Hollow porous molecularly imprinted polymer nanosphere for fast and efficient recognition of bisphenol A†
Xue-Bin
Zhang
,
Jin
Li
,
Bo
You
,
Guo-Ping
Yong
,
Hong-Wu
Tong
and
Shao-Min
Liu
*
Department of Chemistry, University of Science and Technology of China, Hefei 230026, P. R. China. E-mail: liusm@ustc.edu.cn; Fax: +86-0551-3492065; Tel: +86-0551-3492147
First published on 28th August 2012
Abstract
Hollow porous molecularly imprinted polymer (HPMIP) nanospheres were successfully synthesized by an in situ polymerization technique using mesoporous silica as a sacrificial support, and were used for the recognition of bisphenol A (BPA). The HPMIPs thus obtained exhibit a faster adsorption rate and a higher affinity compared with other BPA molecularly imprinted polymers.
Although bisphenol A (BPA) is widely used in the plastics industry as a monomer for the production of polycarbonate and epoxy resin, it is a typical endocrine-disrupting chemical that can fully or partially mimic the natural hormones in the body, increase the likelihood of cancer, reduce immune functions, and impair reproduction.1 Therefore, there has been rising international concern over the monitoring of BPA released into our environment. However, as BPA has a low concentration and exists in a complex matrix, its analysis often requires extensive sample preparation.2 For effective separation and enrichment of BPA, various molecularly imprinted polymers (MIPs) with good binding affinity, stability, and specificity towards target molecules have been widely investigated.3 Although numerous studies have been conducted for the development of an effective molecular imprinting technology, the main challenges to their successful and widespread use include heterogeneous binding sites, template leakage, low binding capacity, and slow mass transfer of MIPs.4 To overcome the above-mentioned problems, methods such as surface molecular imprinting, porous molecular imprinting, and hollow molecular imprinting strategies have been developed.5 MIPs prepared using the above methods have a high density of effective recognition sites on their surface, which facilitates the transport of the template. This property of MIPs contributes to their high adsorption capacity and fast kinetics to uptake target molecules. Mostly organic-functionalized SiO2 spheres or polymer microspheres are used as hard templates for the synthesis of MIPs. Mesoporous materials that possess high surface areas and a narrow pore size distribution6 have attracted the attention of many researchers. Unfortunately, the use of mesoporous silica spheres as a sacrificial support in the synthesis of hollow porous molecularly imprinted polymers (HPMIPs) has rarely been reported, particularly for the recognition of BPA.
In this work, we synthesized, using mesoporous silica spheres as a sacrificial support, an HPMIP that possesses a high adsorption affinity for BPA, (see Scheme 1). Firstly, the imprint precursor mixture was perfused to the inside of MCM-48 spheres by ultrasonic impregnation, and then polymerized in the internal surfaces of the MCM-48 spheres to form MCM-48–MIP composites. Finally, HPMIP was obtained by removing SiO2 and imprinted molecules with ethanol solution containing HF. The above strategy showed at least three advantages: (1) the prepared HPMIP basically retained the particle size, shape, and pore structure of the mesoporous silica hard template; (2) the formation of a high density of recognition sites on the surface helped improve the bonding capacity and bonding rate; (3) matrix MCM-48 and imprinted molecules could be effectively removed by treating with a HF/ethanol solution.
