Concise and efficient one-pot copper-catalyzed synthesis of H-pyrazolo[5,1-a]isoquinolines†
Xiaobo
Yang
a,
Yue
Luo
a,
Yibao
Jin
b,
Hongxia
Liu
b,
Yuyang
Jiang
b and
Hua
Fu
*ab
aKey Laboratory of Bioorganic Phosphorus Chemistry and Chemical Biology (Ministry of Education), Department of Chemistry, Tsinghua University, Beijing 100084, P. R. China. E-mail: fuhua@mail.tsinghua.edu.cn
bKey Laboratory of Chemical Biology (Guangdong Province), Graduate School of Shenzhen, Tsinghua University, Shenzhen 518057, P. R. China
First published on 20th July 2012
Abstract
A new, concise and efficient one-pot copper-catalyzed method for the synthesis of H-pyrazolo[5,1-a]isoquinolines has been developed, and the H-pyrazolo[5,1-a]isoquinolines containing various functional groups were prepared in moderate to good yields. The method should provide a simple and practical strategy for this kind of N-fused heterocycles.
N-Heterocycles widely occur in a variety of biologically active substances,1 and they have been assigned as privileged structures in drug development because N-heterocyclic moieties often show improved solubility and can facilitate salt formation, both of which are important for oral absorption and bioavailability.2 While pyrazoles are an important motif of man-made biologically active compounds such as celecoxib, fipronil, lonazolac, viagra, they are rarely found in natural products.3 The isoquinoline motif is commonly found in natural products4 and pharmaceutical compounds.5 For example, the isoquinoline derivatives were applied as potential PET radioligands for imaging peripheral benzodiazepine receptor,6a and as orally bioavailable Kv1.5 antagonists for the treatment of atrial fibrillation.6b The N-fused heterocycles of pyrazoles and isoquinoline frameworks, H-pyrazolo[5,1-a]isoquinolines (Fig. 1), exhibit promising biological activities for the inhibition of CDC25B, TC-PTP, and PTP1B,7 and some efficient methods for their synthesis have been developed by Wu7,8 and other groups.9 Recently, there have been great achievements made in copper-catalyzed coupling reactions,10 and various N-heterocycles were constructed through these reactions by other groups11 and us.12 Herein, we report a novel, concise and efficient one-pot copper-catalyzed synthesis of H-pyrazolo[5,1-a]isoquinolines.
![H-Pyrazolo[5,1-a]isoquinoline as N-fused heterocycle of pyrazole and isoquinoline.](/image/article/2012/RA/c2ra21305b/c2ra21305b-f1.gif) | ||
| Fig. 1 H-Pyrazolo[5,1-a]isoquinoline as N-fused heterocycle of pyrazole and isoquinoline. | ||
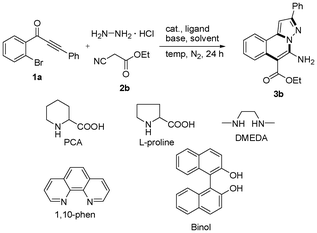
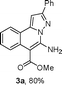
As shown in Table 1, a three-component reaction of 1-(2-bromophenyl)-3-phenylprop-2-yn-1-one (1a), hydrazine hydrochloride and ethyl 2-cyanoacetate (2b) was chosen as the model to optimize reaction conditions including copper-catalysts, ligands, bases, solvents and temperature under nitrogen atmosphere. Effect of ligands was investigated using 10 mol% as the catalyst, K3PO4 as the base, DMF as the solvent at 100 °C under nitrogen atmosphere (entries 1–6), and a highest yield (82%) was provided in the absence of ligand (entry 6). When CuBr (entry 7) and Cu(OAc)2 (entry 8) were used as the catalysts, low yields were obtained. Other bases were also attempted (entries 9 and 10), and they showed weak results. DMSO, 1,4-dioxane and toluene were used as the solvents (entries 11–13), and they were inferior to DMF (entry 5). A lower yield was obtained when temperature was decreased to 80 °C (entry 14). Therefore, the standard conditions for the copper-catalyzed three-component reactions of substituted 1-(2-bromophenyl)-3-phenylprop-2-yn-1-ones, hydrazine hydrochloride and alkyl 2-cyanoacetates are as follows: 10 mol% CuI as the catalyst, K3PO4 as the base and DMF as the solvent, and the reactions were carried out at 100 °C under nitrogen atmosphere.
