 Open Access Article
Open Access ArticleConcentrated seawater brines for use in solar-powered desiccant cooling cycles
George
Lychnos
a,
Ridha
Amdouni
b and
Philip A.
Davies
*a
aSustainable Environment Research Group, School of Engineering and Applied Science, Aston University, Birmingham, B4 7ET, UK. E-mail: p.a.davies@aston.ac.uk
bCompagnie Générale des Salines de Tunisie, CO.TU.SAL, Gabes Road P.O Box 86, 3018 Sfax, Tunisia
First published on 13th July 2012
Abstract
We have directly measured properties of concentrated seawater brines produced through solar evaporation in salt works. They are sufficiently hygroscopic for use in desiccant cooling cycles which can cool air to 8.0–10.9 °C below ambient. This compares to only 3.8–8.7 °C with simple evaporative cooling. Desiccant cooling can extend the growing seasons of greenhouse crops thus providing an adaptive measure against climate change.
Several recent reviews have highlighted the risks to global food security arising fundamentally from population growth, which is likely to increase the demand for food by 70% with the world population reaching some 9 billion by 2050.1–4 These risks are exacerbated by changing patterns of climate which are expected to affect food production both indirectly and directly. Areas of cultivable land with adequate irrigation water are likely to diminish and, where suitable land remains available, plant physiology and viability may suffer directly from increased temperatures. By the end of the 21st century surface temperatures are predicted to rise by 0.6–4 °C.5,6 The results of studies to predict the impact of increased temperatures are variable, but the averaged predictions indicate decreased yields at lower latitudes where species are already at the threshold of heat intolerance.7 Although the singular effects of extreme temperature at specific stages of crop development make precise predictions difficult, historical case studies tend to confirm that heat waves have indeed disrupted crop production in the past.8
The aim of this study is to investigate the technical feasibility of a system that can reduce temperatures in spaces, such as greenhouses, for protected cultivation. The system would use inputs of solar energy and salts found in seawater – resources that are readily available in many coastal locations near to centres of population requiring food supplies. Specifically, we pursue the idea that concentrated brines obtained by solar evaporation of seawater may be used to remove moisture from the air in the first stage of a desiccant cooling process.
A common approach to space cooling uses vapour-compression heat pumps. This would be too energy intensive, however, for greenhouse cooling as solar gain in greenhouses amounts to several hundred watts per square metre of plan area and commensurately large inputs of electrical power would be needed. This obstacle is overcome in evaporative cooling systems which are widely used to cool greenhouses currently. Such systems exchange sensible heat for latent heat and thus require no essential energy input.9 Nevertheless, evaporative cooling systems can only reduce temperature to the ambient wet-bulb temperature at best; whereas desiccant cooling systems can achieve lower temperatures and take advantage of low grade sources of heat such as solar energy.10,11
In the desiccant cooling system proposed here (Fig. 1a), seawater is concentrated in evaporation ponds and the resulting brine is fed to an air contactor (desiccator) incorporating a recuperative heat exchanger which is supplied with raw seawater at the ambient wet-bulb temperature. The brine removes moisture from the air while the raw seawater removes the latent heat of desiccation. Thus the relative humidity is brought below ambient conditions while the temperature is also lowered. Subsequently, the air enters an evaporative cooler where it comes into direct contact with raw seawater. The psychrometric chart of Fig. 1b illustrates the desiccant cooling process and contrasts it with the more conventional process comprising simple evaporative cooling.