 | ||
| Scheme 1 Fabrication of HPMIP. | ||
The MCM-48 spheres were prepared according to the literature method with some modifications.7 As shown in Fig. 1a and 1b, mesoporous silica nanospheres have an average diameter of 350 nm, and show smooth and good dispersion. The high-resolution transmission electron microscope (HRTEM) and X-ray diffraction (XRD) patterns (Fig. S1†) confirmed the synthesized mesoporous silica nanospheres to have the typical structure of MCM-48.7 During the preparation of MCM-48–MIP composites, the amount of toluene (as solvent) played a crucial role. On decreasing the solvent dosage from 12 to 6 mL, a weak diffraction peak of the prepared HPMIP appeared at a 2θ value of about 2°, and the 2θ of (211) MCM-48 was 2.3° (Fig. S2†). These findings suggested that HPMIP copied the pore structure of hard template MCM-48 to some extent.8 On decreasing the solvent dosage further, only partially dipped MCM-48 was found, so 6 mL solvent was chosen for the following experiment. As shown in Fig. 1c and 1d, if the imprint precursor was 0.6 times the pore volume of MCM-48 (0.98 cm3 g−1), the synthesized MIP showed a hollow structure with a 60 nm thick shell. By increasing the dosage of imprint precursor from 0.8 to 1.0 times the pore volume of MCM-48, the thickness of the shell of HPMIPs increased from 80 to 110 nm (Fig. S3†). Meanwhile, the inner diameter of HPMIPs showed a decreasing trend with an increasing amount of imprint precursor, which indicated that the imprint precursor polymerized from the outside to the inside of the MCM-48 sphere. The spherical MCM-48 has a cubic mesoporous structure. Hence, when it is mixed with an imprint precursor, the imprint precursor will permeate into the MCM-48 sphere from outside to the inside by ultrasonic dipping.
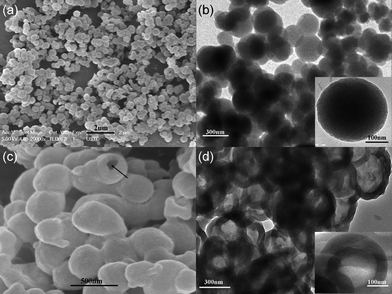 | ||
| Fig. 1 (a, b) SEM and TEM images of the MCM-48 spheres. (c, d) SEM and TEM images of the synthesized HPMIPs with the imprint precursor being equal to 0.6 times the pore volume of MCM-48. | ||
Nitrogen adsorption was used to measure the porosity of the obtained HPMIP at −196 °C. The isotherm in Fig. 2a exhibits the characteristics of an IV curve.9 The average pore size was 2.8 nm (Fig. 2b), corresponding to the wall thickness of MCM-48. This also indicated that the prepared HPMIP copied the template structure of MCM-48 (Fig. S4†). As estimated by the standard BET method, the specific surface areas of the obtained HPMIPs with different thicknesses of 60, 80 and 110 nm were 607, 536 and 423 m2 g−1, respectively.
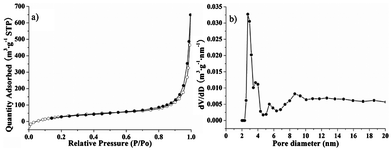 | ||
| Fig. 2 (a) N2 sorption isotherms and (b) pore size distribution curves of HPMIP. | ||
Usually, a mixture of organic solvents (methanol and acetic acid mixed in the ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) is applied to remove the imprint molecule;3 however, this process is time consuming and also shows lower extraction efficiency. Aqueous solution of HF is also widely used to etch the silica matrix.10 Unfortunately, it could not be used for the effective removal of the template (Fig. S5†), because of the lower solubility of BPA in water. On the other hand, BPA is highly soluble in ethanol—a non-toxic, low-cost solvent. Hence, ethanol was mixed with HF for etching the silica matrix and removing the imprint molecule. Interestingly, the silica matrix and the imprint molecule were simultaneously removed by the HF–ethanol solution (Fig. S5†). As is known, HF is a stronger acid than acetic acid; hence, when combined with ethanol it can effectively break the hydrogen bonds between BPA and 4-VP (4-vinyl pyridine), and extract BPA from the as-synthesized MCM-48–MIP composites. As indicated by the FT-IR spectrum of HPMIP (Fig. S6†), the disappearance of the SiO2 bands characteristic of stretching vibrations at ∼1080, ∼1631, and 960 cm−1, and their appearance at ∼1731, ∼1640, and 1158 cm−1 demonstrated that the silica matrix MCM-48 was removed and the MIP was formed.5g Thermogravimetric analysis (TGA) revealed an obvious weight loss for HPMIP in the temperature range 300–400 °C, and 95% of its quality was lost at 600 °C. This finding also confirmed the removal of the matrix MCM-48 (Fig. S7†).
