DOI:
10.1039/C2RA21406G
(Review Article)
RSC Adv., 2012,
2, 9763-9777
Recent advances in new multicomponent synthesis of structurally diversified 1,4-dihydropyridines
Received
6th May 2012
, Accepted 8th August 2012
First published on 14th August 2012
Abstract
1,4-Dihydropyridines (DHPs) are one of the most important classes of heterocyclic compounds as privileged pharmacophores. They are therefore attractive synthetic targets of organic chemistry. Multicomponent reactions (MCRs) are the most efficient strategies for the synthesis of 1,4-DHPs in terms of providing both sufficient structural diversity and numbers for compound libraries. Following the classical multicomponent synthesis of 1,4-DHPs by the Hantzsch reaction, chemists have developed a large number of new MCRs to access 1,4-DHPs with significantly extended structural diversity, which is pivotal for the process of discovering new lead compounds and drugs of 1,4-DHPs. The advances on the synthesis of structurally diversified 1,4-DHPs through new MCRs beyond the classical Hantzsch reaction is reviewed.
 Jie-Ping Wan | Dr Jie-Ping Wan obtained his Bacholer's degree by studying chemistry in Nanchang University in 2000–2004. He continued his graduate study in Zhejiang University in 2005–2010 in Professor Yuanjiang Pan’s group and obtained his Ph.D degree in 2010. In July, 2010, he joined the College of Chemistry and Chemical Engineering in Jiangxi Normal University as an assistant professor. In September 2011, under the support of Chinesisch-Deutsche Zentrum für Wissenschaftsförderung Dr Wan joined Prof. Dieter Enders group in RWTH Aachen University as a postdoctoral fellow working in organocatalysis. His present research interest contains multicomponent reactions, organocatalysis and metal-catalyzed synthesis. |
 Yunyun Liu | Dr Yunyun Liu was born in 1983 in Shandong Province, China. She obtained her Bachelor Degree in Qufu Normal University in 2005. She then moved to Zhejiang University to continue her graduate study in the Department of Chemistry. Under the supervision of Professor Weiliang Bao, she worked in the field of copper-catalyzed Ullmann coupling reaction and related tandem reactions for her doctorate study. She obtained her doctorate degree in 2010 and presently is an assistant professor in Jiangxi Normal University. She is currently interested in the research of metal-catalyzed organic synthesis and the development of new cascade organic reactions. |
1. Introduction
Among the known strategies of drug discovery, high throughput screening is obviously the one that is serving most efficiently to the pharmaceutical industry. To guarantee the success on acquiring lead compounds via this kind of screening tactic, sources of large amount of molecular libraries are important preconditions.1 To satisfy such requirements, new synthetic strategies with robust efficiency in providing a massive amount of structurally diverse organic products are the only solution. MCRs which employ three or more reactants to furnish products containing structure or substructure of all starting materials in one-pot and one set of fixed reaction conditions are such examples with powerful productivity.2 The Hantzsch reaction (Scheme 1) which provides 1,4-DHPs as products is one of the earliest and most well-known MCRs owing to the excellent pharmaceutical profile of 1,4-DHPs.3 The most typical examples of 1,4-DHPs pharmaceuticals are those broadly prescribed calcium channel blockers, for example, Nifedipine (1), Felodipine (2), Nicardipine (3) and Nimodipine (4), to name but a few (Scheme 2). In addition, other versatile biological profiles of 1,4-DHPs such as anticonvulsant activity,4 selective adenosine-A3 receptor antagonism,5 radioprotective activity,6 sirtuin activation and inhibition,7etc. have also been reported. Therefore, it is not surprising that 1,4-DHPs receive daily increasing interests as synthetic targets.
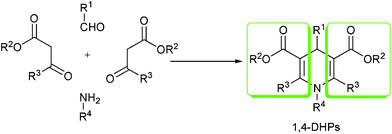 |
| | Scheme 1 Classical Hantzsch reaction. | |
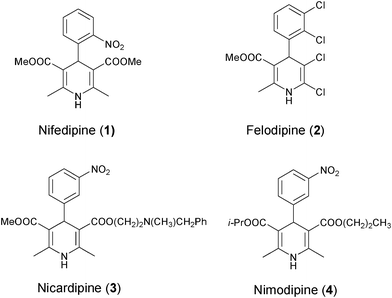 |
| | Scheme 2 1,4-DHPs used as clinical drugs. | |
Conventionally, 1,4-DHPs could be accessed via the Hantzsch reaction, reduction of pyridines, addition to pyridines or cycloadditions etc.8 As a facile and broadly tolerable protocol, the Hantzsch reaction remains as a frequently employed tactic for the synthesis of 1,4-DHPs in a large number of areas such as stereoselective synthesis and green chemistry.9 On the other hand, following the rapid progress of chemical and pharmaceutical industries, some limits have also been noticed on this classical multicomponent synthetic route. A main limit of the Hantzsch reaction is that the 1,4-DHPs provided by the reaction are all symmetrical in the heterocyclic fragment since this unit was constructed by employing two molecules of 1,3-dicarbonyl compounds. Although Hantzsch reactions using two different dicarbonyl compounds had been later achieved, the symmetrical 1,4-DHPs formed by the homo-condensation of same dicarbonyl compound remained more or less as side products in these entries. In terms of biological screening, acquiring diversified derivatives of a known pharmacophore is a main and efficient approach for discovering new biologically active lead compounds.10 Even though many reported biologically active 1,4-DHPs are symmetrical, this was not the requirement of biological receptors, but the results of employing the Hantzsch reaction for 1,4-DHPs synthesis in most cases. For the sake of finding more 1,4-DHPs possessing new and improved biological functions, the synthesis of structurally diverse 1,4-DHPs such as unsymmetrical 1,4-DHPs is pivotal work. Therefore, new MCRs that are capable of generating novel 1,4-DHPs have attracted significant attention in recent years. Considering the merits of 1,4-DHPs for the development of chemical biology and medicinal chemistry as well as the impressive progress being made in this field, it is therefore necessary to summarize the research advances on the multicomponent synthesis of 1,4-DHPs with different new MCRs. We present herein the first review on the advances of 1,4-DHPs synthesis with new MCRs beyond the Hantzsch reaction. Based on the analysis of all known literature on this topic, we classified these reactions into different sections according to the amino sources employed for the construction of the dihydropyridine ring. The main content of this review covered the period from 2004 to 2012.
2. Multicomponent synthesis of 1,4-DHPs using amine as amino source
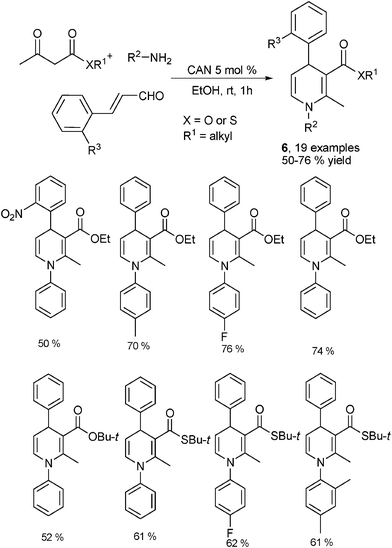
Amines are obviously the most easily available and widely utilized amino sources in organic synthesis. They have been frequently employed as the amine component in the classical Hantzsch reaction. Similarly, amines are also favorable amino sources in the devisal of new multicomponent synthesis of 1,4-DHPs. For example, employing α,β-unsaturated aldehydes, 1,3-dicarbonyl compounds and amines has been found as a very practical approach for the synthesis of unsymmetrical 1,4-DHPs. During their work of synthesizing 1,4-DHPs 6 by two-component condensation of β-enamino esters 5 and α,β-unsaturated aldehydes, Renaud et al. conducted tentative investigations on direct three-component reaction of 1,3-dicarbonyl compounds, amines and α,β-unsaturated aldehydes to access 1,4-DHPs with Lewis acid catalysis. Moderate to excellent yields have been obtained by performing the reactions in a stepwise manner (Scheme 3).11 Later on, the one step, three-component reaction of a 1,3-dicarbonyl compound, primary amine and α,β-unsaturated aldehyde has been realized by Menéndez and coworkers. Their method was established by employing cerium ammonium nitrate (CAN) as a Lewis acid catalyst. This protocol has been demonstrated with excellent substrate tolerance by providing a sound number of 1,4-DHPs of type 6 with moderate to good yields at room temperature. And a noteworthy feature of this methodology was that β-ketothioesters could be efficiently employed as the dicarbonyl component to correspondingly give 1,4-DHPs containing a reactive thioester group as shown by typical results outlined in Scheme 4.12 In addition, as a widely employed catalysis tactic, organocatalysis had also turned out to be practical for this kind of three-component 1,4-DHPs synthesis according to the results presented by Kumar et al. While a series of different organocatalysts such as (−)-cinchonidine, (−)-ephedrine, L-lysine, L-histidine, L-glutamic acid, L-asparatic acid, L-pipecolic acid and L-proline have been screened, L-proline displayed the best activity as an organocatalyst. By performing the reactions of different α,β-unsaturated aldehydes, amines and dicarbonyl compounds at room temperature and solvent free conditions, various 1,4-DHPs of type 6 have been obtained with generally excellent yields. For example, the reaction of cinnamaldehyde, aniline and 2-acetyl ethyl acetate provided the corresponding 1,4-DHP in 90% yield.13 Following these results, some other different catalysts such as nano CuO,14 silica-supported perchloric acid (HClO4·SiO2)15 and InCl316 were also discovered as practical catalysts for this three-component 1,4-DHPs synthesis.