| Entry | Cat. | Ligand | Base | Solvent | T/°C | Yield (%)b |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1-(2-bromophenyl)-3-phenylprop-2-yn-1-one (1a) (0.25 mmol), hydrazine hydrochloride (1.2 equiv, 0.3 mmol), ethyl 2-cyanoacetate (2b) (5 equiv, 1.25 mmol), catalyst (0.1 equiv, 0.025 mmol), ligand (0.2 equiv, 0.05 mmol), base (5 equiv, 1.25 mmol), solvent (2 mL) under nitrogen atmosphere. Reaction time (24 h). b Isolated yield. c PCA = piperidine-2-carboxylic acid. | ||||||
| 1 | CuI | PCAc | K3PO4 | DMF | 100 | 69 |
| 2 | CuI | L-proline | K3PO4 | DMF | 100 | 40 |
| 3 | CuI | DMEDA | K3PO4 | DMF | 100 | 36 |
| 4 | CuI | 1,10-phen | K3PO4 | DMF | 100 | 21 |
| 5 | CuI | Binol | K3PO4 | DMF | 100 | 75 |
| 6 | CuI | — | K3PO4 | DMF | 100 | 82 |
| 7 | CuBr | — | K3PO4 | DMF | 100 | 71 |
| 8 | Cu(OAc)2 | — | K3PO4 | DMF | 100 | 54 |
| 9 | CuI | — | K2CO3 | DMF | 100 | 43 |
| 10 | CuI | — | Li2CO3 | DMF | 100 | trace |
| 11 | CuI | — | K3PO4 | DMSO | 100 | 62 |
| 12 | CuI | — | K3PO4 | 1,4-Dioxane | 100 | 17 |
| 13 | CuI | — | K3PO4 | Toluene | 100 | 0 |
| 14 | CuI | — | K3PO4 | DMF | 80 | 70 |
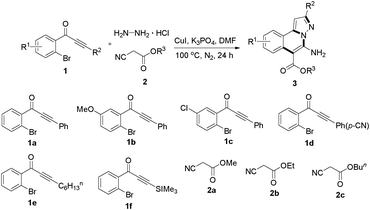
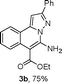
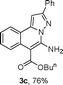
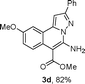
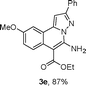
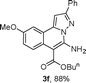
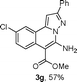
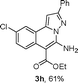
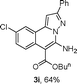
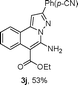
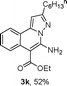
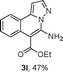
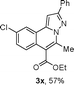
As shown in Table 2, the substrate scope of the copper-catalyzed three-component reactions was investigated, and the examined substrates provided moderate to good yields. For substituent R1, the substrates with electron-donating groups showed higher reactivity than those with electron-withdrawing groups. For substituent R2, the aromatic substrates (1a–d) provided higher yields than aliphatic ones (1e–f), and the trimethylsilyl group was deprotected for substrate 1f (see product 3l). Interestingly, the synthesized products contain amino and carboxylate moieties, which provides opportunity for further derivatization in synthesis of diverse molecules. The method can tolerate functional groups in the substrates including ether (3d–f), C–Cl bond (3g–i), cyano (3j) and ester (3a–l).
