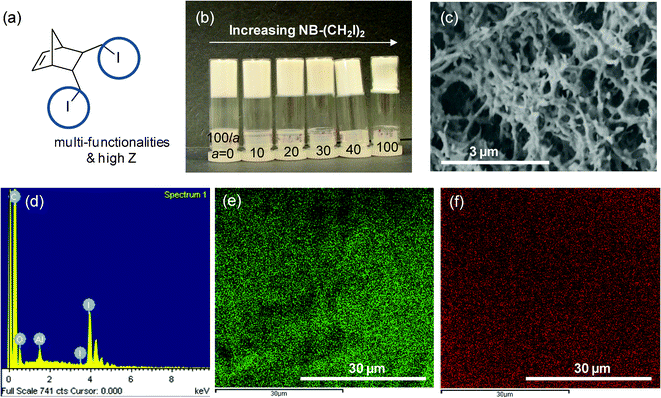 | ||
| Fig. 4 Introduction of chemical functionality into aerogels: (a) bisiodo norbornene monomer 12 as an example of a functional monomer, (b) a photo of dried P(DCPD-r-12) (100/a) (wt/wt) monoliths, (c–f) typical examples of (c) an SEM image and (d) energy-dispersive X-ray spectrum (EDS), (e) C-mapping and (f) I-mapping images of the P(DCPD-r-12) (100/100) aerogel. | ||
Further quantitative information on the material composition was obtained from Rutherford backscattering spectrometry (RBS) because of the unique ability of RBS to deliver highly accurate film compositions without the use of standards. Fig. 5a shows a typical RBS spectrum of P(DCPD-r-12) (100/100) acquired with an analyzing ion dose of ∼8 × 1012 cm−2. We found that the iodine concentration in the aerogels increased with the concentration of monomer 12 in the copolymer, as anticipated. However, we also found that He+ ion irradiation during the RBS analysis resulted in composition changes, as shown in Fig. 5b. Thus, the initial sample composition at zero dose of the analyzing beam for each sample was determined by extrapolating dose dependencies. The iodine concentrations in P(DCPD-r-12) (100/20) and (100/100) samples were determined to be about 0.63 and 1.90 (at. %), respectively. From the assumption of the chemical structure of P(DCPD-r-12) (Fig. 5c), the RBS result is used to determine the molar ratios of NB-R to DCPD in copolymer gels (see details in the ESI†). These values correspond to (100/21) and (100/75) by weight, respectively. This shows that the comonomers were uniformly incorporated throughout the polymer gels with a relatively good yield from feed ratios.
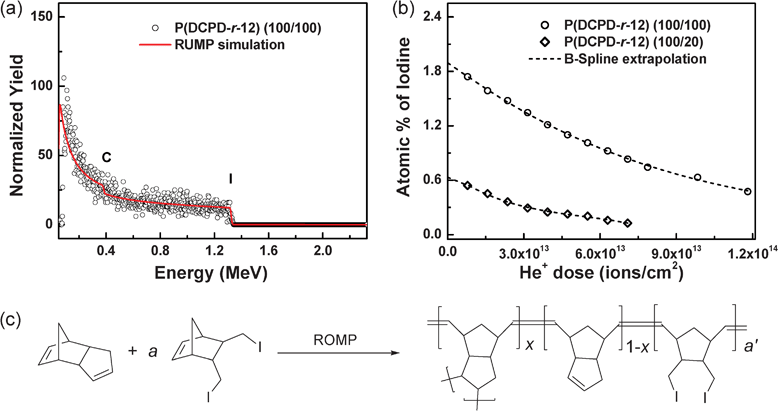 | ||
| Fig. 5 Rutherford backscattering spectrometry (RBS) analysis of P(DCPD-r-12) (100/a) (wt/wt) aerogels: (a) A typical RBS spectrum (symbols) and a RUMP-code simulation (solid line), (b) the atomic concentration of iodine in P(DCPD-r-12) samples as a function of the dose of the analyzing ion beam (1.5 MeV He), and (c) the chemical structure of P(DCPD-r-12) used for the determination of the composition of the samples. | ||
4. Conclusion
In summary, we have explored the potential of ring opening metathesis polymerization (ROMP) as a simple and efficient synthetic route to polymeric aerogels with a target density of 0.02–0.05 g cm−3. The copolymerization of dicyclopentadiene (DCPD) with various substituted norbornene (NB-R) monomers highlights the versatility of the ROMP approach. Significant enhancements in the coating behavior