DOI:
10.1039/C2RA21485G
(Paper)
RSC Adv., 2012,
2, 10238-10244
H3PO4-imbibed three-dimensional polyacrylamide/polyacrylamide hydrogel as a high-temperature proton exchange membrane with excellent acid retention
Received
18th July 2012
, Accepted 24th August 2012
First published on 28th August 2012
Abstract
We herein report the use of polyacrylamide/polyacrylamide interpenetrating polymer network (PAM/PAM IPN) hydrogel as a matrix to imbibe proton conducting H3PO4, forming a robust proton exchange membrane (PEM) suitable for high-temperature PEM fuel cells by combining excellent acid retention, simple synthesis, and low cost. Its extraordinary ability to absorb large quantity of aqueous solution is fully utilized to achieve high H3PO4 loading, showing a proton conductivity of 0.0833 S cm−1 at 183 °C in dry air. The synthesized membrane also shows excellent acid retention even under mechanical load and high humidity. These profound advantages along with simple and low-cost synthesis promise the new membrane to be a strong candidate as a high-temperature PEM.
1 Introduction
High-temperature proton exchange membrane fuel cells (PEMFCs) operating at T > 100 °C are currently being pursued as an advanced option to the state-of-the-art 80 °C Nafion-based equivalents.1,2 The potential benefits gained from operating at T > 100 °C include increased CO and H2S tolerances, eliminated water management, improved electrode kinetics, and likelihood of using non-precious metal catalysts.3,4 One key enabling material for high-temperature PEMFCs is the proton exchange membrane (PEM), which should be solely proton conducting, chemically stable and mechanically strong at T > 100 °C. The majority of high-temperature PEM systems contain polymers having Lewis base centers in their structure doped with strong inorganic acids. To date, phosphoric acid (H3PO4) doped polybenzimidazole (PBI) membranes are the most developed high-temperature PEMs, showing high proton conductivity, excellent chemical compatibility, thermal stability, and nearly zero water drag coefficient.5 However, conventional PBI-based PEMs have low molecular weights (intervening sequence of 0.5–0.8 dL g−1), limited H3PO4 loading (6–10 moles of H3PO4 per mole of repeat unit), and poor H3PO4 retention.6–9 Low H3PO4 loading, typically less than 60 wt%, confines proton conductivity to the range of 0.01–0.05 S cm−1 at 180 °C and in 5–30% relative humidity. To improve the H3PO4 loading and thereby proton conductivity, Benicewicz et al. have developed a sol–gel poly(phosphoric acid) process to imbibe H3PO4 into PBI membranes. The results are impressive: as high as 95 wt% of H3PO4 loading and 0.35 S cm−1 in proton conductivity have been achieved at 180 °C.10,11 However, one of the major issues associated with PBI-based PEMs is that H3PO4 can be easily washed out by steam produced at the cathode during normal operation and by liquid water formed during startup and shutdown. A gradual loss of H3PO4 can lead to a decrease in proton conductivity, degradation of the cell performance and thus the lifetime of PEMFCs.
In the search for more robust high-temperature PEM materials, we have recently investigated the use of polyacrylamide (PAM) as the polymer matrix for imbibing proton conducting phase H3PO4. PAM is a highly hydrophilic and stable hydrogel. Its remarkable ability to absorb large quantity of water or aqueous solution ensures high H3PO4 loading into the PAM matrix network, thus high proton conductivity. On the other hand, by double crosslinking PAM into a 3D interpenetrating polymer network (IPN), the mechanical strength of the PAM-based hydrogels can be significantly enhanced.
The early work on using acid-doped PAMs as proton conducting membranes were mainly focused on a working temperature range from ambient to 100 °C12–17 for applications in electrochromic devices. The H3PO4-doped PAMs characterized by Stevens et al. in a hydrated state exhibited room-temperature conductivity in the order of 10−2 S cm−1, which can be further increased to 10−1 S cm−1 at 100 °C.14 However, the high conductivity at elevated temperatures suffered from substantial decay with time due to the loss of water from the membranes. In a similar study conducted by Lassègues et al.,13 the proton conductivity of an anhydrous blend of non-crosslinked PAM and H3PO4 (H3PO4/PAM = 1–2) was shown to be in the order of 10−3 S cm−1 at 30 °C, demonstrating its potential to be a proton conducting membrane. However, the linear PAM used for the study is susceptible to water attack (partially soluble in water), low H3PO4 loading, poor thermal, chemical and mechanical stabilities.
