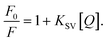PAMAM dendrimer for mitigating humic foulant†
Priyanka
Bhattacharya
ab,
Nathan
Conroy
c,
Apparao M.
Rao
a,
Brian A.
Powell
c,
David A.
Ladner
c and
Pu Chun
Ke
*a
aDepartment of Physics and Astronomy, Clemson University, Clemson, SC 29634, USA. E-mail: pcke11@clemson.edu
bApplied Materials Science, Pacific Northwest National Laboratory, PO Box 999, Richland, WA 99352, USA
cDepartment of Environmental Engineering and Earth Sciences, Clemson University Clemson, SC 29634, USA
First published on 16th July 2012
Abstract
Poly(amidoamine) (PAMAM) dendrimers have been established as efficient ligands for the removal of humic acid (HA) foulant, based upon electrostatic and hydrophobic interactions, as well as hydrogen bonding. This proof-of-concept study indicates the broad utility of dendritic nanotechnology for water treatment and environmental applications.
Humic substances, particularly humic acid (HA), form principle components of natural organic matter (NOM) which result from biodegradation and a chemical breakdown of animal and plant residues. Structurally, HA is a heterogeneous mixture of various aromatic and aliphatic molecules containing amines, catechols, quinones, carboxyl and phenolate functional groups which provide some hydrophilic character above pH 2. HA is resistant to further degradation and can therefore persist in natural and engineered systems. This may be problematic as HA can (i) pose as an environmental hazard by binding to trace metals, radionuclides and/or toxic and carcinogenic contaminants,1a–c (ii) regulate the transport and bioavailability of contaminants, and (iii) reduce the efficiency of water filtration membranes through fouling.2a–c Moreover, the complex and highly variable nature of HA often hinders their efficient removal.
NOM poses a major challenge in drinking water purification systems owing to their high degree of fouling and disinfectant byproduct formation.2a–c,3 The most commonly used methods for removal of NOM from drinking water include coagulation and flocculation, followed by sedimentation/flotation and sand filtration.4 Even though most of the NOM can be removed by coagulation, the hydrophobic fraction and high molar mass compounds of NOM are removed more efficiently than the hydrophilic fraction and the low molar mass compounds. Amongst the coagulants used, organic cationic polyelectrolytes such as chitosan and poly(DADMAC) have proved most efficient.5 Regardless, a high dose of coagulants is usually required for the efficient removal of NOM, thus reducing the cost efficiency of the process. Furthermore, some of the coagulants also exhibit toxic effects.4 Therefore, alternative materials with enhanced coagulation properties must be developed for the better removal of NOM.
Dendritic nanotechnology is a promising new technology for the removal of NOM from water; these polymeric materials possess exceptional adsorption properties and their porous structures are ideal for trapping organic molecules. Accordingly, this study specifically examines removal of HA using PAMAM dendrimers. PAMAM dendrimers are a class of engineered macromolecules consisting of a series of branches emanating from an inner core.6a Their physicochemical properties, including high surface area, good aqueous solubility, presence of inner cavities, high degree of surface functionality and biocompatibility (PAMAM dendrimers of generation 6 or lower show minimal toxicity),6b–c render them highly desirable for environmental applications.7a–c Recent studies have shown that dendrimers such as PAMAM and poly(propylene imine) are capable of encapsulating polyaromatic hydrocarbons, inorganic solutes, and metal cations and anions, and then reversibly releasing the contaminant loads upon changing the solvent pH and electrolyte strength, or by a UV trigger.8a,b Such versatile capacity implies that dendrimers can act as a host for a variety of chemical species and serve as a “nanosponge” for the remediation of contaminated water and soils. The low viscosity, nano-size, and strong contaminant affinity of dendrimers facilitates enhanced contaminant removal via ultrafiltration with minimal membrane fouling, allowing the system to be operated typically at low pressures.8a However, despite these apparent advantages, little attention has been paid to using dendrimers for membrane defouling. Herein, we report a first study on the use of PAMAM dendrimers as an efficient nanotechnology for defouling of humic substances. The present work is a laboratory-scale feasibility study, and its translation to membrane-based separations will be briefly discussed.