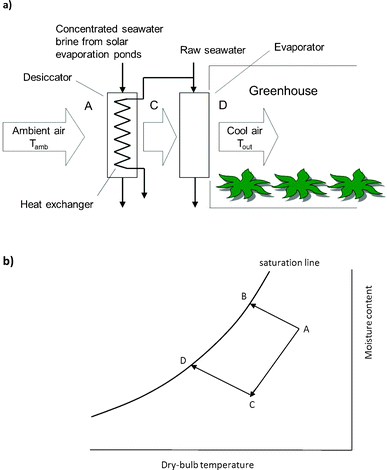 | ||
| Fig. 1 Hygroscopic seawater brine may be used to enhance evaporative cooling through prior removal of moisture from the air. (a) Process schematic of proposed arrangement. (b) Psychrometric chart illustrating the desiccant cooling operation (ACD), contrasting it with simple evaporative cooling (AB) which is limited by the wet-bulb temperature. The process ACD consists of a desiccant step (AC) followed by an evaporative cooling step (CD). Note that the processes illustrated are ideal processes, in which mass and heat transfer proceed to equilibrium; real processes will fall short of the ideal case. | ||
Concentrated seawater brines occur in salt works which have traditionally been used to produce sea salt for centuries. (The supernatant brines from the evaporation process are sometimes referred to as bitterns due to their bitter taste.) To test the hypothesis that these brines (bitterns) are sufficiently hygroscopic to drive a desiccant cooling process, we measured the vapour pressures of brine samples taken from the solar salt works situated to the south of the city of Sfax in the south-east of Tunisia.
The Sfax salt works covers an area of about 1700 ha and is divided into several evaporation ponds. The brine is transferred from one pond to the next, such that the salt concentration gradually increases. After crystallization of sodium chloride in the primary ponds, the residual brine (magnesium content > 40 kg m−3) is pumped to the magnesium chloride ponds (labeled R in Fig. 2) where it spends the winter. Production of magnesium brines starts towards the end of April and lasts until August. It begins with a gradual feeding by gravity of the circuit S by the waters from R. Once they have reached a magnesium concentration of at least 110 kg m−3, the brines are pumped to storage ponds (labelled L in Fig. 2). The annual production of the Sfax works is around 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of brines. For this study samples were taken from the storage ponds L1, L2, L3 and L4.
000 tonnes of brines. For this study samples were taken from the storage ponds L1, L2, L3 and L4.
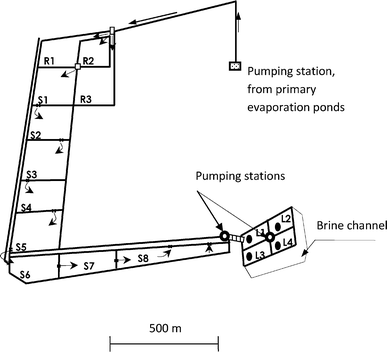 | ||
| Fig. 2 The magnesium chloride ponds at the Sfax salt works. The locations of the sampling points L1, L2, L3 and L4 are shown. | ||
The results for vapour pressures are expressed as percentages (referred to as equilibrium relative humidity, ERH) of that of pure water at the standard temperature of 25 °C. The ERH is in the range 32.4–37.4% (Fig. 3a). To compare these measurements with previous ones of relevance, we note that Rothbaum12 concentrated seawater in the laboratory to densities of 1203–1293 kg m−3 and measured ERH values of 63.1–93% . While consistent with the results obtained here, these values represented brines too dilute for use as liquid desiccant coolant. More recently, Lychnos et al.13 made up laboratory brines to simulate those occurring in salt works and measured vapour pressures down to 34% ERH, which are also consistent with the present results. The present study differs from previous ones in that it reports measurements on highly concentrated brines sampled directly from solar salt works.