Here we report the study of an anhydrous 3D PAM/PAM IPN hydrogel imbibed with H3PO4 as a potential PEM for operating in the temperature range of 100–200 °C. To the best of our knowledge, there is no report in the literature that uses acid-imbibed PAM/PAM IPN hydrogel as a PEM operating at 100–200 °C.
2 Experimental section
2.1 Synthesis of PAM/PAM IPN composites
The PAM/PAM interpenetrating polymer network (IPN) was synthesized according to a simple two-step method as described in our previous work.18,19 In detail, a mixture solution 1 containing 5 g acrylamide monomer (99+%, electrophoresis grade) and 0.002 g crosslinker N,N′-methylenebisacrylamide (NMBA, 96%) was agitated with 7.5 mL DI-water in a water-bath at 90 °C. Under the vigorous stirring, 0.015 g ammonium persulfate (APS, 98%) was added to the above mixture. The acrylamide monomers were initiated by the thermal decomposition of APS to form PAM prepolymers. With the proceeding of polymerization, the viscosity increased. When the viscosity of the PAM prepolymers reached around 140 mPa s−1, another homogeneous solution 2 consisting of 5 g acrylamide and 0.002 g NMBA was added. To continue the polymerization, another 0.015 g APS was added into the above reagent solution until the viscosity of the system reached around 180 mPa s−1. Finally, the reagent was poured into a Petri dish and cooled to room temperature until an elastic transparent gel was formed. PAM/PAM discs with ∅ 3 cm were then cut, washed with DI water, and finally dried under vacuum at 50 °C for 24 h.
2.2 Preparation of H3PO4-imbibed PAM/PAM IPN membranes
The dried PAM/PAM IPN membranes were immersed in an H3PO4 aqueous solution with concentration varying from 0.1 to 10 M. The absorption process was carried out in a sealed bottle at room temperature for 3 days to reach absorption equilibrium. The resultant products were filtrated and dried under vacuum at 60 °C for 2 days to drive off all water and obtain the final H3PO4-imbibed PAM/PAM hydrogel materials. The H3PO4 loading (wt%) was determined by titration with 0.1 M sodium hydroxide as the neutralizer. Every sample was first mixed in 20 mL of previously boiled (to remove carbon dioxide) DI-water and allowed to stir for at least 30 min, followed by titrating using a Metrohm 716 DMS Titrino titrator. The first equivalence point was used to determine the volume of sodium hydroxide necessary for neutralization.
2.3 Electrochemical characterizations
The proton conductivity of the H3PO4-imbibed PAM/PAM membranes in either hydrated or dried state were characterized with ac-impedance spectroscopy using a Zahner IM6 Electrochemical Workstation (ZAHNER-Electrik Gmbh & Co., Kronach, Germany) in a frequency range of 0.05 Hz–3 MHz and an ac amplitude of 10 mV in a temperature range of 25–183 °C. The self-adhesive carbon conductive tapes in a diameter of 1.18 cm were used as the electrodes. The ohmic resistance associated with the membrane was determined from the high frequency intersection of the spectrum with the Z′ axis, from which the proton conductivity can be calculated out.
2.4 Evaluation of H3PO4 retention
To evaluate the H3PO4 retention ability of H3PO4-imbibed PAM/PAM membrane, the membranes with 68.7 wt% H3PO4 loading were placed in an oven at 80 °C and mechanical pressure under 2.1 × 104 Pa with or without moist air of 100% RH. The residual H3PO4 loading was also determined by titration method at intervals.
2.5 Other characterizations
The morphologies of the hydrated and dried H3PO4-imbibed PAM/PAM were captured with a Zeiss Ultra Plus field emission scanning electron microscopy (FESEM). For the hydrated H3PO4-imbibed PAM/PAM membranes, it was cut into thin films and kept at freezing temperature to remove the water. Fourier transform infrared spectrometry (FTIR) spectra were recorded on a PerkinElmer spectrum 100 FTIR spectrometer.
3 Results and discussion
3.1 Synthesis and H3PO4 loading
The synthesis of PAM/PAM IPN hydrogel is a simple, two-step process schematically illustrated in Fig. 1.18,19 The polymerization reaction of PAM/PAM hydrogel is an example of free radical polymerization, in which APS is used as thermal initiator. Homolytic cleavage of each peroxide bond (–O–O–) in the APS provides two SO4˙− radical anions, which react with water to form hydroxyl radical (OH˙). The OH˙ radicals serve as the initiator for the process of polymerization by generating the free radicals on the >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C< bonds of NMBA and acrylamide. During propagation, crosslinking of PAM by NMBA occurs to form the 3D PAM network because of the macrobiradical nature of NMBA. In the absence of crosslinker NMBA, the resultant PAM would be in linear structure and mechanically weaker. In fact, the as-obtained PAM/PAM system is a complicated structure comprising of NMBA crosslinked PAM and physical entanglement of NMBA crosslinked PAM networks. Physical entanglement of the networks in combination with the chemical bonds and hydrogen bonds results in the formation of a high-mechanical strength PAM/PAM composite.
