Platinum nanoparticle immobilization onto carbon nanotubes using Pt-sputtered room-temperature ionic liquid†
Kazuki
Yoshii
a,
Tetsuya
Tsuda
*ab,
Takashi
Arimura
c,
Akihito
Imanishi
cd,
Tsukasa
Torimoto
e and
Susumu
Kuwabata
*ad
aDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamada-oka, Suita, Osaka, 565-0871, Japan. E-mail: kuwabata@chem.eng.osaka-u.ac.jp
bFrontier Research Base for Global Young Researchers, Graduate School of Engineering, Osaka University, 2-1 Yamada-oka, Suita, Osaka, 565-0871, Japan. E-mail: ttsuda@chem.eng.osaka-u.ac.jp
cDepartment of Chemistry, Graduate School of Engineering Science, Osaka University, 1-3 Machikaneyama, Toyonaka, Osaka, 560-8531, Japan
dCore Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency (JST), Kawaguchi, Saitama, 332-0012, Japan
eDepartment of Crystalline Materials Science, Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, Aichi, 464-8603, Japan
First published on 24th July 2012
Abstract
Establishment of a facile Pt nanoparticle–SWCNT composite fabrication method that never requires a laborious pretreatment of SWCNTs or any chemical reagent was achieved by using Pt-sputtered RTILs.
Metal nanoparticles have received great attention over the past few decades because of their distinctive characteristics including large surface area, specific catalytic activities, and quantum size effect.1 Much effort has been made to produce such useful metal nanoparticles by various methods to date. However, the conventional methods require a stabilizing agent to prevent aggregation behaviour of the obtained metal nanoparticles. The stabilizing agent often affects physicochemical properties of the metal nanoparticles.2
Recently, metal nanoparticle preparation methods using room-temperature ionic liquids (RTILs) have become a hot topic in materials chemistry because it works not only as a reaction medium but also as a stabilizing agent, allowing homogeneous dispersion of the produced nanoparticles.3 RTIL is a liquid salt that can keep a liquid state over a wide temperature range, and they possess unique properties, e.g., high thermal and chemical stabilities, relatively-high ionic conductivity, wide electrochemical window, and extremely-low vapour pressure.4 Of special note is that the peculiar liquid salt can be handled at 298 K or less. A couple years ago, Dupont et al. reviewed synthetic methods for various nanoparticles in RTILs.5 Janek, Endres, and Baba reported a distinct metal nanoparticle preparation method in RTIL by plasmaelectrochemistry.6,7 Although most RTIL-used metal nanoparticle preparations are conducted by chemical methods, we found that magnetron sputtering that is one of physical material tailoring methods is a useful technique to yield metal nanoparticles uniformly dispersed in RTIL.8 This method, which is named RTIL-sputtering method, has enabled the synthesis of a variety of metal or metal oxide nanoparticles.9 However there is no report about immobilization of metal nanoparticles prepared by this method on carbon nanotubes (CNTs) that is a promising supporting material for catalytically active nanoparticles due to favourable physicochemical properties and large surface area to volume ratio.10 Regardless of metal nanoparticle preparation methods, it is very difficult for us to immobilize metal nanoparticles on CNTs consisting of a chemically inert surface. But, a couple of successful approaches to immobilize metal nanoparticle onto the CNT surface have already been proposed. A commonly-used approach is a CNT oxidation method with a strong acid.11 By this approach, aromatic rings on CNTs are partially wrecked, and it offers active sites for metal nanoparticle immobilization, e.g., –COOH and –OSO3H groups. Another popular approach is a wrapping method.12 In this case, the CNT is covered with a functionalized polymer that works as an organic linker. These conventional approaches often have an adverse impact on original electrical property and chemical stability of the CNT owing to partial breakup of the π conjugated skeleton and reduction of contact points among the CNTs. In this article, we report that the RTIL species shown in Fig. S1 (ESI†) affect Pt nanoparticle preparation by the RTIL-sputtering method as pertains to the immobilization of Pt nanoparticles onto the single-walled carbon nanotube (SWCNT). The aim of this study is, using the Pt nanoparticle-dispersed RTILs prepared by the RTIL-sputtering method, to establish a facile approach for Pt nanoparticle-immobilized SWCNT (Pt–SWCNT) composite fabrication without any special pretreatment of the SWCNT.