Generation 5 amine-terminated PAMAM dendrimers (G5-NH2, MW 28,826 g mol−1) in aqueous solutions and HA dissolved in deionized (DI) water were characterized to estimate their hydrodynamic size and zeta potential using dynamic light scattering and a Zetasizer. The hydrodynamic size of G5-NH2 at a concentration between 42–450 mg L−1 ranged between 4.8–5.6 nm at pH 7 (Fig. S1 in ESI†). At this pH value, only the primary amines of the dendrimers remain protonated while the interior of the dendrimers is hydrophobic. The HA at 75–125 mg L−1 (the use of such high concentrations was to accommodate the instrument resolution for DLS, zeta potential and UV-vis measurements) and pH 5.5 showed a high inter-molecular association (d = 62.53 nm). Although the complex nature of HA prevents an accurate characterization of its chemical composition, it is accepted9 that carboxyl and phenol are the major functional groups, and HA at neutral pH is known to form clusters stabilized by the hydrophobic effect and intermolecular hydrogen bonds.10 Operationally, HA is defined as the component of humic substances which is insoluble at and below pH 2, due to protonation of the anionic groups.11 The zeta potential of HA at a pH of 5.5 was measured to be −34.5 mV due to the deprotonation of carboxyl functional groups. In a preliminary batch experiment, G5-NH2 at pH 7.5 was added to a HA solution at pH 5.5 in an Eppendorf tube at several mass ratios to determine the optimum dendrimer concentration at which visual precipitation of the dendrimer–HA complex took place. The mixtures were mounted on a rotary shaker for 24 h and indeed an optimum ratio of HA to G5-PAMAM was observed at which flocculation of the complexes occurred. This suggests that an electrostatic interaction between the cationic dendrimers and the anionic HA led to the formation of strong complexes that cross-linked to form large precipitates.
In order to further assess the binding affinity between the dendrimers and the HA, a fluorescence quenching method was developed for determining the equilibrium constants of the complex. The phenol, amine, catechol, and quinone groups within humic substances give rise to strong fluorescence signals which are characteristic of the source of the humic substance. We observed that complexation with cationic dendrimers quenched the fluorescence of the HA. The fractional decrease in the fluorescence as a function of added dendrimers was examined using the Stern–Volmer equation:
Here, F0 and F are the fluorescence intensities of HA in the absence and presence of the quencher (dendrimers), respectively, and [Q] is the concentration of the quencher. KSV is the Stern–Volmer constant. An HA solution was prepared by diluting the stock in DI water to reach 15 mg L−1, the average concentration of humic substances found in natural ground and surface waters in the United States.12 Increasing concentrations of G5-PAMAM were added to the HA solution. The fluorescence of the HA was recorded between 400–600 nm upon excitation at 320 nm, showing a broad emission spectrum with a peak around 460 nm. Slight quenching of the HA fluorescence was observed upon dilution with DI water, resulting from charge transfer between the HA and dissolved oxygen in water (Fig. S2a in ESI†).13 Dendrimers, in contrast, exhibited negligible fluorescence in the range examined (Fig. S2b in ESI†). However, a significant quenching was observed upon adding dendrimers to the HA solution (Fig. 1a). The Stern–Volmer plot derived from the fluorescence emission was linear (Fig. 1b), and the Stern–Volmer constant KSV was determined to be 1.3 × 107 M−1. At a mass ratio of 5.25 mg of HA per mg of G5-PAMAM, corresponding to a G5-PAMAM concentration of 2.85 mg L−1, the fluorescence intensity of the initial HA solution decreased by approximately 50%, implying that approximately half of the HA molecules were associated with the dendrimers. Linearity of the Stern–Volmer plot may imply either static or dynamic quenching. For dynamic quenching, the Stern–Volmer constant KSV is equal to the bimolecular quenching constant kq multiplied by the fluorescence lifetime in the absence of the quencher. The fluorescence lifetime of the commercial Aldrich HA is of the order of 0.6–8.4 ns (MW 3–50 kDa) at pH 6.3.14 Therefore kq is determined to be 0.16–2.3 × 1016 M−1s−1, much higher than the largest possible value for diffusion-controlled processes in aqueous solution.15 Hence, the quenching process here is concluded as a static process arising from complex formation between G5-PAMAM and HA, primarily mediated by electrostatic interactions. Other interactions such as hydrogen bonding between the hydrogens of the dendrimer amines and the oxygens of the phenol and carboxyl groups of the HA are also feasible. The static Stern–Volmer constant, KSV, can be interpreted as the ground-state association constant for the dendrimers and the HA, indicating a high affinity of HA for the G5-PAMAM dendrimers.