C< bonds of NMBA and acrylamide. During propagation, crosslinking of PAM by NMBA occurs to form the 3D PAM network because of the macrobiradical nature of NMBA. In the absence of crosslinker NMBA, the resultant PAM would be in linear structure and mechanically weaker. In fact, the as-obtained PAM/PAM system is a complicated structure comprising of NMBA crosslinked PAM and physical entanglement of NMBA crosslinked PAM networks. Physical entanglement of the networks in combination with the chemical bonds and hydrogen bonds results in the formation of a high-mechanical strength PAM/PAM composite.
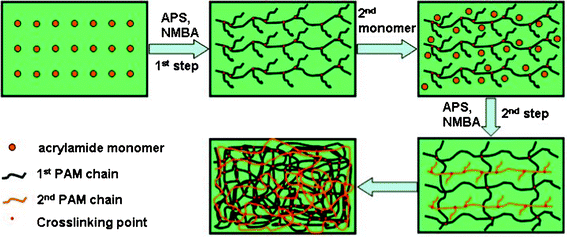 |
| | Fig. 1 Schematic of a “two-step” synthesis of the PAM/PAM IPN membranes. | |
The loading of H3PO4 into PAM/PAM IPN hydrogel is primarily driven by the osmotic pressure across the membrane.20 The uptake of H3PO4 by PAM/PAM causes the PAM/PAM network to stretch and expand considerably in volume, the process of which can be briefly described by the following three steps:21 (1) adsorption of H2O on the surface of the PAM/PAM composite because of the hydrophilicity of amide groups (–CONH2); (2) relaxation of PAM/PAM macromolecule chains, and (3) stretch of whole PAM/PAM macromolecule chains in H3PO4 solution. After removal of H2O, the remaining H3PO4 molecules close to the PAM/PAM network backbones form hydrogen bonds with carbonyl and amide groups. The H3PO4 molecules away from the network backbones are free.22 In our studies, the samples with low H3PO4 loading show rigidity, but become flexible at higher H3PO4 loading, indicating the presence of free H3PO4 molecules in the membrane. The free H3PO4 plays an important role in proton conductivity of the membrane.
The physical appearance of the as-synthesized PAM/PAM IPN hydrogel, shown in Fig. 2a, has a dense microstructure. After the PAM/PAM IPN hydrogel is imbibed in a concentrated aqueous H3PO4 solution, its volume is appreciably enlarged (see Fig. 2c) by the incorporation of water and H3PO4 molecules into the IPN. To reveal the interconnected porous microstructure of the H3PO4-imbibed PAM/PAM IPN hydrogel membrane, a freeze-drying technique is employed and the cross-sectional SEM photograph is also shown in Fig. 2c. It is evident that the resultant PAM/PAM hydrogel membrane is indeed a well-interconnected, interpenetrating network capable of trapping a large quantity of H3PO4 in the microporous structures. After the membrane is dried at 60 °C, Fig. 2b, its volume shrinks considerably to allow the maintenance of the dense microstructure. At this stage, the imbibed H3PO4 molecules are completely trapped inside the PAM/PAM IPN matrix. It is noteworthy to mention that the migration of H3PO4 aqueous solution into PAM IPN is driven by the osmotic pressure gradient across the membrane,23 which is different from the capillary-force driven H3PO4 migration into porous PBI membranes.24,25 The former H3PO4 imbibing process is a molecular-level mixing H3PO4 into PAM/PAM IPN matrix involving water absorption, polymer chain relaxation and stretching.26 Upon removal of water molecules by dehydration, the polymer chains shrink, leaving the imbibed H3PO4 chemically caged in the 3D network. Such a unique property also results in excellent H3PO4 retention ability of the membrane. In contrast, H3PO4 imbibed into a pre-existing porous polymer network has a greater propensity to escape from the structure at conditions different from which H3PO4 was imbibed.27
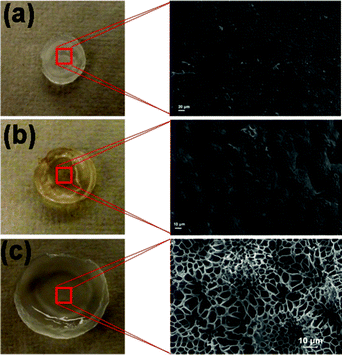 |
| | Fig. 2 Photographs and cross-sectional views of microstructures of PAM/PAM IPN: (a) as-synthesized; (b) after drying, H3PO4-loading: 68.7 wt%; (c) swollen by H3PO4 aqueous solution (H3PO4 loading 68.7 wt%). | |
To illustrate the 3D network in PAM/PAM IPN hydrogel, a swollen hydrated sample was freeze-dried to remove water. The resultant porous microstructure revealed by SEM is shown in Fig. 2b. It is evident that the synthesized PAM/PAM hydrogel is indeed a well-interconnected, interpenetrating network capable of caging a large amount of H3PO4 in its structure.