Fig. S2 (ESI†) depicts a typical TEM image and a size distribution diagram of the Pt nanoparticles prepared in N,N,N-trimethyl-N-propylammonium bis(trifluoromethanesulfonyl)amide ([Me3PrN][Tf2N]) by RTIL-magnetron sputtering method. Provided that the sputtering time was 900 s, the mean particle size of the Pt nanoparticles was estimated to be 2.4 nm and the size distribution width was relatively narrow. The monodispersed state of the Pt nanoparticles was kept for more than three months. The Pt concentration in the Pt-sputtered [Me3PrN][Tf2N] determined by ICP-AES measurements was 21.6 mmol L−1. The concentration varied approximately linearly with the sputtering time but interestingly the mean nanoparticle size was nearly-unchanged (Table S1, ESI†). Similarly spherical Pt nanoparticles were also produced in different RTILs. As summarized in Table S2 (ESI†), the resulting Pt nanoparticles had mean particle size of ca. 1.4–2.4 nm except for that prepared in 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)amide ([EtMeIm][Tf2N]). The particle size was apparently dependent on the RTIL species. As to the [EtMeIm][Tf2N], we could see very small crystals in the Pt-sputtered RTIL but could not estimate the crystal size precisely because their size was considerably small.
The Pt nanoparticle-dispersed RTILs prepared by the RTIL-sputtering method were exploited for the fabrication of Pt nanoparticle-immobilized SWCNT composite material. The fabrication process is depicted in Scheme S1 (ESI†). This approach never needs a laborious pretreatment of the SWCNT or any chemical reagents. One thing that we have to do is to agitate the mixture of the Pt nanoparticle-dispersed RTIL and SWCNT with heating. After completing the agitation process, the resulting mixture was rinsed in dry acetonitrile several times to remove excess RTIL. Eventually we collected the target Pt–SWCNT composite by a centrifuge separation method. The Pt–SWCNT was dried in vacuum before use. As discussed below, we revealed that minuscule amounts of RTIL remains in the Pt–SWCNT by XPS analysis. Fig. 1 represents TEM images of Pt–SWCNT fabricated under different Pt-nanoparticle preparation conditions. If the Pt nanoparticles were prepared in [Me3PrN][Tf2N], many of them were readily immobilized on the SWCNTs by our approach. The amount of the Pt nanoparticles supported on the composite could be controlled by the sputtering time for the Pt nanoparticle preparation (Fig. 1a–c and Table S1, ESI†). The mean particle size was ca. 3.2–3.5 nm independent of the sputtering time although it became larger than original Pt nanoparticle size during the agitating process at 573 K as shown in Scheme S1 (ESI†). EDX data and size distribution diagram of the Pt nanoparticles supported on the SWCNT are presented in Fig. S3 (ESI†). As given in Table S2 (ESI†), mean particle size of the Pt nanoparticles on the Pt–SWCNT composite varied with RTIL species used for the Pt sputtering process (Fig. S3, S4, and S5, ESI†). The value was ca. 2.3–3.5 nm except for [EtMeIm][Tf2N]. The amount of Pt nanoparticles was up to 32.0 wt%, but it was difficult to support the Pt nanoparticles dispersed in imidazolium-based RTILs onto the SWCNT. The Pt content in the Pt–SWCNT was quite low compared with other RTIL systems having alkylammonium cations and saturated heterocyclic cations. For example, there are no measurable Pt nanoparticles on the Pt–SWCNT fabricated by using [EtMeIm][Tf2N] (Fig. 1d), and the Pt content was 1.30 wt.%. Considering that imidazolium cation-based RTILs can form an ordered layer-by-layer liquid structure on a solid interface,13,14 this unexpected result may be caused by the ordered layer structure onto both Pt nanoparticle and SWCNT. Under such conditions, a thick RTIL layer between the Pt nanoparticles and SWCNT is likely formed, and then it seems to be hard to immobilize the Pt nanoparticle on the SWCNT because the fluidity of the RTIL inevitably increases with increasing the layer thickness. We believe this is the reason why the unexpected result was obtained in the imidazolium-based RTILs. However, as shown in Table S2 (ESI†), the Pt content in the Pt–SWCNT composites produced by imidazolium-based RTILs tended to increase when the alkyl chain on the imidazolium cation became longer. This would be caused by an increase in the molecular fluctuation in the cation leading to the destruction of the stable RTIL layer structure on the Pt nanoparticle and the SWCNT. These results support the discussion reported by Endres, et al.15 What is interesting is that our procedure can readily change the amount of Pt nanoparticles supported on the SWCNT composite and the particle size by just controlling the Pt sputtering conditions and/or the RTIL species. This feature is a great advantage for our approach.