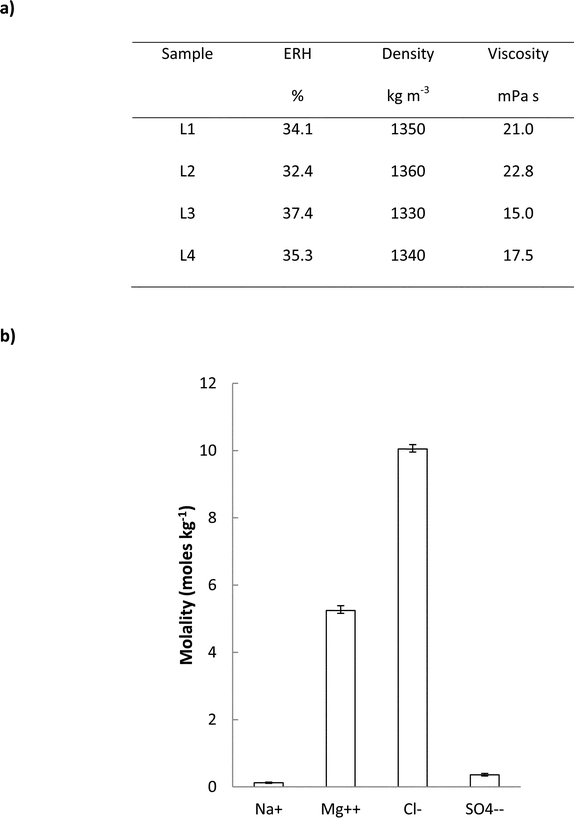 | ||
| Fig. 3 Properties of the concentrated brine samples taken at Sfax. (a) Physical properties: vapour pressure (expressed as equilibrium relative humidity, ERH), density and dynamic viscosity (experimental errors: ERH% ±1.5, density ±1 kg m−3, viscosity ±0.35%). (b) Concentrations of the principal ions, expressed as moles per kg of water (molality), showing mean and range for the 4 samples (experimental error ±0.5%). Potassium and calcium ions were also detected but at concentrations below 0.1 mole kg−1. | ||
In addition to vapour pressure, we measured the density and viscosity of each sample (Fig. 3a). Compared to water, the brines are about 35% denser and 20 times more viscous. These results are relevant to the energy needed to pump the brines around a cooling system comprising pipes, valves and distributors. In most practical pipework arrangements, flow is turbulent and thus pump power to overcome friction is expected to be approximately proportional to density and less affected by viscosity. We also measured concentrations of the principal ions thus confirming that the main constituent of the brines was magnesium chloride (Fig. 3b).
To investigate the degree of cooling possible using the seawater brines in a liquid desiccant cycle, we used a simulation model created in gPROMS®. The model had been calibrated against laboratory experiments with a desiccator of cross-flow design, consisting of porous packing perfused with desiccant solution and incorporating a heat exchanger comprising tube bundles.14,15 For the simulations, we assumed a thickness of 0.2 m for the desiccator and an effectiveness of 0.8 for the evaporator. Thus we predict, for a desiccant solution with ERH equivalent to sample L2, the temperature reduction relative to ambient and compare it to that possible using a simple evaporative cooling system corresponding to only the second stage in Fig. 1a. The model has been applied to Sfax and four other coastal cities in various climates as classified by the Köppen-Geiger system.16Fig. 4 shows the predictions of average temperature reduction over the daytime from 10.00 to 17.00 h, for the most and least humid days occurring during the three hottest months of a typical meteorological year. The advantage of desiccant cooling over evaporative cooling is greatest on the most humid days.