observed for P(DCPD-r-NB-R) copolymer gels make it possible to fabricate uniform low density polymeric aerogel coatings on various substrates. The copolymerization approach can also be used to modify the pore structure and morphology of aerogels as well as to introduce chemical functionality. The approach described herein could potentially expand the use of hydrocarbon aerogel materials in a wide range of applications in the fields of material, environmental, and industrial science.Acknowledgements
This work was performed under the auspices of the U.S. Department of Energy by the Lawrence Livermore National Laboratory under contract DE-AC52-07NA27344. We thank Dr Brian P. Mayer and Dr Ian D. Hutcheon for their valuable assistance in the execution of the NMR and SEM experiments.References
- R. W. Pekala, J. Mater. Sci., 1989, 24, 3221–3227 CrossRef CAS.
- W. W. Lukens and G. D. Stucky, Chem. Mater., 2002, 14, 1665–1670 CrossRef CAS.
- S. Bag, I. U. Arachchige and M. G. Kanatzidis, J. Mater. Chem., 2008, 18, 3628–3632 RSC.
- D. R. Rolison, J. W. Long, J. C. Lytle, A. E. Fischer, C. P. Rhodes, T. M. McEvoy, M. E. Bourg and A. M. Lubers, Chem. Soc. Rev., 2009, 38, 226–252 RSC.
- J. Biener, M. Stadermann, M. Suss, M. A. Worsley, M. M. Biener, K. A. Ross and T. F. Baumann, Energy Environ. Sci., 2011, 4, 656–667 CAS.
- K. Nagai, H. Azechi, F. Ito, A. Iwamoto, Y. Izawa, T. Johzaki, R. Kodama, K. Mima, T. Mito, M. Nakai, N. Nemoto, T. Norimatsu, Y. Ono, K. Shigemori, H. Shiraga and K. A. Tanaka, Nucl. Fusion, 2005, 45, 1277–1283 CrossRef CAS.
- K. Nagai, T. Norimatsu and Y. Izawa, Fusion Sci. Technol., 2004, 45, 79–83 CAS.
- U. Meyer, F. Svec, J. M. J. Fréchet, C. J. Hawker and K. Irgum, Macromolecules, 2000, 33, 7769–7775 CrossRef CAS.
- J. Streit and D. Schroen, Fusion Sci. Technol., 2003, 43, 321–326 CAS.
- C. Dawedeit, S. H. Kim, T. Braun, M. A. Worsley, S. A. Letts, K. J. Wu, C. C. Walton, A. A. Chernov, J. H. Satcher Jr., A. V. Hamza and J. Biener, Soft Matter, 2012, 8, 3518–3521 RSC.
- J. Biener, C. Dawedeit, S. H. Kim, T. Braun, M. A. Worsley, A. A. Chernov, C. C. Walton, T. M. Willey, S. O. Kucheyev, S. J. Shin, Y. M. Wang, M. M. Biener, J. R. I. Lee, B. J. Kozioziemski, T. van Buuren, K. J. J. Wu, J. H. Satcher Jr. and A. V. Hamza, Nucl. Fusion, 2012, 52, 062001 CrossRef.
- (a) P. A. Levkin, F. Svec and J. M. J. Frechet, Adv. Funct. Mater., 2009, 19, 1993–1998 CrossRef CAS; (b) J. Hiller, J. D. Mendelsohn and M. F. Rubner, Nat. Mater., 2002, 1, 59–63 CrossRef CAS.
- J. K. Lee and G. L. Gould, J. Sol-Gel Sci. Technol., 2007, 44, 29–40 CrossRef CAS.