 | ||
| Fig. 1 (a) Fluorescence emission of HA in the presence of decreasing amounts of HA per G5-PAMAM (g/g): 21, 10.5, 7, 5.25, 4.2, 3.5, 2.62, 2.1, 1.4, and 1.05. (b) Stern–Volmer plot for G5-HA complexes. The linearity of the plot indicates static quenching due to complex formation. | ||
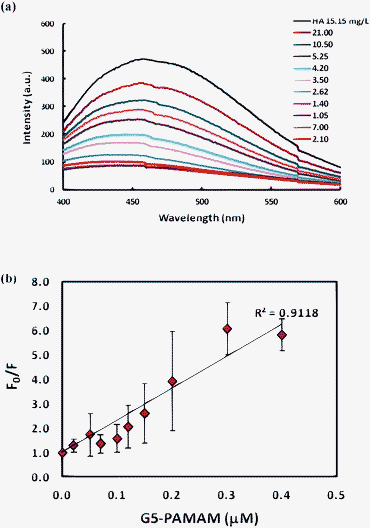
Given the high affinity of HA for dendrimers, it is of interest to assess the efficiency of the removal of dissolved HA in aqueous solutions by dendrimers. For this purpose, we carried out a UV-vis absorbance measurement to quantify the amount of HA removed from the solution. HA has a broad absorption spectrum which decreases with increasing wavelength in an approximately exponential fashion, exhibiting no distinct bands. This observed tailing in the HA absorption spectrum is attributed to the intra-molecular charge transfer between the hydroxyl-aromatic donors and quinoid acceptors formed by the partial oxidation of lignin precursors.16 HA solutions of 75–125 mg L−1 at pH 5.5 were prepared by diluting the stock solution with DI water. G5-PAMAM of gradient concentrations were then added to the HA solutions. The final pH of the mixture was noted to be neutral. The mixtures were allowed to incubate on a rotor for 24 h, after which, visible precipitates were observed in the tube for optimal dendrimer concentrations (Fig. 2 inset). No visible precipitates were observed for the control HA, confirming strong interactions between the two oppositely charged species. The mixtures were centrifuged for 15 min (ESI†, method 3) and the absorbance of the supernatants was measured. The supernatants showed no new absorbance peaks, but were lower in intensity than the corresponding control HA samples for the entire range. The difference in intensities at 254 nm was then used to infer the fraction of HA left in the aqueous phase. Fig. 2 shows the fraction of HA remaining in the aqueous phase vs. the ratio of HA to G5-PAMAM in solution. The fraction of remaining HA initially decreased followed by an increase with increasing dendrimer concentrations. Narkis and Rebhun17a and Edzwald et al.17b reported that cationic polyelectrolytes can destabilize high molecular weight polymers such as humic acid by charge neutralization. Such charge neutralization results in the formation of neutral species which can aggregate to form relatively large molecular weight compounds and precipitate out of the solution. The electrostatic interaction between the cationic G5-PAMAM and the anionic HA led to the formation of complexes which cross-linked to form floc particles. The optimal concentration of G5-PAMAM at which maximum precipitation was observed was 50 mg L−1, corresponding to ∼2 g HA per gram of G5-PAMAM added. Interestingly, this is also the stoichiometric ratio at which complete fluorescence quenching of HA of 15 mg L−1 occurred (Fig. 1a). At this concentration, only 10% of the HA remained in the aqueous phase. This removal efficiency is twice as good as previously reported by Glaser and Edzwald,18 where 20% HA remained in the aqueous phase when the mass ratio of HA to branched polyethyleneimine (PEI) was ∼1.25. In addition, compared to PEI, PAMAM dendrimers have a well-defined number of surface functionalities and hence a uniform surface charge distribution.