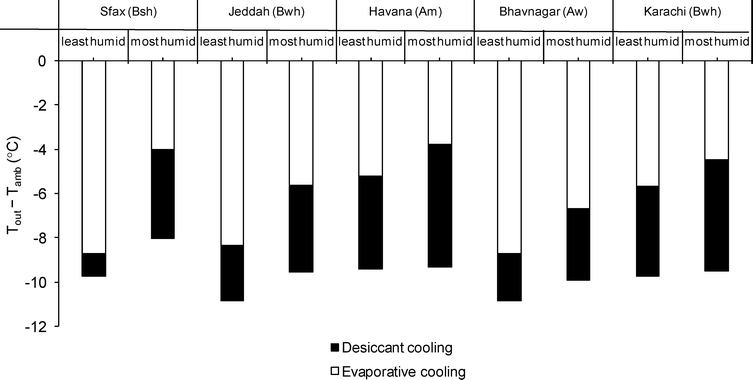 | ||
| Fig. 4 Temperature reductions predicted with the liquid desiccant cooling system in comparison to a simple evaporative cooling system. The air temperature (Tout) at the outlet of the liquid desiccant cooling system of Fig. 1 (approximating process ACD) is lower than ambient temperature (Tamb) and also lower than that at the outlet of a simple evaporative cooling system (approximating process AB). The chart compares the temperature drop achievable with each system, averaged over the daytime from 10.00 to 17.00 h, on the least and most humid days during the hottest three months of a typical meteorological year for five cities with climates classified (in brackets) according to the Köppen-Geiger system. | ||
The climate of Sfax is hot and arid with a dry summer (Köppen-Geiger type Bsh). For Sfax, the desiccant cooling system will provide 8.0–9.7 °C of cooling below ambient (corresponding to the least and most humid days respectively) representing a further 1.0–4.0 °C compared to simple evaporative cooling. For Karachi, whose climate is hot and arid with dry winter (type Bwh), we predict 9.5–9.7 °C of cooling below ambient from the desiccant system, which is an improvement of 4.0–5.1 °C compared to evaporative cooling alone. Jeddah also has climate type Bwh and desiccant cooling here will give 9.6–10.9 °C, representing a more modest improvement of 2.5–3.9 °C. For Bhavnagar (equatorial with dry winter, Aw), desiccant cooling will give 9.9–10.9 °C, which is only a 2.1–3.2 °C improvement. The greatest improvement is for Havana (equatorial monsoon climate, Am) where we predict 9.3–9.4 °C with desiccant cooling – a further 4.1–5.5 °C compared to evaporative cooling.
These locations include populous areas. Karachi's population of 17.4 million places it among the world's megacities.17 The Gujarati coastline around Bhavnagar is densely populated and has extensive salt works, being a major salt producing region of India. Bhavnagar is within 300 km of Mumbai (population 20.8 million) and about 500 km from Karachi. Future work could include studies of brine concentration and output achieved in salt works in these and other locations besides Sfax, to determine how easily sufficiently hygroscopic solutions can be produced.
Protected cultivation represents a rapidly growing segment of global agriculture: it is estimated that in 2010 there were about 1.2 × 106 ha of greenhouse cultivation worldwide compared to only 0.7 × 106 ha in the year 2000.18,19 Greenhouses using simple evaporative cooling are already common in countries such as Saudi Arabia, Oman and the UAE. The use of cooled greenhouses in hot climates is the direct opposite of heated greenhouses in cool climates. Historically, however, there has been a higher level of investment in technology for heated greenhouses in countries such as Holland, than in technology for cooled greenhouses. The rising populations and strengthening economies in the hot regions across the Middle East and the Indian subcontinent could see this situation reversed. For greenhouse cooling technology such as the one presented here, the challenge will be to provide it at an affordable cost in competition with other solutions for meeting the growing food demand.
Conclusion
Directly measured properties of concentrated seawater brines with relevance to desiccant cooling have been reported for the first time. Equilibrium relative humidity is in the range 32.4–37.4%, density is 1330–1360 kg m−3 and viscosity is 15–22.8 mPa s. The use of concentrated brine in a solar-powered desiccant cycle for greenhouse cooling has been simulated for five cities with different types of climate. The simulation results predict that the desiccant cooling system can achieve 8.0–10.9 °C of cooling below ambient depending on location and ambient humidity. This compares to only 3.8–8.7 °C achievable with simple evaporative cooling and represents an improvement of 1.0–5.5 °C.Note on methods
Vapour pressures were measured using an isoteniscope and a manometer filled with dibutyl phthalate, according to an ASTM Standard.20 The isoteniscope was made by a glassblower, to the dimensions provided therein. The viscosity measurements were carried out according to a standard procedure for U-tube viscometers for direct flow, with a BS/U viscometer and a constant temperature bath of ± 0.1 °C accuracy.21 All property measurements were carried out at 25 °C. Concentrations of magnesium and calcium were determined by titration against EDTA and those of chloride and sulphate by silver titration and gravimetric methods. The analyses of sodium and potassium ions were carried out by flame photometry.Acknowledgements
G.L. acknowledges financial support from the Greek State Scholarship Foundation. The authors thank W. T. Norris for his comments on the manuscript.References
-
FAO, High Level Expert Forum – How to Feed the World in 2050, Rome, 2009 Search PubMed
.