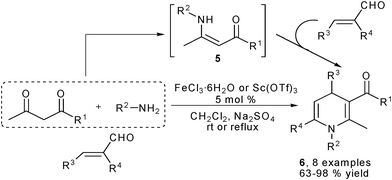 |
| | Scheme 3 Three-component stepwise synthesis of 1,4-DHPs. | |
 |
| | Scheme 4 Typical results on enal-based synthesis of unsymmetrical 1,4-DHPs. | |
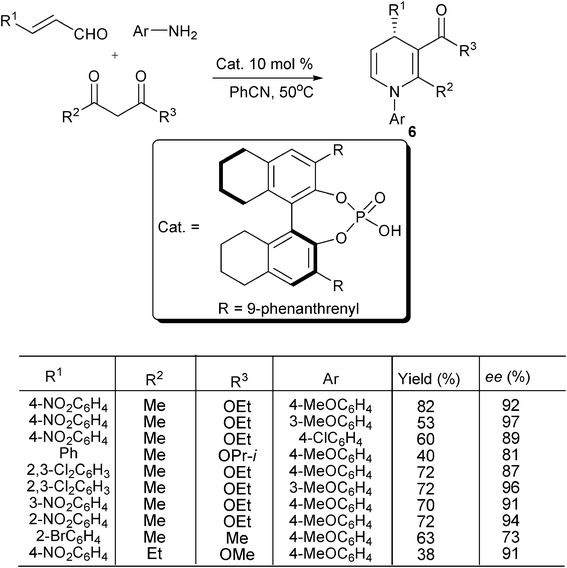
One of the most important properties of unsymmetrical 1,4-DHPs is the chiral center embedded in the central heterocycle. Therefore, performing this kind of multicomponent reactions in an enantioselective manner is of great significance. Few methods of asymmetric catalysis on 1,4-DHPs synthesis are currently available. The representative results on this area was reported by Gong and coworkers, in which a chiral phosphoric acid was used as a Brønsted acid to catalyze the three-component reaction of α,β-unsaturated aldehydes, amines and dicarbonyl compounds giving (S)-6 with good to excellent enantioselectivity in general (66–97% ee) and fair to good yields (31–93%). Some typical results on the synthesized chiral 1,4-DHPs of type 6 are shown in Scheme 5.17 Notably, elegant results on the enantioselective synthesis of 1,4-DHPs by the Hantzsch reaction has also recently been accomplished by Gestwicki and co-workers employing BINOL-phosphoric acid catalysis.18
 |
| | Scheme 5 Enal-based enantioselective synthesis of 1,4-DHPs. | |
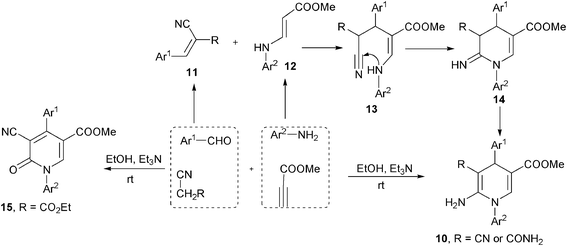
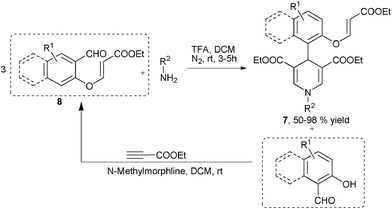
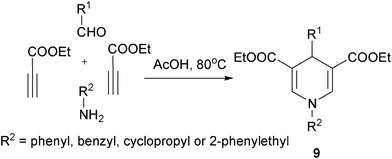
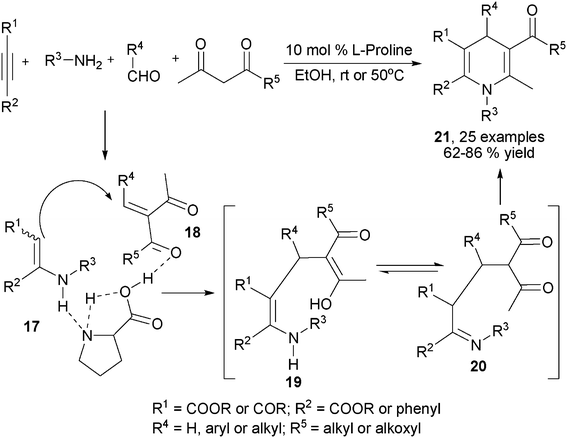
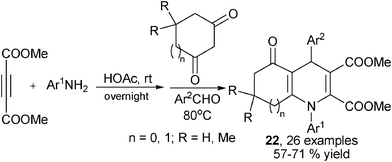
Alkynes were another type of frequently employed building block in the multicomponent synthesis of 1,4-DHPs of both symmetrical and unsymmetrical structures. For example, Wang, et al. developed an interesting multicomponent synthesis of 1,4-DHPs of type 7via TFA-catalyzed assembly of 3 molecules of E-3-(2-formylphenoxy)propenoates 8 and amines (Scheme 6). Corresponding salicaldehydes were generated as side products from 8 after incorporating amines and two ethyl propiolate building blocks. The salicaldehydes were able to be regenerated to 8 by incorporating ethyl propiolate. This transformation has been found to be specifically applicable for the starting materials of type 8 since no corresponding reactions were observed when using either the para-substituted isomers of 8 to incorporate amines or direct three-component reactions of salicaldehyde, aniline and ethyl propiolate/ethyl 3-phenoxyacrylate under standard reaction conditions.19 However, it was later discovered that the direct MCRs of ethyl propiolate, benzaldehyde and a primary amine were capable of providing corresponding 1,4-DHPs 9 by heating the starting materials in acetic acid. The application scope of this protocol, however, was not defined, as only 4 examples of 1,4-DHPs 9 were prepared for the sake of biological research (Scheme 7).7 Also employing propiolates as building blocks for the dihydropyridine ring construction, Yan et al. established a four-component synthesis of 1,4-DHPs 10 from methyl propiolate, aryl amines, aromatic aldehydes and acetonitriles. With the catalysis of triethyl amine, the Knoevenagel condensed intermediates 11 incorporated intermediates 12 from an aza-Michael addition to provide intermediates 13 which underwent subsequent intramolecular cyclization and tautomerization to 1,4-DHPs 10 as final products. On the other hand, when ethyl 2-cyanoacetate was used as the starting material in the place of malonitrile/2-cyanoacetamide, 2-pyridinones 15 were afforded as main or single products in the corresponding entries (Scheme 8).20 As alkyne substrates with similar reactivity as alkyl propiolates, dialkyl acetylenedicarboxylates were also found to be versatile materials for the synthesis of novel functionalized 1,4-DHPs. For example, under the catalysis of organocatalyst L-proline, acetylene-dicarboxylates 16 efficiently reacted with amines to generate β-enamino esters of type 17. Based on this transformation, Jiang and coworkers designed an L-proline catalyzed multicomponent synthetic method starting from acetylenedicarboxylates (alkyl propiolates as well), amines, aldehydes and 1,3-dicarbonyl compounds to synthesize 1,4-DHPs of type 21. As shown in Scheme 9, active intermediates 17 generated from the addition of amines to electron deficient alkynes were believed to directly couple with Knoevenagel condensed intermediates 18 to form 19/20, which were efficiently transformed to 1,4-DHPs 21via further intramolecular condensation. Mild reaction conditions and wide substrate tolerance on all starting materials were advantages in this method.21 Similar to acyclic 1,3-dicarbonyl compounds, cyclic1,3-dicarbonyl compounds also reacted with aromatic amines, aromatic aldehydes and dimethyl acetylenedicarboxylate to give corresponding 1,4-DHPs 22 in a fused structure by employing HOAc as solvent as well as acid catalyst (Scheme 10).22
 |