1, v/v) is applied to remove the imprint molecule;3 however, this process is time consuming and also shows lower extraction efficiency. Aqueous solution of HF is also widely used to etch the silica matrix.10 Unfortunately, it could not be used for the effective removal of the template (Fig. S5†), because of the lower solubility of BPA in water. On the other hand, BPA is highly soluble in ethanol—a non-toxic, low-cost solvent. Hence, ethanol was mixed with HF for etching the silica matrix and removing the imprint molecule. Interestingly, the silica matrix and the imprint molecule were simultaneously removed by the HF–ethanol solution (Fig. S5†). As is known, HF is a stronger acid than acetic acid; hence, when combined with ethanol it can effectively break the hydrogen bonds between BPA and 4-VP (4-vinyl pyridine), and extract BPA from the as-synthesized MCM-48–MIP composites. As indicated by the FT-IR spectrum of HPMIP (Fig. S6†), the disappearance of the SiO2 bands characteristic of stretching vibrations at ∼1080, ∼1631, and 960 cm−1, and their appearance at ∼1731, ∼1640, and 1158 cm−1 demonstrated that the silica matrix MCM-48 was removed and the MIP was formed.5g Thermogravimetric analysis (TGA) revealed an obvious weight loss for HPMIP in the temperature range 300–400 °C, and 95% of its quality was lost at 600 °C. This finding also confirmed the removal of the matrix MCM-48 (Fig. S7†).
The specific binding performances of HPMIP materials with different shell thicknesses for imprint molecules were evaluated. Adsorption kinetics of HPMIPs for BPA showed that the binding equilibrium was obtained within 30 min for HPMIP-0.6, 40 min for HPMIP-0.8, and 60 min for HPMIP-1.0 (Fig. 3a). Fig. 3b shows the adsorption isotherms for HPMIPs and hollow porous non-imprinted polymers (HPNIPs) at various concentrations of BPA solutions ranging from 0.1 to 5 mmol L−1. It can easily be seen that HPMIP-0.6 exhibits a stronger adsorption towards the target BPA, with the adsorption capacity (up to 670 μmol g−1) being about twice that of HPNIP-0.6 (up to 350 μmol g−1). It has also been found that HPMIP-0.8 and HPMIP-1.0 exhibit a slightly lower adsorption capacity towards the target BPA than HPMIP-0.6. A comparison of the adsorption capacity and kinetics between HPMIP and BPA–MIPs synthesized by other methods is given in Table 1. It is, therefore, clear that the prepared HPMIP shows a higher adsorption capacity and faster adsorption rate than other kinds of BPA–MIPs. This might be because the presence of binding sites close to the surface, a high surface area, and better steric matching with its imprint molecules help HPMIP achieve a faster template loading rate and adsorb more imprint molecules.5 To find out its further applicability, the selective adsorption of BPA by HPMIP-0.6 and HPNIP-0.6, in the mixture of BPA, 4,4-dihydroxybiphenyl (BIP), phenol, o-phenylphenol (OPP), and p-tert-butylphenol (PTBP), was determined. As can be seen from Fig. S8†, HPMIP-0.6 has a much higher binding capacity for BPA than for BIP, phenol, OPP, and PTBP. However, even though HPNIP-0.6 has a lower binding capacity for BPA than HPMIP-0.6, the former exhibits a slightly higher binding capacity for BIP, phenol, OPP, and PTBP than the latter. The relative selectivity coefficient (k′) values of HPMIP-0.6 for BIP, phenol, OPP, and PTBP were calculated to be 1.31, 2.09, 2.14 and 2.33, respectively. These results proved the existence of strong interactions between the template and the functional monomer that favoured the formation of high-affinity binding sites and improved the selectivity of the polymers. Also, the obtained HPMIP supplied an attractive sorbent for selective pre-concentration of BPA.