| 3, Yieldb | ||
|---|---|---|
| a Reaction conditions: substituted 1-(2-bromophenyl)-3-alkylprop-2-yn-1-one (1) (0.25 mmol), hydrazine hydrochloride (1.2 equiv, 0.3 mmol), alkyl 2-cyanoacetate (2) (5 equiv, 1.25 mmol), CuI (0.1 equiv, 0.025 mmol), K3PO4 (5 equiv, 1.25 mmol), DMF (2 mL) under nitrogen atmosphere. Reaction time (24 h). b Isolated yield. | ||
|
|
|
|
|
|
|
|
|
|
|
|
As shown in Scheme 1, we attempted control experiments in order to explore the copper-catalyzed three-component reaction mechanism. Coupling of 1-(2-bromophenyl)-3-phenylprop-2-yn-1-one (1a) with hydrazine hydrochloride in the presence of K3PO4 led to 4-(5-(2-bromophenyl)-1H-pyrazol-3-yl)benzonitrile in 87% yield (Ia) (Scheme 1A), whose structure was confirmed by NMR and MS. Copper-catalysis with Ia and ethyl 2-cyanoacetate (2b) provided product 3b in 91% yield under the standard conditions (Scheme 1B). Therefore, a possible mechanism for this copper-catalyzed three-component reaction of substituted 1-(2-bromophenyl)-3-phenylprop-2-yn-1-ones, hydrazine hydrochloride and alkyl 2-cyanoacetates leading to alkyl 5-amino-2-alkyl-H-pyrazolo[5,1-a]isoquinoline-6-carboxylates (3) is proposed in Scheme 2. First, coupling of substituted 1-(2-bromophenyl)-3-phenylprop-2-yn-1-one (1) with hydrazine hydrochloride provides I in the presence of base (K3PO4). Copper-catalyzed coupling of I with alkyl 2-cyanoacetate (C-arylation) gives II, intramolecular nucleophilic attack of the NH of the pyrazolo group to cyano in II leads to III, and isomerization of III affords the target product (3).
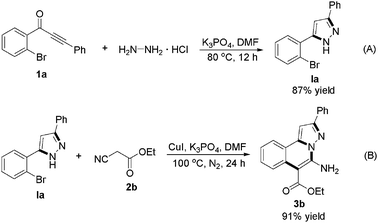 | ||
| Scheme 1 (A) Coupling of 1-(2-bromophenyl)-3-phenylprop-2-yn-1-one (1a) with hydrazine hydrochloride leading to 5-(2-bromophenyl)-3-phenyl-1H-pyrazole (Ia). (B) Copper-catalyzed of Ia with ethyl 2-cyanoacetate (2b) leading to 3b. | ||
![Possible mechanism for synthesis of alkyl 5-amino-2-alkyl-H-pyrazolo[5,1-a]isoquinoline-6-carboxylates (3).](/image/article/2012/RA/c2ra21305b/c2ra21305b-s2.gif) | ||
| Scheme 2 Possible mechanism for synthesis of alkyl 5-amino-2-alkyl-H-pyrazolo[5,1-a]isoquinoline-6-carboxylates (3). | ||
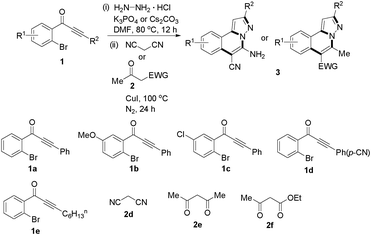
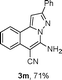
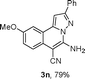
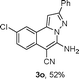
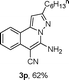
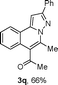
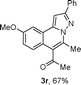
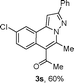
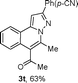
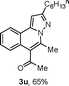
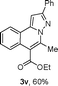
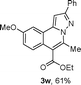
We extended the three-component reactions to other substrates including malononitrile (2d), pentane-2,4-dione (2e) and ethyl 3-oxobutanoate (2f). Unfortunately, some by-products were observed. A major reason was that hydrazine could react with 2d–f during treatment of 1 with hydrazine. Therefore, one-pot two-step reactions of three-components were performed as shown in Table 3: coupling of 1 with hydrazine hydrochloride led to intermediate I (see Scheme 2) in DMF in the presence of K3PO4 or Cs2CO3 at 80 °C for 12 h, and then treatment of I with 2d–f provided the target product (3) under copper-catalysis at 100 °C for 24 h. The one-pot two-step reactions exhibited the similar results to Table 2. In addition, the synthesized products possess amino, cyano, carbonyl and ester groups.
| 3, yieldb | ||
|---|---|---|
| a Reaction conditions: substituted 1-(2-bromophenyl)-3-alkylprop-2-yn-1-one (1) (0.25 mmol), hydrazine hydrochloride (1.2 equiv, 0.3 mmol), alkyl 2-cyanoacetate (2) (5 equiv, 1.25 mmol), CuI (0.1 equiv, 0.025 mmol), K3PO4 for 3m–p (5 equiv, 1.25 mmol), Cs2CO3 for 3q–x (5 equiv, 1.25 mmol), DMF (2 mL) under nitrogen atmosphere. Reaction time for the first step (12 h); for the second step (24 h). b Isolated yield for the two steps. | ||
|
|
|
|
|
|
|
|
|
|
|
|
In summary, we have developed a convenient and efficient copper-catalyzed method for synthesis of H-pyrazolo[5,1-a]isoquinolines from the readily available starting materials. The synthesized H-pyrazolo[5,1-a]isoquinolines own various key functional groups including halo, amino, ester, cyano and carbonyl, which provided opportunity for the construction of diverse biologically active molecules.