| | Scheme 6 Three-component synthesis of 1,4-DHPs initiated by E-3-(2-formylphenoxy)propenoates. | |
 |
| | Scheme 7 Direct synthesis of symmetrical 1,4-DHPs from propiolate. | |
 |
| | Scheme 9 Synthesis of fused 1,4-DHPs using cyclic diketones. | |
 |
| | Scheme 10 Synthesis of 1,4-DHPs using aldehyde, amine, a 1,3-dicarbonyl compound and electron deficient alkynes. | |
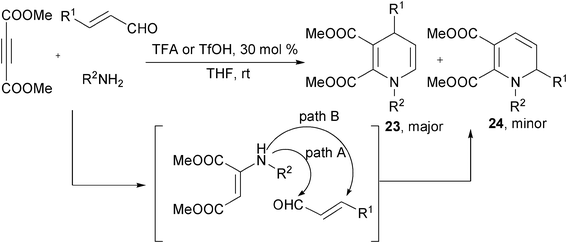
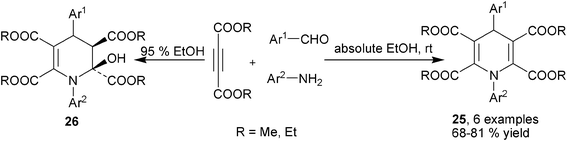
Instead of enone intermediates 18, α,β-unsaturated aldehydes could be directly employed as building blocks to prepare 1,4-DHPs by reacting with acetylenedicarboxylates and primary amines. In the presence of TFA or TfOH, 1,4-DHPs 23 were easily afforded by the three-component reactions of dimethyl acetylenedicarboxylate, amines and α,β-unsaturated aldehydes at room temperature in THF. 1,2-DHPs 24 were formed as minor side products in these reactions. Based on the proposed reaction mechanism, the initial N-nucleophilic site in β-enamino esters attack the different electrophilic sites in α,β-unsaturated aldehydes was believed to determine the regioselectivity. N-attacking led to the formation of 1,4-DHPs, while minor 1,2-DHPs products was proposed to be provided by the aza-Michael addition of the N-nucleophilic site to enals (Scheme 11).23 Interestingly, the homo-condensation of acetylenedicarboxylates with amines and aldehydes were also capable of delivering 1,4-DHPs. Yan et al. discovered the synthesis of fully substituted 1,4-DHPs 25 by directly reacting acetylenedicarboxylates with aromatic amines and aromatic aldehydes in absolute ethanol. When 95% EtOH was employed, 2-hydroxylhydropyridines 26 were obtained diastereoselectively probably by the addition of H2O to 25 (Scheme 12).24
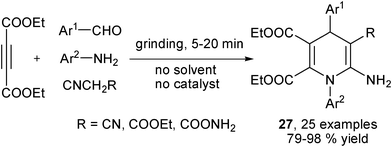
Recently, Kumar et al. discovered that malonitrile or ethyl cyanoacetate was able to efficiently react with diethyl acetylenedicarboxylate, aromatic amines and aromatic aldehydes to provide 1,4-DHPs of type 27 by a solvent- and catalyst-free grinding protocol. This clean synthetic method proceeded also via enamino esters and Knoevenagel condensed intermediates (Scheme 13).25
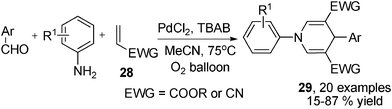
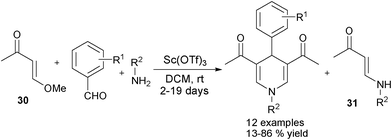
Besides those aforementioned alkynes, alkenes have also been found as applicable substrates in 1,4-DHPs synthesis. In 2011, Jiang et al. reported an interesting multicomponent synthesis of 1,4-DHPs directly employing aldehydes, amines and electron deficient alkenes 28 with palladium(II) catalysis in O2 atmosphere. The 1,4-DHPs of type 29 were given in moderate to good yields. Compared with benzaldehyde derivatives, heteroaromatic aldehydes were not good reaction partners as lower yields of the corresponding products were afforded (Scheme 14).26 Another multicomponent synthesis of 2,6-unsubstituted 1,4-DHPs of type 29 employing electron rich alkenes 30 as precursors of enaminone intermediates 31 was reported recently. In the presence of the Lewis acid Sc(OTf)3, corresponding 1,4-DHPs were afforded in fair to good yields. However, 2–19 days of reaction time and the presence of side products 31 as well as other types of side products in the reactions were the main restrictions of this method (Scheme 15).27
 |
| | Scheme 14 Electron deficient alkene-based synthesis of 1,4-DHPs. | |
 |
| | Scheme 15 Alkoxyl activated alkene initiated synthesis of 1,4-DHPs. | |
As reactants possessing analogous reactivity with 30, the β-ester acetals 32 could also be employed as C-nucleophile sources for 1,4-DHP synthesis by serving as the precursors of β-enamino esters. The in situ generated β-enamino esters from starting acetals and aromatic amines efficiently reacted with aldehydes in the manner of homo-condensation to provide 1,4-DHPs 33 by heating at 90 °C in dioxane in the presence of Lewis acid Yb(OTf)3 (Scheme 16).28
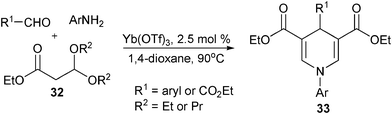 |
| | Scheme 16 β-Ester acetal initiated synthesis of 1,4-DHPs. | |
In addition to the utility of new C-nucleophiles for devising new MCRs assembling 1,4-DHPs, the employment of a new electrophilic species to replace traditional aldehyde electrophiles has also been recently developed as practical tactic. And some well defined electrophilic species such as isatin and analogous 1,2-dicarbonyl compounds have been found particularly interesting in this regard by affording spirocyclic 1,4-DHPs derivatives through MCRs. For example, isatin 34 as well as analogous dicarbonyl substrate 36 have been both successfully utilized to react with cyclic 1,3-dicarbonyl compounds and aromatic primary amines respectively to construct polycyclic 1,4-DHPs 3529 and 3730 which containing both spiro- and fused architecture (Scheme 17). It should be noted that the essence of these reactions is identical as that of the Hantzsch reaction.