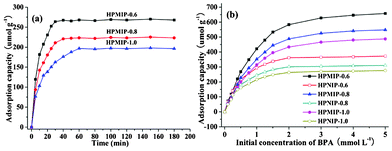 | ||
| Fig. 3 (a) Binding kinetics of HPMIPs for BPA (0.5 mmol L−1), (b) binding isotherms of HPMIPs and HPNIPs for BPA at concentrations in the range of 0.1–5 mmol L−1. | ||
| BPA–MIP synthesis strategy | BPA–MIP shape | Hard template | Molecular template | Bonding capacity (μmol g−1) | Bonding rate (min) | Ref. |
|---|---|---|---|---|---|---|
| a Not detected. b The experiments for 200 s and 1000 min are performed by US bath incubation and contact incubation, respectively. c TBBPA = 3,3,5,5-tetrabromobisphenol A, an analogue of BPA. | ||||||
| Bulk imprinting | Irregular | nda | BPA | 3.6 | nda | 11 |
| Sphere | nda | BPA | 26.9 | (200 s/1800 min)b | 12 | |
| Surface imprinting | Sphere | SiO2 | BPA | 17.3 | 50 | 13 |
| Fe3O4 | BPA | 30.26 | 180 | 14 | ||
| Fe2O3 | BPA | 71.4 | 30 | 15 | ||
| SiO2 | TBBPAc | 958 | 40 | 16 | ||
| HPMIP | Sphere | MCM-48 | BPA | 670 | 30 | This paper |
In summary, HPMIP has been fabricated using a mesoporous MCM-48 sphere as a hard template. To the best of our knowledge, this is the first study presenting a promising approach for fabricating this type of MIP for BPA. The obtained HPMIP has a high specific surface area of 607 m2 g−1 and a faster adsorption rate (30 min) compared with other BPA–MIPs. The results are very attractive and promising for the enrichment and separation of BPA. In addition, these excellent properties make HPMIPs promising candidates in various applications, especially in advanced separation technologies.
Acknowledgements
We gratefully acknowledge the Commonwealth Scientific Foundation for Industry of Chinese Inspection and Quarantine (No. 201210071), of the Ministry of National Science and Technology of ChinaReferences
- (a) C. A. Staples, P. B. Dorn, G. M. Klecka, S. T. O'Block and L. R. Harris, Chemosphere, 1998, 36, 2149 CrossRef CAS PubMed; (b) H. Hiroi, O. Tsutsumi, M. Momoeda, Y. Tatai, Y. Osuga and Y. Taketani, Endocr. J., 1999, 46, 773 CrossRef CAS PubMed; (c) J. H. Kang, F. Kondo and Y. Katayama, Toxicology, 2006, 226, 79 CrossRef CAS PubMed.
- (a) M. Rezaee, Y. Yamini, S. Shariati, A. Esrafili and M. Shamsipur, J. Chromatogr., A, 2009, 1216, 1511 CrossRef CAS PubMed; (b) Y. Q. Cai, G. B. Jiang, J. F. Liu and Q. X. Zhou, Anal. Chem., 2003, 75, 2517 CrossRef CAS PubMed.
- (a) Y. Watabe, K. Hosoya, N. Tanaka, T. Kubo, T. Kondo and M. Morita, J. Chromatogr., A, 2005, 1073, 363 CrossRef CAS; (b) F. Navarro-Villoslada, B. San Vicente and M. C. Moreno-Bondi, Anal. Chim. Acta, 2004, 504, 149 CrossRef CAS; (c) X. M. Jiang, W. Tian, C. D. Zhao, H. X. Zhang and M. C. Liu, Talanta, 2007, 72, 119 CrossRef CAS PubMed; (d) Y. M. Yin, Y. P. Chen, X. F. Wang, Y. Liu, H. L. Liu and M. X. Xie, J. Chromatogr., A, 2012, 1220, 7 CrossRef CAS PubMed.
- L. X. Chen, S. F. Xua and J. H. Li, Chem. Soc. Rev., 2011, 40, 2922 RSC.