Acknowledgements
The authors wish to thank the National Natural Science Foundation of China (Grant Nos. 20972083 and 21172128), and the Ministry of Science and Technology of China (Grant No. 2012CB722605) for financial support.References
- R. W. DeSimone, K. S. Currie, S. A. Mitchell, J. W. Darrow and D. A. Pippin, Comb. Chem. High Throughput Screening, 2004, 7, 473 CrossRef CAS.
- P. D. Leeson and B. Springthorpe, Nat. Rev. Drug Discovery, 2007, 6, 881 CrossRef CAS.
- (a) A. Strecker, Justus Liebigs Ann. Chem., 1850, 75, 27 CrossRef; (b) A. Döling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef; (c) C. O. Kappe, Acc. Chem. Res., 2000, 33, 879 CrossRef CAS; (d) K. Kumari, D. S. Raghuvanshi, V. Jouikov and K. N. Singh, Tetrahedron Lett., 2012, 53, 1130 CrossRef CAS.
- K. W. Bentley, The Isoquinoline Alkaloids, Hardwood Academic, Amsterdam, Vol. 1, 1998 Search PubMed.
- Selected examples: (a) F. Dzierszinski, A. Coppin, M. Mortuaire, E. Dewally, C. Slomianny, J.-C. Ameisen, F. DeBels and S. Tomavo, Antimicrob. Agents Chemother., 2002, 46, 3197 CrossRef CAS; (b) D. Kletsas, W. Li, Z. Han and V. Papadopoulos, Biochem. Pharmacol., 2004, 67, 1927 CrossRef CAS; (c) U. R. Mach, A. E. Hackling, S. Perachon, S. Ferry, C. G. Wermuth, J.-C. Schwartz, P. Sokoloff and H. Stark, ChemBioChem, 2004, 5, 508 CrossRef CAS; (d) D. E. Muscarella, K. A. O'Brian, A. T. Lemley and S. E. Bloom, Toxicol. Sci., 2003, 74, 66 CrossRef CAS.
- (a) W. Yu, E. Wang, R. J. Voll, A. H. Miller and M. M. Goodman, Bioorg. Med. Chem., 2008, 16, 6145 CrossRef CAS; (b) B. W. Trotter, K. K. Nanda, N. R. Kett, C. P. Regan, J. J. Lynch, G. L. Stump, L. Kiss, J. Wang, R. H. Spencer, S. A. Kane, R. B. White, R. Zhang, K. D. Anderson, N. J. Liverton, C. J. McIntyre, D. C. Beshore, G. D. Hartman and C. J. Dinsmore, J. Med. Chem., 2006, 49, 6954 CrossRef CAS.
- Z. Chen and J. Wu, Org. Lett., 2010, 12, 4856 CrossRef CAS.
- Selected papers: (a) S. Li and J. Wu, Org. Lett., 2011, 13, 712 CrossRef CAS; (b) S. Ye, X. Yang and J. Wu, Chem. Commun., 2010, 46, 5238 RSC; (c) X. Yu, S. Ye and J. Wu, Adv. Synth. Catal., 2010, 352, 2050 CrossRef CAS; (d) X. Yu, Z. Chen, X. Yang and J. Wu, J. Comb. Chem., 2010, 12, 374 CrossRef CAS; (e) Z. Chen, X. Yang and J. Wu, Chem. Commun., 2009, 3469 RSC; (f) Z. Chen, Q. Ding, X. Yu and J. Wu, Adv. Synth. Catal., 2009, 351, 1692 CrossRef CAS; (g) Z. Chen, M. Su, X. Yu and J. Wu, Org. Biomol. Chem., 2009, 7, 4641 RSC.