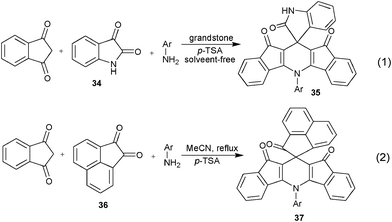 |
| | Scheme 17 Synthesis of spiro-1,4-DHPs with 1,2-dicarbonyl substrates. | |
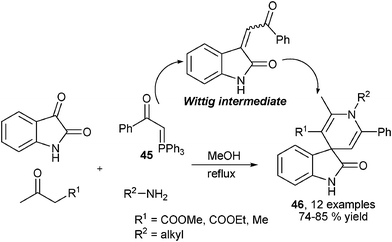
Reasonably, following the consideration on designing new MCRs for the synthesis of structurally diverse 1,4-DHPs employing aldehydes as C-electrophiles, new types of MCRs employing electrophilic isatins had also been developed for 1,4-DHP library synthesis. Through an analogous route described in Scheme 13, Perumal et al. performed MCRs for synthesis of spiro-1,4-DHPs 40 wherein isatin took the place of the aldehyde component in Scheme 13. With the catalysis of triethylamine, key Knoevenagel condensed intermediates 38 and enamino ethers 39 assembled to provide corresponding products in generally good yields (Scheme 18).31 On the other hand, Alizadeh et al. employed diamines 41 and 1,1-bis(methylthio)-2-nitroethylene 42 as precursors of enaminone intermediates 43, which incorporated 38 to provide spiro- and fused 1,4-DHPs 44 under the promotion of piperidine (Scheme 18).32 After their synthesis of 1,4-DHPs 44, Alizadeh et al. later developed another four-component reaction for 1,4-DHP synthesis. The protocol employed Wittig reagent 45 to react with isatin to form an electron deficient Wittig intermediate which was efficiently captured by the enamino ester intermediates in situ generated from ketoesters and amines to give 1,4-DHPs products 46 in good to excellent yields (Scheme 19).33 On the other hand, Sambri and coworkers developed an unconventional multicomponent protocol for the synthesis of 1,4-DHPs 47 from ethyl propiolate, two 1,3-dicarbonyl compounds and primary amines wherein ethyl propiolate served as electrophile (Scheme 20).34
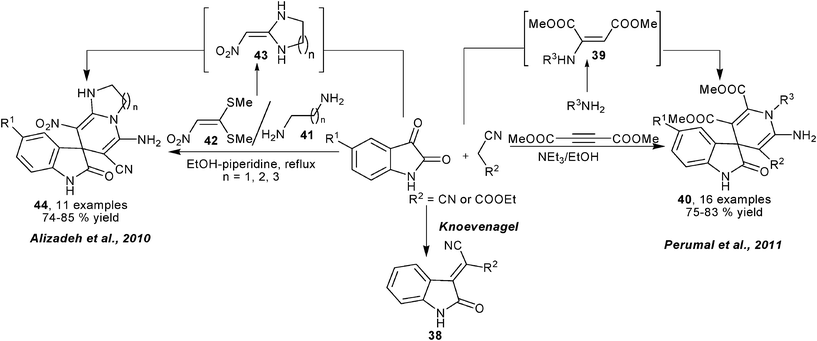 |
| | Scheme 18 Multicomponent synthesis of spiro-1,4-DHPs using isatins as carbonyl electrophiles. | |
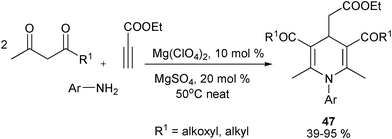 |
| | Scheme 20 Propiolate as electrophile in 1,4-DHPs synthesis. | |
3. Multicomponent synthesis of 1,4-DHPs using ammonia as amino source
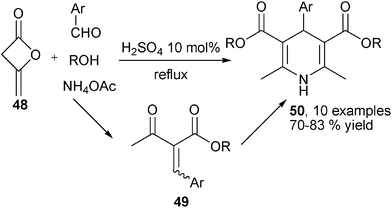
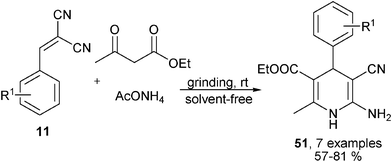
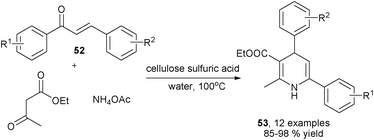
Ammonia was used as the amino source in the earliest version of the Hantzsch 1,4-DHPs synthesis, the reactivity of ammonia was identical as primary amines in most 1,4-DHPs synthesis, the difference was that 1,4-DHPs containing a free NH-group in the DHP ring were the products when ammonia was utilized as the amino source. Similar to the cases of amine-based reactions, novel MCRs have been known for the synthesis of new 1,4-DHPs libraries bearing a free NH group in the central ring by using ammonia as the amino source. For instance, starting from aldehyde, alcohols, diketene 48 and ammonia acetate, an alternative four-component protocol for synthesizing Hantzsch-type 1,4-DHPs 50 in the presence of 10 mol% H2SO4 had been achieved. The reactions proceeded via key intermediates 49 which were generated from 4-methyleneoxetan-2-one 48, aldehydes and alcohols (Scheme 21).35 In another example, the (2-aryl)methylene malononitriles 50 which were prepared from aldehydes and malonitrile have been successfully employed to react with ethyl acetoacetate and ammonia acetate to provide 1,4-DHPs 51 by grinding under solvent-free conditions (Scheme 22).36 Safari et al. developed a new three-component synthesis of NH-free 1,4-DHPs of type 53 using chalcones 52, ethyl acetoacetate and ammonia. Excellent yields of products were obtained in refluxing water with the catalysis of cellulose sulfuric acid (Scheme 23).37
 |
| | Scheme 22 Three-component synthesis of 1,4-DHPs using dienemalononitriles. | |
 |
| | Scheme 23 Chalcone-based three-component 1,4-DHPs synthesis. | |
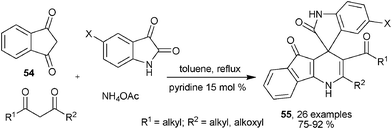
Ammonia acetate has also been used successfully as the reaction partner of cyclic 1,3-diketone 54. Acylic 1,3-dicarbonyl compounds and isatin for multicomponent synthesis of 1,4-DHPs, unsymmetrical sipro- and fused cyclic scaffolds 55 were provided in good to excellent yield by subjecting the four-component reactions to refluxing toluene in the presence of 15 mol% pyridine (Scheme 24).38
 |
| | Scheme 24 Ammonia-based synthesis of fused spiro-1,4-DHPs. | |
4. Multicomponent synthesis of 1,4-DHPs using enaminone, β-enamino esters or enamines as amino source
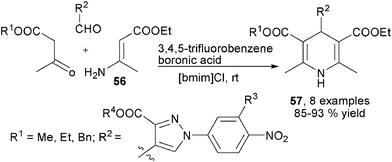
