A novel base-promoted cyclization: synthesis of substituted benzo[b]furans†
Krishna
Damera‡
,
Bowen
Ke‡
,
Ke
Wang
,
Chaofeng
Dai
,
Lifang
Wang
and
Binghe
Wang
*
Department of Chemistry, Center for Diagnostics and Therapeutics and Center for Biotechnology and Drug Design, Georgia State University, Atlanta, Georgia 30303-4098, USA. E-mail: wang@gsu.edu; Tel: +1 404-413-5545
First published on 3rd July 2012
Abstract
A new base-promoted cyclization for the synthesis of substituted benzo[b]furans is described. This method is simple and inexpensive and gives good yields.
Benzo[b]furan is an important structural scaffold in pharmaceutical research because compounds containing this moiety show a wide range of pharmacological activities.1–4 For example, the benzofuran moiety is found in antidepressant (−)-BPAP,5 angiotensin II inhibitors,6 5-lipoxygenase inhibitors,6 nerokinin-2 receptor antagonist,7 cathepsin K inhibitors,3 and calcium entry blockers. In addition, there are also many benzofuran-containing natural products including (−)-concentricolide,8 (+)-frondosin B9 and the eupomatenoid family.10 Therefore, there is a very high level of interest in finding efficient ways of constructing the benzo[b]furan core structure.
Several approaches have been reported in the literature.11–15 Most recent approaches have focused on the use of transition-metal-catalyzed synthesis of benzo[b]furan compounds16,17 including metal-catalyzed, one-pot synthesis from 2-halophenols and terminal18,19 or internal alkynes20 by Sonogashira coupling21 followed by cyclization and transition-metal-catalyzed heteroannulation of 2-alkynyl phenols or 2-alkynylphenol ethers.22 In most published literature, the synthesis of benzo[b]furans is via tandem Sonogashira coupling/5-endo-dig cyclization using a palladium catalyst or copper salt26 and oxidative pathway.27
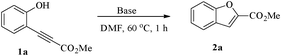
However, most of these approaches require expensive metal reagents and harsh reaction conditions and have limited functional group tolerance. Herein we describe a new base-promoted synthesis of 2-substituted benzo[b]furans from heteroannulation of 2-alkynylphenols. In the present work, heating a mixture of compound 1a and NaN3 in the presence of CuI in DMF at 60 °C led to the unexpected formation of compound 2a as the major product. Further examination of the reaction conditions found that CuI was not necessary for the reaction. Thus we suspected that deprotonation of the phenol hydroxyl group by a base was probably the driving force for the reaction. With this in mind, we screened this reaction under different conditions by altering the base used and found that Cs2CO3 was the most effective and Et3N was ineffective in catalyzing the reaction (Table 1). With such results we concluded that, in the original reaction, NaN3 was acting merely as a base.
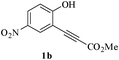
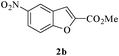
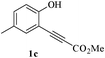
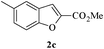
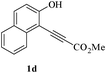
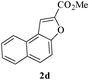
In order to explore the scope of this cyclization reaction, we were interested in examining the effect of various substituents on the reaction. The study started with the preparation of substituted phenols 1a–d. These compounds (1a–d) were subjected to the ring closure reaction conditions (60 °C in dry DMF, Cs2CO3). Substituted benzo[b]furans with different functional groups were synthesized in good yields (76–88%, Table 2). Especially worth mentioning is the synthesis of 5-nitrobenzo[b]furan. It is well known that isomers form under direct nitration of benzo[b]furan,23 so most nitrobenzo[b]furans are synthesized via intramolecular condensation of suitably substituted nitrobenzenes.24 Thus far, only a few metal-catalyzed heteroannulation techniques have been applied to the synthesis of nitrobenzo[b]furan. Since the nitro group is sensitive to strong basic conditions at high temperature, many approaches to the synthesis of benzo[b]furans have proven too harsh for the nitro substituents.25 So the smooth cyclization method described here should be very useful.