- (a) S. Hernández, R. SanMartin, I. Tellitu and E. Domínguez, Org. Lett., 2003, 5, 1095 CrossRef; (b) J. J. Mousseau, A. Fortier and A. Charette, Org. Lett., 2010, 12, 516 CrossRef CAS; (c) E. E. Schweizer, M. Nelson and W. Stallings, J. Org. Chem., 1980, 45, 4795 CrossRef CAS; (d) X. Li and M. Zhao, J. Org. Chem., 2011, 76, 8530 CrossRef CAS; (e) D. B. Huple, C.-H. Chen, A. Das and R.-S. Liu, Adv. Synth. Catal., 2011, 353, 1877 CrossRef CAS.
- For recent reviews on copper-catalyzed cross couplings, see: (a) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS; (b) K. Kunz, U. Scholz and D. Ganzer, Synlett, 2003, 2428 CrossRef CAS; (c) I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337 CrossRef CAS; (d) D. Ma and Q. Cai, Acc. Chem. Res., 2008, 41, 1450 CrossRef CAS; (e) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef CAS; (f) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954 CrossRef CAS; (g) H. Rao and H. Fu, Synlett, 2011, 745 CAS and references cited therein.
- For recent studies on the synthesis of N-heterocycles through Ullmann-type couplings, see: (a) R. Martin, M. R. Rivero and S. L. Buchwald, Angew. Chem., Int. Ed., 2006, 45, 7079 CrossRef CAS; (b) G. Evindar and R. A. Batey, J. Org. Chem., 2006, 71, 1802 CrossRef CAS; (c) F. Bonnaterre, M. Bois-Choussy and J. Zhu, Org. Lett., 2006, 8, 4351 CrossRef CAS; (d) B. Zou, Q. Yuan and D. Ma, Angew. Chem., Int. Ed., 2007, 46, 2598 CrossRef CAS; (e) Y. Chen, X. Xie and D. Ma, J. Org. Chem., 2007, 72, 9329 CrossRef CAS; (f) X. Yuan, X. Xu, X. Zhou, J. Yuan, L. Mai and Y. Li, J. Org. Chem., 2007, 72, 1510 CrossRef CAS; (g) B. Wang, B. Lu, Y. Jiang, Y. Zhang and D. Ma, Org. Lett., 2008, 10, 2761 CrossRef CAS; (h) G. Altenhoff and F. Glorius, Adv. Synth. Catal., 2004, 346, 1661 CrossRef CAS.
- For selected papers, see: (a) X. Liu, H. Fu, Y. Jiang and Y. Zhao, Angew. Chem., Int. Ed., 2009, 48, 348 CrossRef CAS; (b) C. Huang, Y. Fu, H. Fu, Y. Jiang and Y. Zhao, Chem. Commun., 2008, 6333 RSC; (c) D. Yang, H. Fu, L. Hu, Y. Jiang and Y. Zhao, J. Org. Chem., 2008, 73, 7841 CrossRef CAS; (d) D. Yang, H. Liu, H. Yang, H. Fu, L. Hu, Y. Jiang and Y. Zhao, Adv. Synth. Catal., 2009, 351, 1999 CrossRef CAS; (e) F. Wang, H. Liu, H. Fu, Y. Jiang and Y. Zhao, Org. Lett., 2009, 11, 2469 CrossRef CAS; (f) J. Lu, X. Gong, H. Yang and H. Fu, Chem. Commun., 2010, 46, 4172 RSC; (g) H. Zhao, H. Fu and R. Qiao, J. Org. Chem., 2010, 75, 3311 CrossRef CAS; (h) X. Yang, H. Fu, R. Qiao, Y. Jiang and Y. Zhao, Adv. Synth. Catal., 2010, 352, 1033 CrossRef CAS; (i) X. Gong, H. Yang, H. Liu, Y. Jiang, Y. Zhao and H. Fu, Org. Lett., 2010, 12, 3128 CrossRef CAS; (j) S. Xu, J. Lu and H. Fu, Chem. Commun., 2011, 47, 5596 RSC; (k) D. Yang, Y. Wang, H. Yang, T. Liu and H. Fu, Adv. Synth. Catal., 2012, 354, 477 CrossRef CAS; (l) T. Liu, R. Wang, H. Yang and H. Fu, Chem.–Eur. J., 2011, 17, 6765 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General procedure for synthesis, characterization data, and 1H and 13C NMR spectra of compounds 3a–x. See http://dx.doi.org/10.1039/c2ra21305b/ |
| This journal is © The Royal Society of Chemistry 2012 |