![TEM images of Pt nanoparticle–SWCNT composite fabricated by Scheme S1 (ESI). The RTIL was (a–c) [Me3PrN][Tf2N] and (d) [EtMeIm][Tf2N]. The sputtering time was (a) 300 s, (b) 600 s, and (c, d) 900 s.](/image/article/2012/RA/c2ra21243a/c2ra21243a-f1.gif) | ||
| Fig. 1 TEM images of Pt nanoparticle–SWCNT composite fabricated by Scheme S1 (ESI†). The RTIL was (a–c) [Me3PrN][Tf2N] and (d) [EtMeIm][Tf2N]. The sputtering time was (a) 300 s, (b) 600 s, and (c, d) 900 s. | ||
Because a facile approach to immobilize metal nanoparticles onto sp2 carbon nanomaterials including CNTs and graphene is strongly required in various fields, it is considerably important to clarify the driving force for the Pt–SWCNT composite material fabrication reported in this article, in terms of design for future functional materials composed of metal nanoparticle and sp2 carbon nanomaterials. We utilized X-ray photoelectron spectroscopy to reveal the driving force and investigated the interactions in the Pt–SWCNT composite material. Fig. 2 exhibits XPS spectra of neat [Me3PrN][Tf2N], Pt nanoparticle-dispersed [Me3PrN][Tf2N] prepared by the magnetron sputtering method, and Pt–SWCNT fabricated from the Pt-dispersed RTIL. In the case of neat RTIL, there is no XPS valence band for Pt (Fig. 2a). As to the Pt nanoparticle-dispersed RTIL, XPS peaks related to platinum appeared at 73.3 (Pt 4f7/2) and 76.4 eV (Pt 4f5/2). These values are well accorded with the binding energies for Pt(II), not Pt(0).16 In fact, several research groups have already reported that Pt nanoparticles prepared in RTIL have a minimal positive charge on the nanoparticle surface and the charged Pt nanoparticle is stabilized with anions in the RTIL.17–19 The anomalous spectrum obtained by our XPS analysis supports this. After fabrication of the Pt–SWCNT, the peaks shifted to lower binding energy, 71.1 (Pt 4f7/2) and 74.4 eV (Pt 4f5/2). These peaks were attributed to Pt(0), suggesting that Pt nanoparticles exist on the SWCNT in a metal state. Fig. 2b and 2c show XPS spectra associated with N 1s and F 1s, respectively. The XPS spectra for N 1s and F 1s observed in neat [Me3PrN][Tf2N] is confusingly similar to those in the Pt nanoparticle-dispersed RTIL. However, with regard to the Pt–SWCNT composite, XPS peak for N 1s spectrum at 403 eV, which is due to the cationic species in RTIL,20 shifted to a higher binding energy, and that for F 1s spectrum moved to a lower binding energy relative to other two samples. This indicates that there is a strong interaction between the N atom in [Me3PrN]+ and F atom in [Tf2N]− although the Pt–SWCNT was rinsed in dry acetonitrile thoroughly to remove any RTIL. These results imply that the remaining RTIL plays a key role to immobilize Pt nanoparticle on the SWCNT surface. Given both the XPS results obtained in this study and the fact that cationic species in RTIL interact with π electron clouds on the SWCNT surface,21,22 we can give a plausible model for the Pt–SWCNT fabrication as shown in Fig. 3. We believe that the RTIL exists as nanoglue in between the Pt nanoparticles and the SWCNT. Thus, the Pt–SWCNT composite can be fabricated without any pretreatment processes for SWCNT.