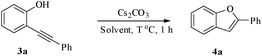
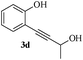
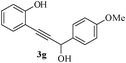
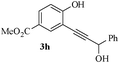
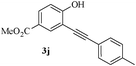
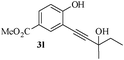
Having successfully prepared a range of benzofuran-2-carboxylates (2a–d), we extended this methodology to the synthesis of 2-substitued benzo[b]furans from o-alkynylphenol. For optimization of the cyclization reactions, we chose compound 3a and screened different solvents and temperatures. The data (Table 3) revealed that DMF is the best solvent for cyclization.
| Entry | Solvent | T (°C) | Yield (%) |
|---|---|---|---|
| 1 | DMF | 60 | 82 |
| 2 | DMF | RT | NR |
| 3 | CH3CN | 60 | 45 |
| 4 | MeOH | 60 | NR |
| 5 | DMSO | 60 | 66 |
| 6 | 1,4-Dioxane | 60 | NR |
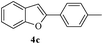
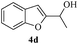
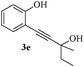
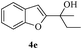
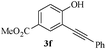
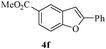
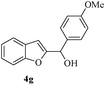
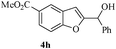
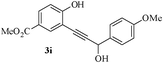
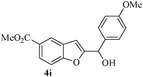
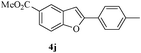
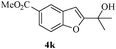
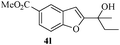
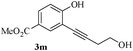
In most published literature, the synthesis of benzo[b]furans is via tandem Sonogashira coupling/5-endo-dig cyclization using a palladium catalyst or copper salt26 and oxidative pathway.27 Using our protocol, we were able to cyclize o-alkynylphenols (3a–m) to the corresponding benzo[b]furans (4a–m) in moderate to good yields (Table 4). It should be noted that many of the same cyclizations, such as 4a, 4c28 and 4d,29 have been noted under Pd catalysis. Our results suggest that metal catalysis is not necessary in some of the literature procedures.
In conclusion, we have reported a new base-promoted synthesis of benzo[b]furans with good yields. This method can be applied to the synthesis of 5-nitrobenzofuran, which is difficult to produce using other methods. This synthetic route is palladium/copper free and avoids the use of expensive or air-sensitive reagents and represents a novel method for the easy synthesis of benzo[b]furans.
Acknowledgements
Partial financial support from the National Institutes of Health (GM086925 and GM084933) is gratefully acknowledged.References
- D. J. Sall, D. L. Bailey, J. A. Bastian, J. A. Buben, N. Y. Chirgadze, A. C. Clemens-Smith, M. L. Denney, M. J. Fisher, D. D. Giera, D. S. Gifford-Moore, R. W. Harper, L. M. Johnson, V. J. Klimkowski, T. J. Kohn, H. S. Lin, J. R. McCowan, A. D. Palkowitz, M. E. Richett, G. F. Smith, D. W. Snyder, K. Takeuchi, J. E. Toth and M. Zhang, J. Med. Chem., 2000, 43, 649–663 CrossRef CAS.
- R. Hili and A. K. Yudin, Nat. Chem. Biol., 2006, 2, 284–287 CrossRef CAS.
- D. S. Yamashita, R. W. Marquis, R. Xie, S. D. Nidamarthy, H. J. Oh, J. U. Jeong, K. F. Erhard, K. W. Ward, T. J. Roethke, B. R. Smith, H. Y. Cheng, X. Geng, F. Lin, P. H. Offen, B. Wang, N. Nevins, M. S. Head, R. C. Haltiwanger, A. A. Narducci Sarjeant, L. M. Liable-Sands, B. Zhao, W. W. Smith, C. A. Janson, E. Gao, T. Tomaszek, M. McQueney, I. E. James, C. J. Gress, D. L. Zembryki, M. W. Lark and D. F. Veber, J. Med. Chem., 2006, 49, 1597–1612 CrossRef CAS.
- A. Gangjee, R. Devraj, J. J. McGuire and R. L. Kisliuk, J. Med. Chem., 1995, 38, 3798–3805 CrossRef CAS.
- S. Shimazu, K. Takahata, H. Katsuki, H. Tsunekawa, A. Tanigawa, F. Yoneda, J. Knoll and A. Akaike, Eur. J. Pharmacol., 2001, 421, 181–189 CrossRef CAS.