- (a) F. Barahona, E. Turiel, P. A. G. Cormack and A. Martín-Esteban, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1058 CrossRef CAS; (b) D. M. Gao, Z. P. Zhang, M. H. Wu, C. G. Xie, G. J. Guan and D. P. Wang, J. Am. Chem. Soc., 2007, 129, 7859 CrossRef CAS PubMed; (c) J. Tan, H. F. Wang and X. P. Yan, Anal. Chem., 2009, 81, 5273 CrossRef CAS PubMed; (d) K. Qian, G. Z. Fang and S. Wang, Chem. Commun., 2011, 47, 10118 RSC; (e) C. Y. He, Y. Y. Long, J. L. Pan, K. Lia and F. Liu, J. Mater. Chem., 2008, 18, 2849 RSC; (f) E. Yilmaz, O. Ramström, P. Möller, D. Sanchezc and K. Mosbach, J. Mater. Chem., 2002, 12, 1577 RSC; (g) F. G. Tamayo, M. M. Titirici, A. Martin-Esteban and B. Sellergren, Anal. Chim. Acta, 2005, 542, 38 CrossRef CAS; (h) C. Baggiani, P. Baravalle, C. Giovannoli, L. Anfossi, C. Passini and G. Giraudi, J. Chromatogr., A, 2011, 1218, 1828 CrossRef CAS PubMed; (i) S. F. Xu, L. X. Chen, J. H. Li, W. Qin and J. P. Ma, J. Mater. Chem., 2011, 21, 12047 RSC; (j) W. Z. Xu, W. Zhou, P. P. Xu, J. M. Pan, X. Y. Wu and Y. S. Yan, Chem. Eng. J., 2011, 172, 191 CrossRef CAS.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- K. Schumacher, P. I. Ravikovitch, A. Du Chesne, A. V. Neimark and K. K. Unger, Langmuir, 2000, 16, 4648 CrossRef CAS.
- (a) J. Y. Kim, S. B. Yoon, F. Kooli and J.-S. Yu, J. Mater. Chem., 2001, 11, 2912 RSC; (b) H. F. Yang and D. Y. Zhao, J. Mater. Chem., 2005, 15, 1217 CAS.
- (a) H. Jiang, T. Sun, C. Z. Li and J. Ma, RSC Adv., 2011, 1, 954 RSC; (b) A. Vinu, D. P. Sawant, K. Ariga, M. Hartmann and S. B. Halligudi, Microporous Mesoporous Mater., 2005, 80, 195 CrossRef CAS.
- (a) B. You, J. Yang, Y. Q. Sun and Q. D. Su, Chem. Commun., 2011, 47, 12364 RSC; (b) S. A. Johnson, P. J. Ollivier and T. E. Mallouk, Science, 1999, 283, 963 CrossRef CAS PubMed; (c) N. Griffete, H. Frederich, A. Maìtre, S. Ravaine and M. M. Chehimi, Langmuir, 2012, 28, 1005 CrossRef CAS PubMed.
- F. Navarro-Villoslada, B. S. Vicente and M. C. Moreno-Bondi, Anal. Chim. Acta, 2004, 504, 149 CrossRef CAS.
- M. C. Cela-Pérez, M. M. Castro-López, A. Lasagabáster-Latorre, J. M. López-Vilariño, M. V. González-Rodríguez and L. F. Barral-Losada, Anal. Chim. Acta, 2011, 706, 275 CrossRef PubMed.
- N. Griffete, H. Li, A. Lamouri, C. Redeuilh, K. Chen, C.-Z. Dong, S. Nowak, S. Ammar and C. Mangeney, J. Mater. Chem., 2012, 22, 1807 RSC.
- Y. M. Ren, W. Q. Ma, J. Ma, Q. Wen, J. Wang and F. B. Zhao, J. Colloid Interface Sci., 2012, 367, 355 CrossRef CAS PubMed.
- J. Z. Liu, W. Z. Wang, Y. F. Xie, Y. Y. Huang, Y. L. Liu, X. J. Liu, R. Zhao, G. Q. Liu and Y. Chen, J. Mater. Chem., 2011, 21, 9232 RSC.
- W. H. Zhao, N. Sheng, R. Zhu, F. D. Wei, Z. Cai, M. J. Zhai, S. H. Du and Q. Hu, J. Hazard. Mater., 2010, 179, 223 CrossRef CAS PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental details, characterization and performance evaluation results of HPMIPs. See DOI: 10.1039/c2ra21166a |
| This journal is © The Royal Society of Chemistry 2012 |