3.2 Hydrated conductivity
The proton conductivity of the PEMs is highly dependent on the H3PO4 loading (measured by titration method). Therefore, the membranes are imbibed in the highly concentrated H3PO4 solution for high H3PO4 loading. In previous H3PO4-doped PBI studies, 11 to 14 M solutions are always employed as the optimal concentrations of H3PO4 solutions,28–31 However, the results shown in Fig. 3 are in apparent disagreement with this empirical rule of thumb. This is because of the “salt-resistance” effect prevalent in hydrogel materials governed by the Flory equation:32,33| | 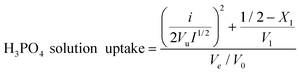 | (1) |
where i/Vu is the concentration of the fixed charges referred to the un-swollen polymer, I is ionic strength in the external solution, Ve/V0 is the crosslinking density of the polymer, and (1/2−X1)/V1 is relative to the water affinity of the polymer. In the case of swelling of the same sample in various concentrated H3PO4 solution, i/Vu, Ve/V0, and (1/2−X1)/V1 are constant, leading to the following simplified Flory equation:| | | H3PO4 solution uptake = kI−1 + c | (2) |
where k is a parameter related to the ratio of charge density to crosslinking density, and c is a parameter related to the ratio of water affinity of polymer to crosslinking density. From eqn (1) and (2), it can be seen that the H3PO4 solution loading decreases with solution concentration as a result of an increase in ionic strength of solution. After systematic variation of concentration of H3PO4 aqueous solution, we found that 7 M is the optimal concentration leading to the highest H3PO4 loading into PAM/PAM IPN membranes. The room-temperature proton conductivity of hydrated PAM/PAM IPN has a similar trend with the highest conductivity of 0.0763 S cm−1 occurred at 7 M. Unless specified, a 7 M H3PO4 aqueous solution is always used for imbibing H3PO4 into PAM/PAM IPN membranes.
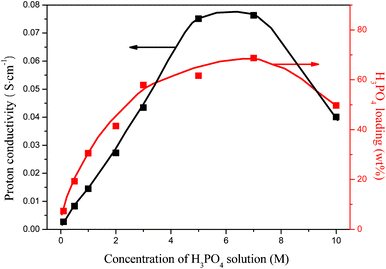 |
| | Fig. 3 Proton conductivity of the H3PO4-imbibed PAM/PAM IPN membrane as a function of H3PO4 concentration at fully hydrated equilibrium state. The measurement was carried out at room temperature. | |
Different from other PEMs, the room-temperature conductivity of the hydrogel-based membranes is highly dependent on both water content and swelling volume ratio (defined as Vswollen/Vdry). Data on the room-temperature conductivity of swollen H3PO4-imbibed PAM/PAM IPN membranes are shown in Fig. 4. A several-fold increase in volume compared to the dried state can be achieved after membranes are hydrated. As expected, for a given swelling volume ratio, higher H3PO4 loading yields higher proton conductivity. For a given H3PO4 loading, the proton conductivity experiences drastic change with the swelling volume ratio. The “onset” swelling volume ratio at which proton conductivity becomes flattened-out decreases with increasing H3PO4 loading. This behavior can be reasonably explained by the fact that proton conduction relies upon the “solvating” effect of limited number of H3PO4 molecules in the IPN. Therefore, the state of hydration has a significant effect on the proton conductivity. As the H3PO4 loading increases, the presence of water molecules is no longer critical to proton conduction since H3PO4 molecules can play a major role in proton conduction.