- (a) N. Leventis, C. Sotiriou-Leventis, D. P. Mohite, Z. J. Larimore, J. T. Mang, G. Churu and H. Lu, Chem. Mater., 2011, 23, 2250–2261 CrossRef CAS; (b) D. P. Mohite, Z. Larimore, C. Sotiriou-Leventis and N. Leventis, 242nd National Meeting of the American-Chemical-Society (185-PMSE), Denver, 2011 Search PubMed.
- L. R. Doolitle, Nucl. Instrum. Methods Phys. Res., Sect. B, 1985, 9, 344–351 CrossRef.
- A. G. M. Barrett, B. T. Hopkins, A. C. Love and L. Tedeschi, Org. Lett., 2004, 6, 835–837 CrossRef CAS.
- S. D. Drouin, F. Zamanian and D. E. Fogg, Organometallics, 2001, 20, 5495–5497 CrossRef CAS.
- A. Bell, B. Knapp, D. Jablonski and P. W. Newsome, US Pat., 0 099 906, 2010 Search PubMed.
- D. D. Reynolds and W. O. Kenyon, J. Am. Chem. Soc., 1950, 72, 1593–1596 CrossRef CAS.
- T. W. Gibson and W. F. Erman, J. Am. Chem. Soc., 1969, 91, 4771–4778 CrossRef CAS.
- J. J. Gajewski and J. L. Jimenez, J. Am. Chem. Soc., 1986, 108, 468–474 CrossRef CAS.
- G. C. Vougioukalakis and R. H. Grubbs, Chem. Rev., 2010, 110, 1746–1787 CrossRef CAS.
- S. R. White, N. R. Sottos, P. H. Geubelle, J. S. Moore, M. R. Kessler, S. R. Sriram, E. N. Brown and S. Viswanathan, Nature, 2001, 409, 794–797 CrossRef CAS.
- X. Sheng, J. K. Lee and M. R. Kessler, Polymer, 2009, 50, 1264–1269 CrossRef CAS.
- X. Sheng, M. R. Kessler and J. K. Lee, J. Therm. Anal. Calorim., 2007, 89, 459–464 CrossRef CAS.
- A. D. Martina, J. G. Hilborn and A. Mühlebach, Macromolecules, 2000, 33, 2916–2921 CrossRef.
- T. A. Davidson, K. B. Wagener and D. B. Priddy, Macromolecules, 1996, 29, 786–788 CrossRef CAS.
- J. D. Rule and J. S. Moore, Macromolecules, 2002, 35, 7878–7882 CrossRef CAS.
- P. J. Hine, T. Leejarkpai, E. Khosravi, R. A. Duckett and W. J. Feast, Polymer, 2001, 42, 9413–9422 CrossRef CAS.
- P. J. Halley and M. E. Mackay, Polym. Eng. Sci., 1996, 36, 593–609 CAS.
- N. Leventis, C. Chidambareswarapattar, D. P. Mohite, Z. J. Larimore, H. Lu and C. Sotiriou-Leventis, J. Mater. Chem., 2011, 21, 11981–11986 RSC.
- N. Leventis, C. Sotiriou-Leventis, N. Chandrasekaran, S. Mulik, Z. J. Larimore, H. Lu, G. Churu and J. T. Mang, Chem. Mater., 2010, 22, 6692–6710 CrossRef CAS.
- C. Chidambareswarapattar, Z. Larimore, C. Sotiriou-Leventis, J. T. Mang and N. Leventis, J. Mater. Chem., 2010, 20, 9666–9678 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Details on the experimental procedure for the synthesis of cyclic olefin monomers, rheological measurements of G′, G′′, and η* during the gelation of P(DCPD-r-NB) (100/x) samples, the procedure to determine the molar ratios of NB-R to DCPD in copolymer gels from the RBS results, and the gel time, photos, and morphologies of P(DCPD-r-NB-R) with a different composition. See DOI: 10.1039/c2ra21214e |
| This journal is © The Royal Society of Chemistry 2012 |