 | ||
| Fig. 2 Fraction of HA remaining in solution vs. ratio of HA per G5-PAMAM added. Insets: Images of G5-HA precipitates in the left tube and HA alone in the right tube for the same HA concentration corresponding to 2 g HA per gram of G5-PAMAM. | ||
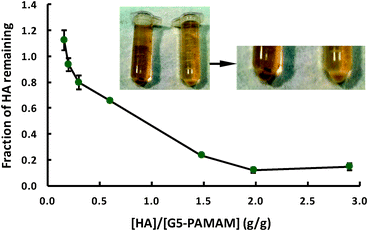
In our experiment, a pseudo-exponential increase in the remaining fraction was observed for dendrimer concentrations above the optimal concentration (i.e. data points <2.0 g/g in Fig. 2). This can be explained as follows. Initially, flocculation of the dendrimer–HA complexes was rapid due to the presence of a large number of sub-micrometer anionic HA particles and cationic dendrimers (Scheme 1, I). As the complexes started to aggregate through cross-linking, mediated by hydrophobic interactions, the number of charged particles/complexes decreased (Scheme 1, II). Once the charge neutralization is achieved at the optimal concentration of added dendrimers, further addition of the dendrimers re-stabilized the neutral hydrophobic flocs by providing steric and electrostatic repulsions between the formed complexes and hence increased absorbance of the HA. Since G5-PAMAM dendrimers each possess 128 surface amines, the optimal amount of the dendrimer amines is 224 μM (6.45 g L−1) to neutralize a 100 mg L−1 HA solution. Potentiometric titrations19 of the dissociation of Aldrich HA provide its total acidity to be 648 μM. This implies that ∼2–3 HA molecules are neutralized by a single G5-PAMAM dendrimer. This is further confirmed by the dynamic light scattering (DLS) and zeta potential measurements of the supernatants. Results from the DLS measurement showed formation of sub-micrometer sized soluble complexes at the optimal dendrimer dose (Fig. S1 in ESI†). However, partial dissociations of the complexes occurred below and above the optimal dendrimer concentration. The zeta potential of the complexes shifted from negative (−18.3 mV) for dendrimers below the optimal concentration, to neutral (−2.24 mV) at the optimal concentration, and positive (+16.1 mV) for just above the optimal concentration. For higher doses, zeta potential measurements were unattainable due to the small sizes of the complexes (<3 nm) that were below the instrument resolution. This corroborates our hypothesis that further addition of dendrimers beyond the optimal concentration caused fragmentation of the large micron-sized aggregates into smaller complexes stabilized by cationic dendrimers (Scheme 1, III). Below the optimal concentration, all dendrimers were complexed with the HA and precipitation was observed. The negative zeta potentials under this condition were therefore indicative of the remaining HA in the solution. At the optimal concentration, hydrophobic interactions between the charge neutral complexes resulted in the formation of precipitates. Further absence of aggregation between the complexes may be attributed to steric effects and entropically driven release of encapsulated water and structural reorganization.20 Such entropic gain could compensate for the hydrophobic interactions between the complexes. Above the optimal concentration, excess dendrimers restabilized the neutralized complexes via electrostatic repulsions by interacting with them through hydrogen bonding.