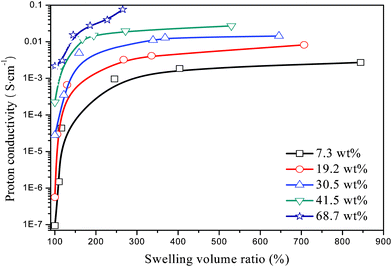 |
| | Fig. 4 Room-temperature proton conductivity of the hydrated H3PO4-imbibed PAM/PAM IPN membranes as a function of swelling volume ratio. | |
3.3 Structural analysis
The polymer–acid interactions of the synthesized anhydrous H3PO4-imbibed PAM/PAM IPN hydrogel were characterized by FTIR. It is apparent from Fig. 5 that the characteristic bands of functional hosts of baseline pure PAM/PAM IPN have been shifted towards lower wavenumbers by the presence of H3PO4 in the structure. The shifts from 1687 to 1673 cm−1 (υ, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1473 to 1430 cm−1 (υ, C–N), and 994 to 951 cm−1 (ω, NH2), along with the disappearance of band at 1228 cm−1 (γ, NH2) in FTIR spectra suggest that H3PO4 does not protonate NH2 to NH3+ in PAM but interacts with it by O–H⋯O
O), 1473 to 1430 cm−1 (υ, C–N), and 994 to 951 cm−1 (ω, NH2), along with the disappearance of band at 1228 cm−1 (γ, NH2) in FTIR spectra suggest that H3PO4 does not protonate NH2 to NH3+ in PAM but interacts with it by O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, O–H⋯N–C, O–H⋯NH2 to form hydrogen bonds.13 The formation of these hydrogen bonds plays an important role in the conductivity behavior of the membranes.
C, O–H⋯N–C, O–H⋯NH2 to form hydrogen bonds.13 The formation of these hydrogen bonds plays an important role in the conductivity behavior of the membranes.
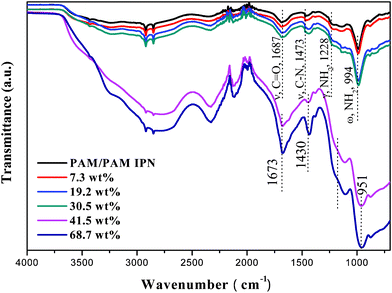 |
| | Fig. 5 FTIR spectra of pure PAM/PAM IPN and H3PO4-imbibed PAM/PAM IPN membranes. | |
3.4 High-temperature conductivity
The proton conductivity of the membrane measured in dry air from 25–183 °C follows reasonably well with Arrhenius relationship in Fig. 6a. The highest proton conductivity achieved is 0.0833 S cm−1 at 183 °C, which compares favorably with the reported values of H3PO4-doped mesoporous PBI24,25 and of ionic liquid doped membranes.3,34 It is also interesting to note that the activation energy, Ea, does not change with the H3PO4 loading at lower loading, say <41.5 wt%. This trend is different from that of H3PO4-doped PBI membranes.6 Only at a higher H3PO4 loading is Ea closer to that of pure H3PO4 (23.05 kJ mol−1). The lower and invariant Ea at lower H3PO4 loading seems to suggest a different proton transfer mechanism from that of pure H3PO4. We hypothesize based on FTIR results that at lower H3PO4 loading, O–H groups in H3PO4 preferentially form hydrogen bonds with functional group hosts C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–N, or NH2 in the PAM/PAM IPN. These hydrogen bonds can serve as the pathways for proton transfer as illustrated in Fig. 7. The low Ea also seems to suggest that such a proton transfer process is energetically favorable. The low conductivity mainly results from the low concentration of protons or hydrogen bonds formed. As H3PO4 loading increases, H3PO4 forms its own percolative network to transfer protons via Grotthuss mechanism by successive proton transfer and reorientation steps. This is also the reason why Ea at 68.7 wt% H3PO4 loading is closer to that of pure H3PO4. To further confirm the percolating effect, the room-temperature proton conductivity was measured and shown as a function of H3PO4 loading in Fig. 6b. A fitting of the experimental data with percolation theory35 yields a threshold of H3PO4fc = 58.9 wt% and critical exponent β = 2, suggesting that a 3D percolation network has been formed.
O, C–N, or NH2 in the PAM/PAM IPN. These hydrogen bonds can serve as the pathways for proton transfer as illustrated in Fig. 7. The low Ea also seems to suggest that such a proton transfer process is energetically favorable. The low conductivity mainly results from the low concentration of protons or hydrogen bonds formed. As H3PO4 loading increases, H3PO4 forms its own percolative network to transfer protons via Grotthuss mechanism by successive proton transfer and reorientation steps. This is also the reason why Ea at 68.7 wt% H3PO4 loading is closer to that of pure H3PO4. To further confirm the percolating effect, the room-temperature proton conductivity was measured and shown as a function of H3PO4 loading in Fig. 6b. A fitting of the experimental data with percolation theory35 yields a threshold of H3PO4fc = 58.9 wt% and critical exponent β = 2, suggesting that a 3D percolation network has been formed.