-
Food and Agricultural Organization, U. N. O. Food Security and Agricultural Mitigation in Developing Countries: Options for Capturing Synergies, 2009 Search PubMed
.
- United Nations, D. E. S. A., Population Division, World Population Prospects: The 2008 Revision, Highlights, Working Paper No. ESA/P/WP.210, 2009.
- FAOSTAT, http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor, 2011.
-
IPCC, Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, IPCC, Geneva, Switzerland, 2007 Search PubMed
.
- NASA, http://data.giss.nasa.gov/gistemp/, 2010.
- J. Gornall, R. Betts, E. Burke, R. Clark, J. Camp, K. Willett and A. Wiltshire, Implications of climate change for agricultural productivity in the early twenty-first century., Philos. Trans. R. Soc. London, Ser. B, 2010, 365, 2973–2989 CrossRef
.
- D. S. Battisti and R. L. Naylor, Historical Warnings of Future Food Insecurity with Unprecedented Seasonal Heat, Science, 2009, 323, 240–244 CrossRef CAS
.
-
V. Zabeltitz, in Ecosystems of the world, ed. G. Stanhill and H. Z. Enoch, Elsevier science, 1999, pp. 17–69 Search PubMed
.
- G. Grossman, Solar-powered systems for cooling, dehumidification and air-conditioning., Sol. Energy, 2002, 72, 53–62 CrossRef CAS
.
- D. S. Kim and C. A. Infante Ferreira, Solar refrigeration options-a state-of-the-art review., Int. J. Refrig., 2008, 31, 3–15 CrossRef CAS
.
- H. Rothbaum, Vapor Pressure of Sea Water Concentrates., Industrial & Engineering Chemistry Chemical & Engineering Data Series, 1958, 3, 50–52 CAS
.
- G. Lychnos, J. P. Fletcher and P. A. Davies, Properties of seawater bitterns with regard to liquid-desiccant cooling., Desalination, 2010, 250, 172–178 CrossRef CAS
.
- G. Lychnos and P. A. Davies, Modelling and experimental verification of a solar-powered liquid desiccant cooling system for greenhouse food production in hot climates., Energy, 2012, 40, 116–130 CrossRef CAS
.
-
G. Lychnos, Feasibility of a solar-powered liquid desiccant cooling system for greenhouses, PhD Thesis, Aston University, 2010 Search PubMed
.
- M. Kottek, J. Grieser, C. Beck, B. Rudolf and F. Rubel, World Map of the Köppen-Geiger climate classification updated., Meteorol. Z., 2006, 15, 259–263 CrossRef
.
-
T. Brinkhoff, The Principal Agglomerations of the World, 2012, http://www.citypopulation.de, accessed July
.
-
G. W. Hickman, Greenhouse Vegetable Production Statistics, 2010, http://www.cuestaroble.com/ Search PubMed
.
- A. Pardossi, F. Tognoni and L. Incrocci, Mediterranean greenhouse technology, Chron. Hortic., 2004, 44, 28 Search PubMed
.
- ASTM D 2879-97, Standard Test Method for Vapor Pressure-Temperature Relationship and Initial Decomposition Temperature of Liquids by Isoteniscope (reapproved 2002).
- BS188:1977, Methods for determination of the viscosity of liquids.
| This journal is © The Royal Society of Chemistry 2012 |