![XPS spectra of (a) Pt 4f, (b) N 1s, (c) F 1s of (—) neat [Me3PrN][Tf2N], (– – –) Pt-sputtered [Me3PrN][Tf2N] and (···) Pt–SWCNT.](/image/article/2012/RA/c2ra21243a/c2ra21243a-f2.gif) | ||
| Fig. 2 XPS spectra of (a) Pt 4f, (b) N 1s, (c) F 1s of (—) neat [Me3PrN][Tf2N], (– – –) Pt-sputtered [Me3PrN][Tf2N] and (···) Pt–SWCNT. | ||
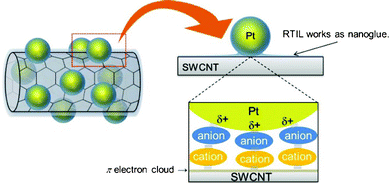 | ||
| Fig. 3 Schematic illustration of Pt–SWCNT composite material fabricated in this study. | ||
Metal nanoparticle–CNT composite materials are expected to be a next-generation electrode catalyst for polymer electrolyte fuel cells (PEFCs). There are many papers on the electrocatalytic characteristics of metal nanoparticle–CNT composites.23 We examined the electrocatalytic activity of the Pt–SWCNT prepared by the Scheme S1 (ESI†) in an oxygen reduction reaction (ORR). The results are summarized in the ESI†. We found that the Pt–SWCNT has a great potential as an electrocatalyst for the PEFC system.
In summary, using Pt-sputtered RTILs, we succeeded in the establishment of a facile Pt nanoparticle–SWCNT composite fabrication method. We found that RTIL can work as nanoglue for sticking Pt nanoparticles against SWCNTs. Interestingly our approach enabled variations in the amount of Pt nanoparticle and Pt particle size supported on the Pt–SWCNT composite by changing the sputtering time and/or RTIL species in the Pt sputtering process. These features are a great advantage for our approach. In addition, the Pt–SWCNT composite has a promising catalytic activity for electrochemical ORR.
Acknowledgements
KY expresses his special thanks for The Global COE Program “Global Education and Research Center for Bio-Environmental Chemistry” of Osaka University. Part of this research was supported by Grant-in-Aid for Scientific Research B, Grant No. 24350071, and for Scientific Research on Innovative Areas (Area No. 2206), Grant No. 23107518, Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT), Kansai Research Foundation for Technology Promotion in Japan, The Noguchi Institute in Japan, and the General Sekiyu R&D Encouragement Assistance Foundation in Japan.References
- G. Schmid, Nanoparticles, Wiley–VCH, Weinheim, 2nd edn, 2010 Search PubMed.
- L. S. Ott and R. G. Finke, Coord. Chem. Rev., 2007, 251, 1075 CrossRef CAS.
- (a) T. Torimoto, T. Tsuda, K. Okazaki and S. Kuwabata, Adv. Mater., 2010, 22, 1196 CrossRef CAS; (b) S. Kuwabata, T. Tsuda and T. Torimoto, J. Phys. Chem. Lett., 2010, 1, 3177 CrossRef CAS; (c) C. Vollmer and C. Janiak, Coord. Chem. Rev., 2011, 255, 2039 CrossRef CAS.
- (a) Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, Germany, 2nd edn, 2008 Search PubMed; (b) T. Tsuda and C. L. Hussey, in Modern Aspects of Electrochemistry, ed. R. E. White, Springer, New York, Vol. 45, 2009, pp. 63–174 Search PubMed; (c) T. Tsuda, K. Kondo, T. Tomioka, Y. Takahashi, H. Matsumoto, S. Kuwabata and C. L. Hussey, Angew. Chem., Int. Ed., 2011, 50, 1310 CrossRef CAS.