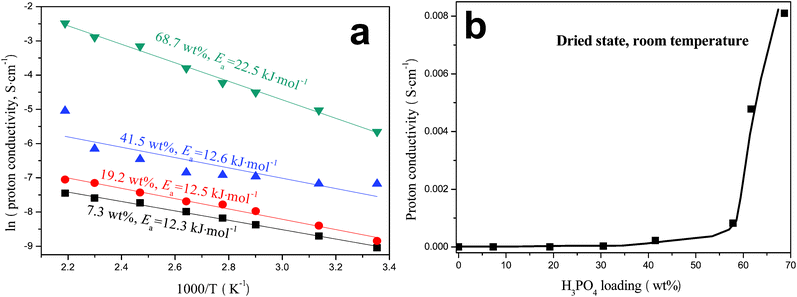 |
| | Fig. 6 (a) Arrhenius plots of H3PO4-imbibed PAM/PAM IPN membranes measured in dry air; (b) variation of room-temperature proton conductivity with H3PO4 loading. | |
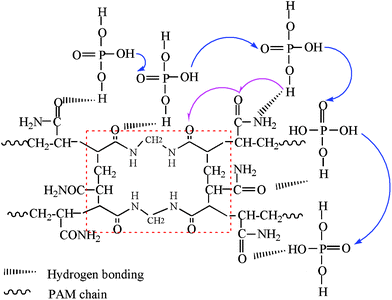 |
| | Fig. 7 A proposed proton transfer mechanism in H3PO4-imbibed PAM/PAM IPN membranes. The red square shows the 3D structure unit of PAM/PAM IPN matrix. | |
Fig. 8 shows the impedance spectra and proton conductivity of H3PO4-imbibed PAM/PAM IPN membrane with 57.8 wt% H3PO4 loading as a function of steam content in air at 132 °C. The conductivity of the membrane was found to improve with increasing humidity, the dependence of which is much smaller than that of Nafion PEMs, but similar to that of H3PO4-doped PBI membranes.36 The incorporation of water into the membrane increases the dissociation of H3PO4 and therefore the number of the protons. Also, lower proton conductivity measured in 2.63% D2O than that in 2.05% H2O is observed. This is attributed to the lower mobility of deuterons in D2O compared to protons in H2O. This result further confirms the nature of proton conduction.
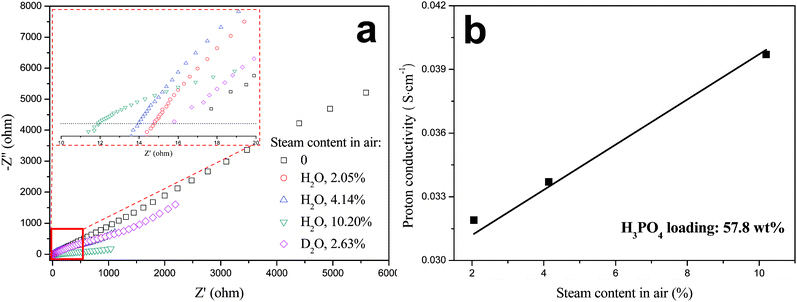 |
| | Fig. 8 (a) Nyquist plot of impedance spectra and (b) proton conductivity of H3PO4-imbibed PAM/PAM IPN membrane as a function of steam content in air at 132 °C. | |
3.5 Stability
The conductivity stability of an anhydrous 68.7 wt% H3PO4-imbibed PAM/PAM IPN hydrogel membrane measured in dry air is shown in Fig. 9a for 132 and 183 °C. Over an 80 h period, it appears that there is an initial decay in conductivity, but it eventually flattens out after 50 h at 183 °C, whereas no obvious sign of degradation can be found for 132 °C. Interestingly, H3PO4 content contained in the IPN shown in Fig. 9b (method of determination is described in the Experimental section) remain unchanged even after being exposed to a mechanical load of 2.1 × 104 Pa and air with 100% RH at the testing temperature of 80 °C for 4 h, suggesting an excellent H3PO4 retention ability owned by the IPN hydrogel membranes. This unique capability is attributed to the aforementioned “caging” effect from PAM/PAM IPN hydrogel incurred during dehydration process.