Enaminones, β-enamino esters and enamines are dinucleophilic species which possess broad reactivity through employing both the amino group and β-nucleophilic sp2 carbon. These compounds have been proved particularly useful for the synthesis of various heterocyclic products. As introduced in the above contents, β-enamino esters were key intermediates in many amine-based MCRs synthesis of 1,4-DHPs, it is therefore not surprising that β-enamino esters as well as analogous substrates were also frequently employed as starting materials for 1,4-DHPs synthesis directly. Actually, during chemists' earliest efforts in devising new synthesis of structurally unsymmetrical 1,4-DHPs, β-enamino esters were selected as ideal candidates as starting building blocks. Usually, β-enamino esters are first prepared by subjecting an amine to incorporate a 1,3-dicarbonyl compound. The obtained β-enamino ester was then employed with an aldehyde and a different 1,3-dicarbonyl compound to provide unsymmetrical 1,4-DHPs in the manner of a three-component reaction. This kind of strategy had been applied for the synthesis of pyrazolyl functionalized 1,4-DHPs 57 by Perumal and co-workers. As a result, acyclic 1,3-dicarbonyl compounds, β-enamino ester 56 and pyrazolyl aldehydes were successfully assembled to provide 57 in ionic liquid [bmim]Cl in the presence of 3,4,5-trifluorobenzene boronic acid (Scheme 25).39 Wang et al. conducted a systematic investigation on this kind of synthesis using cyclic diketone based β-enamino esters 58, aromatic aldehydes and a different cyclic diketone. These reactions were found practical in refluxing water or solvent-free heating conditions to yield 1,4-DHPs 59 (Scheme 26).40
 |
| | Scheme 25 Enaminone-based synthesis of pyrazolyl 1,4-DHPs. | |
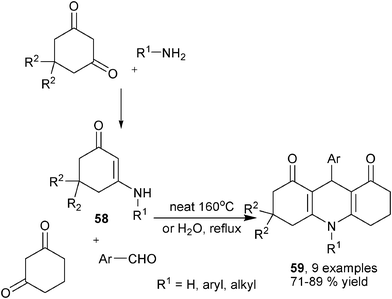 |
| | Scheme 26 Enaminone-based synthesis of fused 1,4-DHPs. | |
An important feature of β-enamino ester/enaminone based MCRs was that they are not only useful in the synthesis of 1,4-DHPs with unsymmetrical structure, but also capable of serving as building blocks in the synthesis of symmetrical 1,4-DHPs. Dondoni et al. synthesized highly functionalized 1,4-DHPs 61 using β-keto esters and β-enamino esters prepared from identical β-keto esters and ammonia to incorporate functionalized aldehyde 60, the products were obtained as enantioenriched compounds owing to the use of functional chiral aldehyde as starting materials (Eq 1, Scheme 27).41 Later on, the same group developed an organocatalyzed three-component reaction of sugar aldehydes 62, enaminones 63 and 1,3-dicarbonyl compounds in the presence of L-proline catalyst, 1,4-DHPs 64 were furnished in excellent diastereoselectivity with the inducement of chiral sugar aldehydes (Eq 2, Scheme 27).42 Mirza-Aghayan et al. developed a three-component synthesis of symmetrical 1,4-DHPs 65 by directly employing two molecules of identical β-enamino esters and aldehydes (Eq 3, Scheme 27).43 In addition, the free NH group functionalized enaminones/β-enamino esters of 66 have also been discovered as practical materials for the synthesis 1,4-DHPs. Li and coworkers successfully performed the assembly of 2 molecules of 66 and aldehydes to provide 2,6-unsubstituted 1,4-DHPs 67 with the catalysis of TsOH·H2O or NaAuCl4·2H2O. Excellent tolerance had been demonstrated with this protocol as R2 and R3 could both be alkyl or aryl groups when incorporating aromatic aldehydes (Eq 4, Scheme 27).44
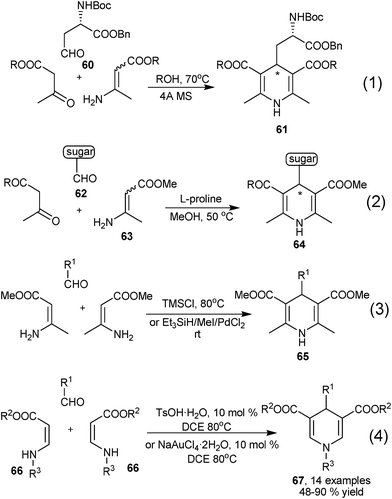 |
| | Scheme 27 Various multicomponent synthesis of symmetrical 1,4-DHPs. | |
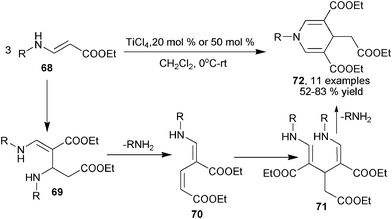
Interestingly, besides serving as dinucleophiles, β-enamino esters could also act as electrophilic acceptors for multicomponent 1,4-DHPs synthesis under proper conditions. Ajavakom et al. discovered that the Lewis acid TiCl4 catalyzes the assembly of three molecules of β-enamino ester 68 to yield symmetrical 1,4-DHPs 72. This three-component process was initiated by a Michael addition between 2 molecules of 66 affording intermediates 69. Upon the elimination of one molecule of amine on 69, intermediates 70 were generated as new Michael acceptors. The addition of one more 69 to 70 led to formation of intermediates 71, and the intramolecular elimination cyclization of 71 provided products 72 (Scheme 28).45
Owing to the excellent reactivity and diverse availability of enaminones, many diversity oriented, enaminone-based synthesis of 1,4-DHPs have been reported by using either new 1,3-dicarbonyl substrates or enaminones/β-enamino esters. For example, enaminone 73 has been found as an ideal reaction partner of isatin and cyclic 1,3-diketones to yield spiro-fused 1,4-DHPs 74 in refluxing water with the catalysis of Brønsted acid p-TSA (Eq 1 in Scheme 29),46 while enaminone 75 with the coumarin backbone has been reported as practical precursor for the synthesis of highly functionalized 1,4-DHPs 76 employing (±) lactic acid and ethyl lactate as catalyst and green solvent, respectively (Eq 2 in Scheme 29).47 Recently, the easily available 6-amino-1,3-dimethyl uracil 77, which was also formally an enaminone building block, has been demonstrated as a useful starting material in the three-component synthesis of 1,4-DHPs 78 by incorporating aromatic aldehydes and β-ketoesters in water (50 °C) in the presence of thiourea dioxide (Eq 3 in Scheme 29).48
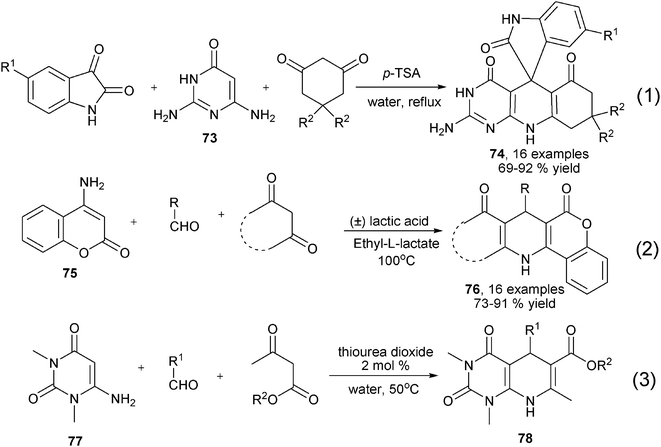 |
| | Scheme 29 Various synthesis of spiro- or fused 1,4-DHPs with different enaminones. | |