 | ||
| Scheme 1 Schematic of complexation (I), aggregation (II) and re-stabilization (III) processes in dendrimer–HA interactions. Dendrimers are shown in green and HA in orange. | ||
To further investigate the specific chemical groups involved in the complex formation, attenuated total reflectance-Fourier transform infrared spectra (ATR-FTIR) were collected on the pellets obtained from experiments at the optimal dendrimer concentration described above. The precipitates were dried in an oven at 45 °C and kept in a dessicator at room temperature overnight. The chemical activity of HA is dominated by a high concentration of oxygen-containing functional groups, particularly carboxyl groups. Despite the complexity of HA molecules, IR spectroscopy is useful in resolving carboxyl groups due to their high polarizability and hence high IR activity. The chemical behaviour of carboxyl groups also depends upon their structural environment, i.e. the types and orientations of other functional groups neighbouring the carboxyls. A carboxyl group gives rise to two main features in the IR absorption spectra depending on its protonation state. The protonated carboxylic acids is characterized by absorption bands corresponding to a carbonyl stretch (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) between 1690 and 1750 cm−1, and C–OH vibrations (νC–OH) between 1200 and 1300 cm−1. Upon deprotonation, the vibrational mode of νC
O) between 1690 and 1750 cm−1, and C–OH vibrations (νC–OH) between 1200 and 1300 cm−1. Upon deprotonation, the vibrational mode of νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O becomes coupled to that of the other deprotonated oxygen and its energy shifts to a lower energy level. This gives rise to an asymmetric stretching feature (νas) between 1540 and 1650 cm−1. The C–OH band also shifts to higher energies upon deprotonation, giving rise to a symmetric COO− mode (νs) between 1300 and 1420 cm−1. The NH stretching vibrations occur near 3300–3500 cm−1. Due to hydrogen bonding in the solid state, these bands are broadened and are characteristic of amides.21
O becomes coupled to that of the other deprotonated oxygen and its energy shifts to a lower energy level. This gives rise to an asymmetric stretching feature (νas) between 1540 and 1650 cm−1. The C–OH band also shifts to higher energies upon deprotonation, giving rise to a symmetric COO− mode (νs) between 1300 and 1420 cm−1. The NH stretching vibrations occur near 3300–3500 cm−1. Due to hydrogen bonding in the solid state, these bands are broadened and are characteristic of amides.21
As shown in Fig. 3, the ATR-FTIR spectrum of the dendrimer–HA complex is a convolution of the spectra of the G5-PAMAM dendrimers and the HA, exhibiting several features suggestive of the interactive sites as follows.
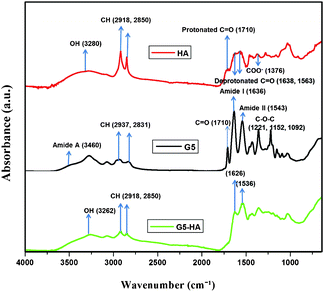 | ||
| Fig. 3 ATR-FTIR spectra of HA (red), G5-PAMAM (black) and G5-HA complex (green). | ||
1. The amide A band (3460 cm−1) originating from a combination of amide II stretching and NH in-plane bending vibrations of the G5-PAMAM broadens to suggest hydrogen bonding with the deprotonated phenolic and/or carboxylic groups of the HA.
2. The amide II band at 1543 cm−1 of the G5-PAMAM and the 1563 cm−1 band of the HA that is associated with the N–H bending vibrations significantly downshifts to 1536 cm−1 for the G5-HA complex, suggesting direct, electrostatic interactions between carboxylic and amine functional groups of the HA and G5-PAMAM, respectively.
3. The asymmetric carbonyl stretch (1612 cm−1) within the HA and the amide I peak (1636 cm−1) within the G5-PAMAM are essentially combined within the G5-HA complex spectra. The apparent broadening of the spectral peaks within the G5-HA complex may be only due to the incorporation of the broad HA peaks. However, the minimal shift in peak positions of approximately 10 cm−1 could imply interactions with the dendrimer primary amines.