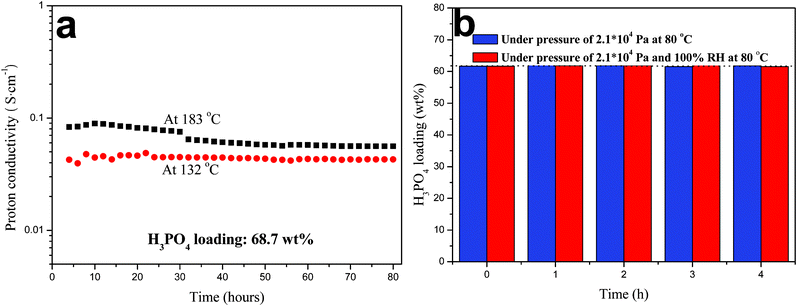 |
| | Fig. 9 (a) Stability of proton conductivity for 68.7 wt% H3PO4-imbibed PAM/PAM IPN membranes in dry air; (b) H3PO4 retention after exposure to mechanical load and an air with 100% RH at the testing temperature. | |
4 Conclusions
In summary, we have demonstrated the use of PAM/PAM IPN hydrogel as a matrix to imbibe proton conducting H3PO4 phase for high-temperature PEM. The proton transport is facilitated by hydrogen bonds formed between O–H in H3PO4 and functional groups such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–N and NH2 in PAM/PAM IPN at lower H3PO4 loading, and hydrogen bonds in H3PO4 itself at higher H3PO4 loading. The highest proton conductivity of the membrane reaches 0.0833 S cm−1 at 183 °C in dry air and remains relatively stable over an 80 h period. The membranes also exhibit excellent acid retention ability. These profound advantages along with low-cost synthesis and easy film castability promise the new membrane to be a strong candidate as high-temperature PEM.
O, C–N and NH2 in PAM/PAM IPN at lower H3PO4 loading, and hydrogen bonds in H3PO4 itself at higher H3PO4 loading. The highest proton conductivity of the membrane reaches 0.0833 S cm−1 at 183 °C in dry air and remains relatively stable over an 80 h period. The membranes also exhibit excellent acid retention ability. These profound advantages along with low-cost synthesis and easy film castability promise the new membrane to be a strong candidate as high-temperature PEM.
Acknowledgements
The authors gratefully acknowledge University of South Carolina for providing Seedling Fund to this project. The authors also thank Prof. Brian C. Benicewicz, Prof. Chuanbing Tang and Dr Kejian Yao for suggestive comments and helping process samples for SEM observation.
References
- V. D. Noto, E. Negro, J. Y. Sanchez and C. Iojoiu, J. Am. Chem. Soc., 2010, 132, 2183 CrossRef.
- H. Zarrin, D. Higgins, Y. Jun, Z. W. Chen and M. Fowler, J. Phys. Chem. C, 2011, 115, 20774 CAS.
- S. Y. Lee, A. Ogawa, M. Kanno, H. Nakamoto, T. Yasuda and M. Watanabe, J. Am. Chem. Soc., 2010, 132, 9764 CrossRef CAS.
- J. Zeng and S. P. Jiang, J. Phys. Chem. C, 2011, 115, 11854 CAS.
- Q. F. Li, J. O. Jensen, R. F. Savinell and N. J. Bjerrum, Prog. Polym. Sci., 2009, 34, 449 CrossRef CAS.
- J. A. Asensio, E. M. Sanchez and P. Gómez-Romero, Chem. Soc. Rev., 2010, 39, 3210 RSC.
- J. A. Asensio, S. Borrós and P. Gómez-Romero, J. Electrochem. Soc., 2004, 151, A304 CrossRef CAS.
- Y. L. Ma, J. S. Wainright, M. H. Litt and R. F. Savinell, J. Electrochem. Soc., 2004, 151, A8 CrossRef CAS.
- J. Mader, L. X. Xiao, T. J. Schmidt and B. C. Benicewicz, Adv. Polym. Sci., 2008, 216, 63 CAS.
- L. Xiao, H. Zhang, E. Scanlon, L. S. Ramanathan, E. W. Choe, D. Rogers, T. Apple and B. C. Benicewicz, Chem. Mater., 2005, 17, 5328 CrossRef CAS.
- S. Yu and B. C. Benicewicz, Macromolecules, 2009, 42, 8640 CrossRef CAS.
- D. Raducha, W. Wieczorek, Z. Florjanczyk and J. R. Stevens, J. Phys. Chem., 1996, 100, 20126 CrossRef CAS.
- D. Rodriguez, C. Jegat, O. Trinquet and J. C. Lassègues, Solid State Ionics, 1993, 61, 195 CrossRef CAS.
- W. Wieczorek and J. R. Stevens, Polymer, 1997, 38, 2057 CrossRef CAS.
- W. Wieczorek, Z. Florjanczyk and J. R. Stevens, Electrochim. Acta, 1995, 40, 2327 CrossRef CAS.