In the process of exploring new MCRs for heterocyclic scaffold synthesis, Li et al. established the synthesis of fused multicyclic 1,4-DHPs 81 from β-ketoester, aldehydes and ketene aminal compounds (cyclic enaminones) 79. It was the 1,4-DHPs 80 that were first generated from a triethyl amine promoted three-component assembly, as intermediate products, 80 underwent efficient intramolecular coupling cyclization with K2CO3 catalysis to produce the final products. On the other hand, fused pyridine derivatives 83 were yielded when Meldrum's acid 82 was used as the dicarbonyl compound for the reaction under standard reaction conditions (Scheme 30).49
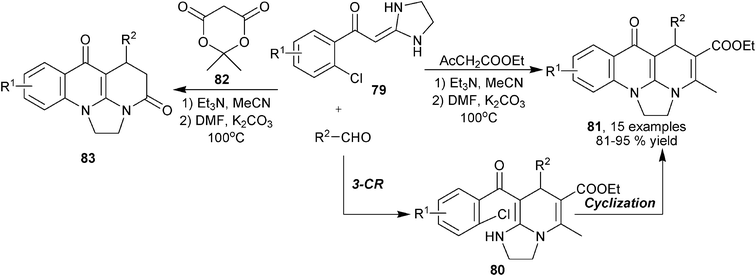 |
| | Scheme 30 1,4-DHPs-based polycycles synthesis via enaminone-based three-component reaction. | |
Similar to the cases of amine-based multicomponent synthesis of 1,4-DHPs, substituted acetonitriles and enals were also frequently employed reaction partners in enaminone/β-enamino ester-based multicomponent synthesis of 1,4-DHPs, particularly for the synthesis of unsymmetrical 1,4-DHPs. For example, starting from enaminones 79, aldehydes and malonitrile, Li et al. attained 1,4-DHPs 84 in refluxing MeCN in the presence of triethylamine, and further treatment with K2CO3 in DMF on 84 led to the production of 85 (Scheme 31).50 On the other hand, interesting chemo-selective formation of 1,4-DHPs were discovered in the three-component reactions of enaminones 86, aldehydes and β-cyanoketones, wherein the selective condensation of the carbonyl instead of cyano group in the β-cyanoketones allowed the production of 3-cyano functionalized 1,4-DHPs 87,51 and a similar transformation was also applied for the synthesis of indolyl substituted 1,4-DHPs 88 by using corresponding indolyl functionalized cyanoketones (Scheme 31).52 More recently, malonitrile and ethyl 2-cyanoacetates were also successfully subjected to incorporate aldehydes and nitro-functionalized cyclic enaminones 89 for 1,4-DHP synthesis. Unlike the reaction process involved in the MCRs using β-cyanoketones, 2-amino functionalized fused 1,4-DHPs 90 were afforded in the reactions via a nucleophilic addition transformation on the cyano group of the β-cyanoester (Scheme 31).53 In the area of multicomponent synthesis of 1,4-DHPs employing enaminone as amino source, Pan and coworkers developed the three-component regioselective synthesis of 1,4-DHPs of type 92 and isomeric 1,2-DHPs 93 using N,N′-dimethyl amino functionalized enaminones 91, primary amines and enals. The designed systematic experiments using primary amines of different electronic and steric properties suggested that a strong electron withdrawing group or bulky ortho-group tend to induce the formation of 1,2-DHPs 93, combined with the observation of compounds 94 in some entries, it was proposed that 94 were the key intermediates of the reaction. The normal transformation of the N-nucleophilic condensation with the formyl group in the enal component led to the production of 1,4-DHPs 92, when a strong electron withdrawing or bulky ortho-group was included in the amine component, the nucleophilicity of the amino group in 94 was significantly hindered, which allowed the nucleophilic carbon atom in 94 to attack the enal formyl first and led to the regioselective formation of 1,2-DHPs 93. This work was the first example on the tunable selective synthesis of 1,4-DHPs and 1,2-DHPs with three-component reactions (Scheme 32).54
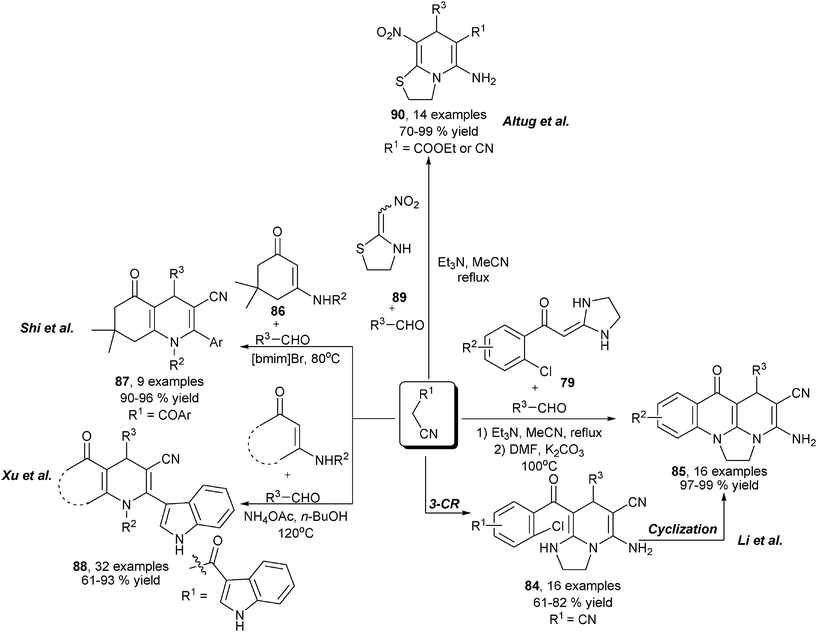 |
| | Scheme 31 Various synthesis of 1,4-DHPs-based heterocycle synthesis with different enaminones. | |
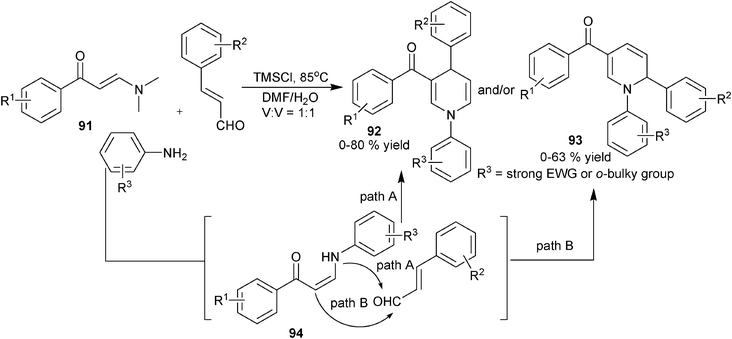 |
| | Scheme 32 Enaminone initiated regioselective synthesis of 1,4- and 1,2-DHPs. | |
Although not frequently available, simple enamines have also been reported to be capable of serving as dinucleophilic building blocks for multicomponent synthesis of 1,4-DHPs by acting similarly as enaminones and β-enamino esters. Quiroga et al. reported the synthesis of elaborated polycyclic products 96 with heteroaryl amines 95, isatin and various 1,3-dicarbonyl substrates. Although 96 are formally fused aromatic heterocycles, the central 1,4-DHP backbone was formed during the transformation via cross-condensed intermediates 97 (Scheme 33).55 Rather recently, a similar three-component transformation using enamine 98, cyclic diketones and aromatic aldehydes have been reported to provide multicyclic elaborated 1,4-DHPs 99 (Scheme 34).56
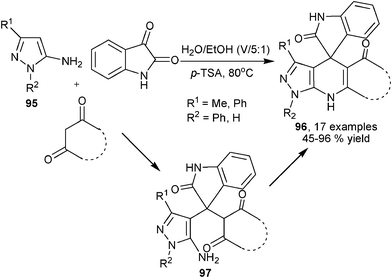 |
| | Scheme 33 Cyclic enamine-based three-component synthesis of highly functionalized 1,4-DHPs. | |
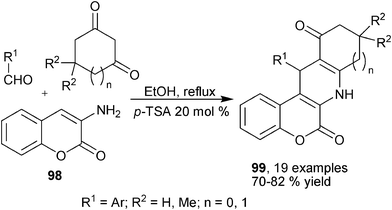 |
| | Scheme 34 Multicyclic 1,4-DHPs synthesis using cyclic enamines. | |
5. Multicomponent synthesis of 1,4-DHPs using other amino sources
According to related results in the known literature, amines, ammonia and enaminone analogs were obviously the most frequently and conventional amino sources used for the synthesis of 1,4-DHPs, however, some other types of N-containing species such as cyanides, imines etc. were also able to serve as the amino sources for the synthesis of novel 1,4-DHPs via MCRs as occasional cases. Depending on the properties of these amino resources, both 1,4-DHPs with novel elaboration and 1,4-DHPs with similar structure as those equivalents obtained from conventional amino resources were possible.