4. The disappearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch at 1710 cm−1 in the G5-HA complex implies deprotonation of the carboxylic acid groups upon formation of the G5-HA complex. This indicates the presence of electron withdrawing groups, such as the dendrimer amines.
O stretch at 1710 cm−1 in the G5-HA complex implies deprotonation of the carboxylic acid groups upon formation of the G5-HA complex. This indicates the presence of electron withdrawing groups, such as the dendrimer amines.
Conclusions
In summary, we have demonstrated efficient removal of dissolved humic acid using biocompatible PAMAM dendrimers. Central to this method is complex formation due to electrostatic interactions between the cationic dendrimers and the anionic HA at neutral pH, as vindicated by the UV-vis and fluorescence measurements. Our ATR-FTIR study further identified the specific chemical groups involved in the dendrimer–HA complexation. Once charge neutralization is reached at a stoichiometric ratio of ∼2 g/g for HA:G5-PAMAM, subsequent fast aggregation occurred resulting from hydrogen bonding and hydrophobic interactions between the neutralized complexes, leading to precipitation of the micron-sized aggregates. However, loading of additional dendrimers re-stabilized and resuspended the aggregates via electrostatic repulsion.Future investigation on the deployment of dendrimer for mitigating humic foulant will include evaluation of membrane-bound dendrimers for HA removal. Based on our current study an HA:G5-PAMAM dendrimer loading ratio of 2 g/g is optimal. Such ratio may decrease slightly in the presence of a membrane due to its partial consumption of dendrimer surface charge. Strategically, cationic PAMAM dendrimers can be immobilized onto anionic ultrafiltration membranes through electrostatic attraction and then such membrane-bound dendrimers may be used to capture HA during water filtration. Upon raising the pH of the dendrimer-bound HA to 10, wherein dendrimers become neutral (pKa of primary amines ∼9) and HA remains highly negatively charged, regeneration of the dendrimers is possible as a result of the much weakened electrostatic interaction between the two species. For large scale applications PAMAM dendrimers may be replaced by functionally comparable and structurally compromised hyperbranched polymers such as PEI for significant cost saving. Thus, the dendritic nanotechnology for the removal of humic foulant described herein may find its utility for the development of sustainable water treatment strategies.
Acknowledgements
This work was supported by NSF CAREER award CBET-0744040 to Ke and US EPA grant RD835182 to Ladner and Ke. Bhattacharya acknowledges a Grant-in-Aid of Research from Sigma Xi and is joining the Pacific Northwest National Laboratory as a Linus Pauling Distinguished Postdoctoral Fellow.References
- (a) J. Buffle, in Complexation Reactions in Aquatic Systems. An Analytical Approach, Ellis Horwood, Chichester, 1988 Search PubMed; (b) E. Tipping, Colloids Surf., A, 1993, 73, 117 CrossRef CAS; (c) P. H. Santschi, K. A. Roberts and L. D. Guo, Environ. Sci. Technol., 2002, 36, 3711 CrossRef CAS.
- (a) S. Hong and M. Elimelech, J. Membr. Sci., 1997, 132, 159 CrossRef CAS; (b) S. G. Yiantsios and A. J. Karabelas, Desalination, 2001, 140, 195 CrossRef CAS; (c) Z. Cai and M. M. Benjamin, Environ. Sci. Technol., 2011, 45, 8935 CrossRef CAS.
- A. Kanan and T. Karanfil, Water Res., 2011, 45, 926 CrossRef CAS.
- A. Matilainen, M. Vepsäläinen and M. Sillanpää, Adv. Colloid Interface Sci., 2010, 159, 189 CrossRef CAS.
- B. Bolto and J. Gregory, Water Res., 2007, 41, 2301 CrossRef CAS.