- J. Dupont and J. D. Scholten, Chem. Soc. Rev., 2010, 39, 1780 RSC.
- S. A. Meiss, M. Rohnke, L. Kienle, S. Z. El Abedin, F. Endres and J. Janek, ChemPhysChem, 2007, 8, 50 CrossRef CAS.
- K. Baba, T. Kaneko and R. Hatakeyama, Appl. Phys. Lett., 2007, 90, 201501 CrossRef.
- T. Torimoto, K. Okazaki, T. Kiyama, K. Hirahara, N. Tanaka and S. Kuwabata, Appl. Phys. Lett., 2006, 89, 243117 CrossRef.
- (a) K. Okazaki, T. Kiyama, T. Suzuki, S. Kuwabata and T. Torimoto, Chem. Lett., 2009, 38, 330 CrossRef CAS; (b) T. Tsuda, K. Yoshii, T. Torimoto and S. Kuwabata, J. Power Sources, 2010, 195, 5980 CrossRef CAS; (c) T. Suzuki, K. Okazaki, S. Suzuki, T. Shibayama, S. Kuwabata and T. Torimoto, Chem. Mater., 2010, 22, 5209 CrossRef CAS.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105 CrossRef CAS.
- R. Yu, L. Chen, Q. Liu, J. Lin, K. L. Tan, S. C. Ng, H. S. O. Chan, G. Q. Xu and T. S. A. Hor, Chem. Mater., 1998, 10, 718 CrossRef CAS.
- (a) M. Okamoto, T. Fujigaya and N. Nakashima, Small, 2009, 5, 735 CrossRef CAS; (b) B. Wu, D. Hu, Y. Kuang, B. Liu, X. Zhang and J. Chen, Angew. Chem., Int. Ed., 2009, 121, 4751 CrossRef.
- R. Atkin and G. G. Warr, J. Phys. Chem. C, 2007, 111, 5162 CAS.
- R. Hayes, S. Z. E. Abedin and R. Atkin, J. Phys. Chem. B, 2009, 113, 7049 CrossRef CAS.
- F. Endres, O. Höfft, N. Borisenko, L. H. Gasparotto, A. Prowald, R. Al-Salman, T. Carstens, R. Atkin, A. Bund and S. Z. E. Abedin, Phys. Chem. Chem. Phys., 2010, 12, 1724 RSC.
- J. Chatain and R. C. King, Jr.Handbook of X-ray Photoelectron Spectroscopy, Physical Electronics, Minnesota, 1995 Search PubMed.
- E. Redel, R. Thomann and C. Janiak, Inorg. Chem., 2008, 47, 14 CrossRef CAS.
- H. Wender, L. F. de Oliveira, P. Migowski, A. F. Feil, E. Lissner, M. H. G. Prechtl, S. R. Teixeira and J. Dupont, J. Phys. Chem. C, 2010, 114, 11764 CAS.
- C. W. Scheeren, G. Machado, S. R. Teixeira, J. Morais, J. B. Domingos and J. Dupont, J. Phys. Chem. B, 2006, 110, 13011 CrossRef CAS.
- M. Shigeyasu, H. Murayama and H. Tanaka, Chem. Phys. Lett., 2008, 463, 373 CrossRef CAS.
- T. Fukushima, A. Kosaka, Y. Ishimura, T. Yamamoto, T. Takigawa, N. Ishii and T. Aida, Science, 2003, 300, 2072 CrossRef CAS.
- Y. Shim and H. J. Kim, ACS Nano, 2009, 3, 1693 CrossRef CAS.
- M. S. Saha and A. Kundu, J. Power Sources, 2010, 195, 6255 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental details and additional results and discussion. See DOI: 10.1039/c2ra21243a/ |
| This journal is © The Royal Society of Chemistry 2012 |