5.1. Using cyanide as amino source
As reactive starting materials, cyanide derivatives such as malonitrile were frequently utilized in the synthesis of 1,4-DHPs in amine, ammonia or enaminone participated MCRs. In these kinds of reactions, as introduced above, it was amine, enamione or ammonia that served as the amino source to construct the heterocyclic backbone while cyano groups were mainly involved as the donor of electrophilic carbons in those MCRs. Interestingly, in some reactions, it was also possible to directly employ the N atom in the cyano group as the amino source of 1,4-DHPs. Fustero and coworkers developed the three-component protocol involving fluorinated nitriles (cyanides) 100, alkyl propiolates and chiral allyl p-tolyl sulfoxide 101 for the synthesis of 1,4-DHPs 104. The reactions proceeded under the promotion of KN(SiMe3)2via two steps of cascade transformations, the first nucleophilic addition of 101 to 100 under catalysis of base gave intermediates 102, the second step addition of 102 to propiolates led to the formation of intermediates 103, and intramolecular Michael addition on 103 provided enantioenriched 1,4-DHPs 104 in generally excellent diastereoselectivity, which was induced by the chiral p-tolyl sulfoxide (Scheme 35).57
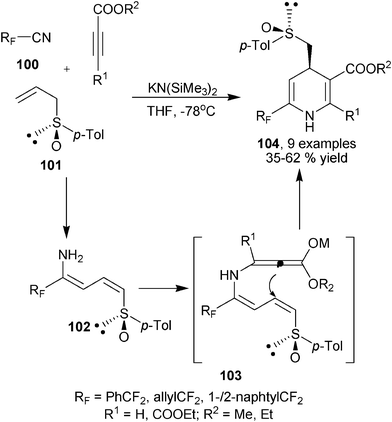 |
| | Scheme 35 Diastereoselective synthesis of 1,4-DHPs using cyanide amino source. | |
5.2. Using imine as amino source
Imines were usually the resulted products from the condensation of amines and aldehydes. Direct employment of aldehydes, amines and propiolates have been a well established protocol for the synthesis of 1,4-DHPs. Interestingly, Fukuzawa et al. developed a three-component reaction of imines 105 and two molecules of propiolates to provide 1,4-DHPs of type 9 (see also Scheme 7) in the presence of 10 mol% Lewis acid catalyst Sc(OTf)3. In these reactions, one key step was the decomposition of imines 105 to aldehydes and amines. Only low to moderate yields of products were obtained by this methodology with imines prepared from the condensation of aromatic aldehydes with aromatic/aliphatic amines (Scheme 36).58
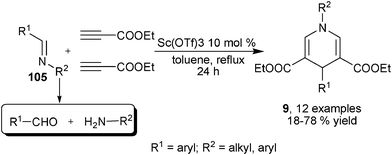 |
| | Scheme 36 Imine in three-component synthesis of 1,4-DHPs. | |
5.3. Using thioamide as amino source
In devising the MCRs synthesis of structurally diverse 1,4-DHPs employing new C-nucleophilic building blocks, β-arylthioamides 106 have been found as useful starting materials in the synthesis of 1,4-DHPs 108 containing a thioester functional group. During the reaction process, 106 tautomerized as 107 to incorporate aldehydes in the presence of electron withdrawing group functionalized acetonitriles and alkyl halide. Microwave irradiation had been found to give superior results over traditional heating conditions to this Et3N-catalyzed 1,4-DHPs synthetic method (Scheme 37).59 On the basis of this transformation, a further devised cascade three-component protocol for the synthesis of fused heterocyclic 1,4-DHPs 112 was reported by employing 2-chlorophenyl functionalized thioamides 109, aldehydes and malonitrile. In the presence of KF/neutral Al2O3 catalysts with PEG6000 and microwave irradiation, 112 were efficiently provided through the intramolecular cyclization of intermediates 110/111 (Scheme 38).60
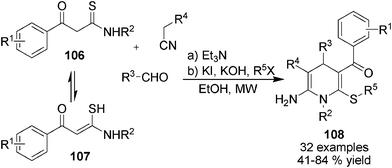 |
| | Scheme 37 Three-component synthesis of thioester functionalized 1,4-DHPs. | |
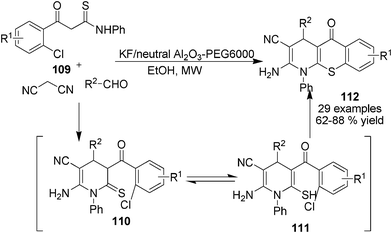 |
| | Scheme 38 Cascade three-component synthesis of sulphur-containing heterocycle fused 1,4-DHPs. | |
6. Conclusions
As a class of typical heterocyclic scaffolds with broad spectrum of biological and pharmaceutical profiles, 1,4-DHPs have been regarded as significant targets of organic synthesis. Following the daily increasing requirement on molecular libraries during the discovering process of new lead compounds and drugs, urgent promotion was simultaneously brought to the chemistry of 1,4-DHPs synthesis. Particularly, the synthesis of 1,4-DHPs possessing novel and diverse elaboration consists of the main effort in this field. With those various types of newly designed MCRs, it is now possible to access highly functionalized 1,4-DHPs with enormously expanded diversity on the basis of the classical Hantzsch multicomponent synthesis. The breakthrough in the field should be ascribed to those highly diversified and reactive starting materials including amine resources, various C-electrophiles such as aldehydes and isatins, bifunctional building blocks such as enals, enones, 1,3-dicarbonyl compounds as well as different alkynes/alkenes etc. In this regard, devising more new MCRs for the synthesis of structurally novel 1,4-DHPs relies on further endeavours in exploring more unconventional building blocks.
In addition, in terms of sustainable synthesis, to develop 1,4-DHPs synthesis with MCRs under milder, greener conditions is also highly desirable in this field. More importantly, as most new MCRs for 1,4-DHPs assembly provide products containing chiral centers, methods of directly accessing enantiomerically pure 1,4-DHPs with MCRs is in urgent requirement. Establishment of efficient methodologies providing chiral 1,4-DHPs is highly expectable to significantly enhance the progress of discovering more medicinally valuable heterocyclic compounds.
Acknowledgements
The authors gratefully acknowledge the financial support from the NSFC of China (21102059), the NSF of Jiangxi Province (20114BAB213005) and a Sponsored Program for Cultivating Youths of Outstanding Ability from Jiangxi Normal University.
References
-
(a) S. L. Schreiber, Science, 2000, 287, 1964–1969 CrossRef CAS;
(b) D. S. Tan, Nat. Chem. Biol., 2005, 1, 74–84 CrossRef CAS;
(c) T. E. Nielsen and S. L. Schreiber, Angew. Chem., Int. Ed., 2008, 47, 48–56 CrossRef CAS;
(d) S. Shang and D. S. Tan, Curr. Opin. Chem. Biol., 2005, 9, 248–258 CrossRef CAS;
(e) G. L. Thomas, E. E. Wyatt and D. R. Spring, Curr. Opin. Drug Discov. Dev., 2006, 9, 700–712 CAS.
-
(a) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef;
(b) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef;
(c) V. Nair, C. Rajesh, A. U. Vinod, S. Bindu, A. R. Sreekanth, J. S. Mathen and L. Balagopal, Acc. Chem. Res., 2003, 36, 899–907 CrossRef CAS;
(d) J.-P. Wan and Y. Liu, Curr. Org. Chem., 2011, 15, 2758–2773 CrossRef CAS.
- A. Hantzsch, Justus Liebigs Ann. Chem., 1882, 215, 1–82 CrossRef.
- K. K. Borowicz, M. Gasior, Z. Kleinrok and S. J. Czuczwar, Eur. J. Pharmacol., 1997, 323, 45–51 CrossRef CAS.
- A. H. Li, S. Moro, N. Forsyth, N. Melman, X.-D. Ji and K. A. Jacobson, J. Med. Chem., 1999, 42, 706–721 CrossRef CAS.
- I. O. Donkor, X. Zhou, J. Schmidt, K. C. Agrawal and V. Kishore, Bioorg. Med. Chem., 1998, 6, 563–568 CrossRef CAS.
- A. Mai, S. Valente, S. Meade, V. Carafa, M. Tardugno, A. Nebbioso, A. Galmozzi, N. Mitro, E. D. Fabiani, L. Altucci and A. Kazantsev, J. Med. Chem., 2009, 52, 5496–5504 CrossRef CAS.
-
(a) For known reviews on 1,4-dihydropyridine chemistry, see: U. Eisner and J. Kuthan, Chem. Rev., 1972, 72, 1–42 CrossRef CAS;
(b) D. M. Stout and A. I. Meyers, Chem. Rev., 1982, 82, 223–243 CrossRef CAS;
(c) A. Sausins and G. Duburs, Heterocycles, 1988, 27, 269–289 CrossRef CAS.
-
(a) For some recent reviews wherein the Hantzsch synthesis of 1,4-DHPs was mentioned, see: C. de Graaff, E. Ruijter and R. V. A. Orru, Chem. Soc. Rev., 2012, 41, 3969–4009 RSC;
(b) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083–3135 CrossRef;
(c) A. Moyano and R. Rios, Chem. Rev., 2011, 111, 4703–4832 CrossRef CAS;
(d) A. Dondoni and A. Massi, Acc. Chem. Res., 2006, 39, 451–463 CrossRef CAS;
(e) N. Isambert, M. M. Sanchez Duque, J.-C. Plaquevent, Y. Génisson, J. Rodriguez and T. Constantieux, Chem. Soc. Rev., 2011, 40, 1347–1357 RSC;
(f) D. Bonne, Y. Coquerel, T. Constantieux and J. Rodriguez, Tetrahedron: Asymmetry, 2010, 21, 1085–1109 CrossRef CAS;
(g) J. Yu, F. Shi and L.-Z. Gong, Acc. Chem. Res., 2011, 44, 1156–1171 CrossRef CAS;
(h) H. Pellissier, Adv. Synth. Catal., 2012, 354, 237–294 CrossRef CAS.