- D. Fattori, M. Porcelloni, P. D'Andrea, R. M. Catalioto, A. Ettorre, S. Giuliani, E. Marastoni, S. Mauro, S. Meini, C. Rossi, M. Altamura and C. A. Maggi, J. Med. Chem., 2010, 53, 4148–4165 CrossRef CAS.
- R. Kiyama, T. Honma, K. Hayashi, M. Ogawa, M. Hara, M. Fujimoto and T. Fujishita, J. Med. Chem., 1995, 38, 2728–2741 CrossRef CAS.
- A. P. Kozikowski, D. W. Ma, L. Du, N. E. Lewin and P. M. Blumberg, J. Am. Chem. Soc., 1995, 117, 6666–6672 CrossRef CAS.
- X. D. Qin, Z. J. Dong, J. K. Liu, L. M. Yang, R. R. Wang, Y. T. Zheng, Y. Lu, Y. S. Wu and Q. T. Zheng, Helv. Chim. Acta., 2006, 89, 127–133 CrossRef CAS.
- A. D. Patil, A. J. Freyer, L. Killmer, P. Offen, B. Carte, A. J. Jurewicz and R. K. Johnson, Tetrahedron, 1997, 53, 5047–5060 CrossRef CAS.
- J. H. Chaplin and B. L. Flynn, Chem. Commun., 2001, 1594–1595 RSC.
- K. O. Kim and J. Tae, Synthesis, 2005, 387–390 CAS.
- O. Miyata, N. Takeda and T. Naito, Org. Lett., 2004, 6, 1761–1763 CrossRef CAS.
- L. M. Geary and P. G. Hultin, Org. Lett., 2009, 11, 5478–5481 CrossRef CAS.
- G. Kumaraswamy, G. Ramakrishna, R. Raju and M. Padmaja, Tetrahedron, 2010, 66, 9814–9818 CrossRef CAS.
- G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644–4680 CrossRef CAS.
- H. Zhang, E. M. Ferreira and B. M. Stoltz, Angew. Chem., Int. Ed., 2004, 43, 6144–6148 CrossRef CAS.
- F. Colobert, A. S. Castanet and O. Abillard, Eur. J. Org. Chem., 2005, 3334–3341 CrossRef CAS.
- M. M. Heravi and S. Sadjadi, Tetrahedron, 2009, 65, 7761–7775 CrossRef CAS.
- A. Arcadi, S. Cacchi, M. DelRosario, G. Fabrizi and F. Marinelli, J. Org. Chem., 1996, 61, 9280–9288 CrossRef CAS.
- R. C. Larock, E. K. Yum, M. J. Doty and K. K. C. Sham, J. Org. Chem., 1995, 60, 3270–3271 CrossRef CAS.
- G. W. Kabalka, L. Wang and R. M. Pagni, Tetrahedron, 2001, 57, 8017–8028 CrossRef CAS.
- L. J. Powers and M. P. Mertes, J. Med. Chem., 1970, 13, 1102–1105 CrossRef CAS.
- W. M. Dai and K. W. Lai, Tetrahedron Lett., 2002, 43, 9377–9380 CrossRef CAS.
- S. L. Graham, J. M. Hoffman, P. Gautheron, S. R. Michelson, T. H. Scholz, H. Schwam, K. L. Shepard, A. M. Smith, R. L. Smith, J. M. Sondey and M. F. Sugrue, J. Med. Chem., 1990, 33, 749–754 CrossRef CAS.
- C. G. Bates, P. Saejueng, J. M. Murphy and D. Venkataraman, Org. Lett., 2002, 4, 4727–4729 CrossRef CAS.
- X.-F. Duan, J. Zeng, Z.-B. Zhang and G.-F. Zi, J. Org. Chem., 2007, 72, 10283–10286 CrossRef CAS.
- A. Ohtaka, T. Teratani, R. Fujii, K. Ikeshita, T. Kawashima, K. Tatsumi, O. Shimomura and R. Nomura, J. Org. Chem., 2011, 76, 4052–4060 CrossRef CAS.
- M. Pal, V. Subramanian and K. R. Yeleswarapu, Tetrahedron Lett., 2003, 44, 8221–8225 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra21302h |
| ‡ These two authors made equal contributions. |
| This journal is © The Royal Society of Chemistry 2012 |