- (a) M. J. Frechet and D. A. Tomalia, in Dendrimers and Other Dendritic Polymers, Wiley and Sons, New York, 2001 Search PubMed; (b) B. Helms and E. W. Meijer, Science, 2006, 313, 929 CrossRef CAS; (c) M. Mortimer, K. Kasemets, M. Heinlaan, I. Kurvet and A. Kahru, Toxicol. in Vitro, 2008, 22, 1412 CrossRef CAS.
- (a) M. S. Diallo, L. Balogh, A. Shafagati, J. H. Johnson, W. A. Goddard III and D. A. Tomalia, Environ. Sci. Technol., 1999, 33, 820 CrossRef CAS; (b) M. Arkas, M. D. Tsiourvas and C. M. Paleos, Chem. Mater., 2003, 15, 2844 CrossRef CAS; (c) P. Chen, Y. Yang, P. Bhattacharya, P. Wang and P. C. Ke, J. Phys. Chem. C, 2011, 115, 12789 CrossRef CAS.
- (a) M. S. Diallo, S. Christie, P. Swaminathan, J. H. Johnson, Jr. and W. A. Goddard III, Environ. Sci. Technol., 2005, 39, 1366 CrossRef CAS; (b) M. S. Diallo, K. Falconer, J. H. Johnson, Jr. and W. A. Goddard III, Environ. Sci. Technol., 2007, 41, 6521 CrossRef CAS.
- M. N. Jones and N. D. Bryan, Adv. Colloid Interface Sci., 1998, 78, 1 CrossRef CAS.
- J. E. Tomaszewski, R. P. Schwarzenbach and M. Sander, Environ. Sci. Technol., 2011, 45, 6003 CrossRef CAS.
- G. Sposito, in The Chemistry of Soils, ed. G. Sposito, Oxford University Press, New York, 2nd edn, 2006, ch. 3, 72 Search PubMed.
- J. A. Leenheer, in Humic Substances in Soil, Sediment, and Water: Geochemistry, Isolation, and Characterization, ed. G. R. Aiken, D. M. McKnight, R. L. Wershaw and P. MacCarthy, John Wiley & Sons, New York, 1985, pp 409–429 Search PubMed.
- J. T. Brownrigg and J. E. Kenny, J. Phys. Chem. A, 2009, 113, 1049 CrossRef CAS.
- C. D. Clark and R. G. Zika, in Marine Chemistry: The Handbook of Environmental Chemistry 5.D, ed. P. J. Wangersky. Springer, New York, 2000, ch. 2, 8 Search PubMed.
- J. R. Lakowicz, in Principles of Fluorescence Spectroscopy, ed. J. R. Lakowicz, Springer, New York, 3rd edn, 2006, ch. 8, 282 Search PubMed.
- R. D. Vecchio and N. V. Blough, Environ. Sci. Technol., 2004, 38, 3885 CrossRef.
- (a) N. Narkis and M. Rebhun, J. AWWA, 1975, 67, 88 Search PubMed; (b) J. K. Edzwald, J. D. Haff and J. W. Boak, Journal of the Environmental Engineering Division., 1977 Search PubMed.
- H. T. Glaser and J. K. Edzwald, Environ. Sci. Technol., 1979, 13, 299 CrossRef CAS.
- T. Andjelkovic, J. Perovic, M. Purenovic, S. Blagojevic, R. Nikolic, D. Andjelkovic and A. Bojic, Ecl. Quim., Sao Paulo, 2006, 31, 39 CAS.
- W. F. Tan, L. K. Koopal and W. Norde, Environ. Sci. Technol., 2009, 43, 591 CrossRef CAS.
- J. Mohan in Organic Spectrosopy: Principles and Applications, ed. J. Mohan, Alpha Science, U.K., 2nd edn, 2004, ch.2, 62 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental details, size distribution graph, fluorescence emission spectra of HA and G5-PAMAM. See DOI: 10.1039/c2ra21245e/ |
| This journal is © The Royal Society of Chemistry 2012 |