- J.-P. Wan and Y. Liu, Synthesis, 2010, 3943–3953 CrossRef CAS.
- R. K.Vohra, C. Bruneau and J.-L. Renaud, Adv. Synth. Catal., 2006, 348, 2571–2574 CrossRef.
- V. Sridharan, P. T. Perumal, C. Avendaño and J. C. Menéndez, Tetrahedron, 2007, 63, 4407–4413 CrossRef CAS.
- A. Kumar and R. A. Maurya, Tetrahedron, 2008, 64, 3477–3482 CrossRef CAS.
- M. L. Kantam, T. Tamani, L. Chakrapani and B. M. Choudary, Catal. Commun., 2009, 10, 370–372 CrossRef CAS.
- D. Ramesh, S. Rajaram, M. Narasimhulu, T. S. Reddy, K. C. Mahesh, G. Manasa and Y. Venkateswarlu, Chin. J. Chem., 2011, 29, 2471–2475 CrossRef CAS.
- S. Maiti, V. Sridharan and J. C. Menéndez, J. Comb. Chem., 2010, 12, 713–722 CrossRef CAS.
- J. Jiang, J. Yu, X.-X. Sun, Q.-Q. Rao and L.-Z. Gong, Angew. Chem., Int. Ed., 2008, 47, 2458–2462 CrossRef CAS.
- C. G. Evans and J. E. Gestwicki, Org. Lett., 2009, 11, 2957–2959 CrossRef CAS.
- S.-L. Cui, J. Wang, X.-F. Lin and Y.-G. Wang, J. Org. Chem., 2007, 72, 7779–7782 CrossRef CAS.
- J. Sun, Y. Sun, E.-Y. Xia and C.-G. Yan, ACS Comb. Sci., 2011, 13, 436–441 CrossRef CAS.
- H. Jiang, R. Mai, H. Cao, Q. Zhu and X. Liu, Org. Biomol. Chem., 2009, 7, 4943–4953 CAS.
- J. Sun, Y. Sun, H. Gao and C.-G. Yan, Eur. J. Org. Chem., 2011, 6952–6956 CrossRef CAS.
- A. T. Khan and M. M. Khan, Tetrahedron Lett., 2011, 52, 3455–3459 CrossRef CAS.
- J. Sun, Q. Wu, E.-Y. Xia and C.-G. Yan, Eur. J. Org. Chem., 2011, 2981–2986 CrossRef CAS.
- A. Kumar and S. Sharma, Green Chem., 2011, 13, 2017–2020 RSC.
- H. Jiang, X. Ji, Y. Li, Z. Chen and A. Wang, Org. Biomol. Chem., 2011, 9, 5358–5361 CAS.
- P. R. Girling, A. S. Batsanov, H. C. Shen and A. Whiting, Chem. Commun., 2012, 48, 4893–4895 RSC.
- S. Sueki, R. Takei, J. Abe and I. Shimizu, Tetrahedron Lett., 2011, 52, 4473–4477 CrossRef CAS.
- R. Ghahremanzadeh, G. I. Shakibaei, S. Ahadi and A. Bazgir, J. Comb. Chem., 2010, 12, 191–194 CrossRef CAS.
- R. Ghahremanzadeh, S. Ahadi, G. I. Shakibaei and A. Bazgir, Tetrahedron Lett., 2010, 51, 499–502 CrossRef CAS.
- S. E. Kiruthika, N. V. Lakshmi, B. R. Banu and P. T. Perumal, Tetrahedron Lett., 2011, 52, 6508–6511 CrossRef CAS.
- A. Alizadeh, T. Firuzyar and A. Mikaeili, Synthesis, 2010, 3913–3917 CrossRef CAS.
- A. Alizadeh and J. Mokhtari, Tetrahedron, 2011, 67, 3519–3523 CrossRef CAS.
- G. Bartoli, M. Bosco, P. Galzerano, R. Giri, A. Mazzanti, P. Melchiorre and L. Sambri, Eur. J. Org. Chem., 2008, 3970–3975 CrossRef CAS.
- A. Alizadeh and S. Rostamnia, Synthesis, 2010, 4057–4060 CrossRef CAS.
- A. M. Zonouz and D. Moghani, Synth. Commun., 2011, 41, 2152–2160 CrossRef CAS.
- J. Safari, S. H. Banitaba and S. D. Khalili, J. Mol. Catal. A: Chem., 2011, 335, 46–50 CrossRef CAS.
- A. Feiz, G. I. Shakibaei, Z. Yasaei, H. R. Khavasi and A. Bazgir, Helv. Chim. Acta, 2011, 94, 1628–1637 CrossRef CAS.
- R. Sridhar and P. T. Perumal, Tetrahedron, 2005, 61, 2465–2470 CrossRef CAS.
- G.-W. Wang and C.-B. Miao, Green Chem., 2006, 8, 1080–1085 RSC.
- A. Dondoni, A. Massi, E. Minghini and V. Bertolasi, Tetrahedron, 2004, 60, 2311–2326 CrossRef CAS.
- D. R. B. Ducatti, A. Massi, M. D. Noseda, M. E. R. Duarte and A. Dondoni, Org. Biomol. Chem., 2009, 7, 1980–1986 CAS.
- M. Mirza-Aghayan, M. K. Langrodi, M. Rahimifard and R. Boukherroub, Appl. Organomet. Chem., 2009, 23, 267–271 CrossRef CAS.
- J. Yang, C. Wang, X. Xie, H. Li and Y. Li, Eur. J. Org. Chem., 2010, 4189–4193 CrossRef CAS.
- T. Sirijindalert, K. Hansuthirakul, P. Rashatasakhon, M. Sukwattanasinitt and A. Ajavakom, Tetrahedron, 2010, 66, 5161–5167 CrossRef CAS.
- K. Jadidi, R. Ghahremanzadeh, P. Mirzaei and A. Bazgir, J. Heterocycl. Chem., 2011, 48, 1014–1018 CrossRef CAS.
- S. Paul and A. R. Das, Tetrahedron Lett., 2012, 53, 2206–2210 CrossRef CAS.
- S. Verma and S. L. Jain, Tetrahedron Lett., 2012, 53, 2595–2600 CrossRef CAS.
- L.-R. Wen, C. Liu, M. Li and L.-J. Wang, J. Org. Chem., 2010, 75, 7605–7614 CrossRef CAS.
- L.-R. Wen, C.-Y. Jiang, M. Li and L.-J. Wang, Tetrahedron, 2011, 67, 293–302 CrossRef CAS.
- J.-X. Wang, G.-L. Shen and D.-Q. Shi, J. Heterocycl. Chem., 2011, 48, 1145–1148 CrossRef CAS.
- T. Chen, X.-P. Xu, H.-F. Liu and S.-J. Ji, Tetrahedron, 2011, 67, 5469–5476 CrossRef CAS.
- C. Altug, A. K. Burnett, E. Caner, Y. Dürüst, M. C. Elliott, R. P. J. Glanville, C. Guy and A. D. Westwell, Tetrahedron, 2011, 67, 9522–9528 CrossRef CAS.
- J.-P. Wan, S.-F. Gan, G.-L. Sun and Y.-J. Pan, J. Org. Chem., 2009, 74, 2862–2865 CrossRef CAS.
- J. Quiroga, S. Portillo, A. Péres, J. Gálves, R. Abonia and B. Insuasty, Tetrahedron Lett., 2011, 52, 2664–2666 CrossRef CAS.
- A. T. Khan and D. K. Das, Tetrahedron Lett., 2012, 53, 2345–2351 CrossRef CAS.
- S. Fustero, S. Catalán, M. Sánchez-Roselló, A. Simón-Fuentes and C. del Pozo, Org. Lett., 2010, 12, 3484–3487 CrossRef CAS.
- S. Kikuchi, M. Iwai, H. Murayama and S.-I. Fukuzawa, Tetrahedron Lett., 2008, 49, 114–116 CrossRef CAS.
- M. Li, Z. Zuo, L. Wen and S. Wang, J. Comb. Chem., 2008, 10, 436–441 CrossRef CAS.
- L. Wen, C. Ji, Y. Li and M. Li, J. Comb. Chem., 2009, 11, 799–805 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here.