- J. R. Stevens, W. Wieczorek, D. Raducha and K. R. Jeffrey, Solid State Ionics, 1997, 97, 347 CrossRef CAS.
- J. Przyluski, Z. Poltarzewski and W. Wieczorek, Polymer, 1997, 39, 4343 CrossRef.
- Q. W. Tang, X. Sun, Q. Li, J. Wu and J. Lin, Colloids Surf., A, 2009, 346, 91 CrossRef CAS.
- Q. W. Tang, X. M. Sun, Q. H. Li, J. H. Wu and J. M. Lin, J. Colloid Interface Sci., 2009, 339, 45 CrossRef CAS.
- R. Luo and H. Li, Acta Biomater., 2009, 5, 2920 CrossRef CAS.
- D. J. Enscore, H. B. Hopfraberg and V. T. Stannett, Polymer, 1977, 18, 793 CrossRef CAS.
- T. Hatakeyema and A. Yamauchi, Eur. Polym. J., 1984, 20, 61 CrossRef CAS.
- R. Luo and H. Li, Acta Biomater., 2009, 5, 2920 CrossRef CAS.
- J. Weber, K. D. Kreuer, J. Maier and A. Thomas, Adv. Mater., 2008, 20, 2595 CrossRef CAS.
- D. Mecerreyes, H. Grande, O. Miguel, E. Ochoteco, R. Marcilla and I. Cantero, Chem. Mater., 2004, 16, 604 CrossRef CAS.
- Q. W. Tang, X. M. Sun, Q. H. Li, J. M. Lin and J. H. Wu, J. Mater. Sci., 2009, 44, 3712 CrossRef CAS.
- J. A. Kerres, J. Membr. Sci., 2001, 185, 3 CrossRef CAS.
- D. Mecerreyes, H. Grande, O. Miguel, E. Ochoteco, R. Marcilla and I. Cantero, Chem. Mater., 2004, 16, 604 CrossRef CAS.
- J. S. Wainright, J. T. Wang, D. Weng, R. F. Savinell and M. Litt, J. Electrochem. Soc., 1995, 142, L121 CrossRef CAS.
- M. Geormezi, V. Deimede, N. Gourdoupi, N. Triantafyllopoulos, S. Neophytides and J. K. Kallitsis, Macromolecules, 2008, 41, 9051 CrossRef CAS.
- J. A. Asensio, E. M. Sánchez and P. Gómez-Romero, Chem. Soc. Rev., 2010, 39, 3210 RSC.
-
P. J. Flory, Principles of polymer chemistry, Cornel University Press, New York, 1953 Search PubMed.
- Q. W. Tang, X. M. Sun, Q. H. Li, J. M. Lin and J. H. Wu, J. Mater. Sci., 2009, 44, 3712 CrossRef CAS.
- S. Y. Kim, S. Kim and M. J. Park, Nat. Commun., 2010, 1, 88 CrossRef.
- W. Wang, K. Fernando, Y. Lin, M. J. Mexiani, L. M. Veca, L. Cao, P. Zhang, M. M. Kimani and Y. Sun, J. Am. Chem. Soc., 2008, 130, 1415 CrossRef CAS.
- Q. Li, R. He, J. O. Jensen and N. J. Bjerrum, Fuel Cells, 2004, 4, 147 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C< bonds of NMBA and acrylamide. During propagation, crosslinking of PAM by NMBA occurs to form the 3D PAM network because of the macrobiradical nature of NMBA. In the absence of crosslinker NMBA, the resultant PAM would be in linear structure and mechanically weaker. In fact, the as-obtained PAM/PAM system is a complicated structure comprising of NMBA crosslinked PAM and physical entanglement of NMBA crosslinked PAM networks. Physical entanglement of the networks in combination with the chemical bonds and hydrogen bonds results in the formation of a high-mechanical strength PAM/PAM composite.
C< bonds of NMBA and acrylamide. During propagation, crosslinking of PAM by NMBA occurs to form the 3D PAM network because of the macrobiradical nature of NMBA. In the absence of crosslinker NMBA, the resultant PAM would be in linear structure and mechanically weaker. In fact, the as-obtained PAM/PAM system is a complicated structure comprising of NMBA crosslinked PAM and physical entanglement of NMBA crosslinked PAM networks. Physical entanglement of the networks in combination with the chemical bonds and hydrogen bonds results in the formation of a high-mechanical strength PAM/PAM composite.


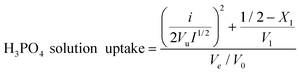


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1473 to 1430 cm−1 (υ, C–N), and 994 to 951 cm−1 (ω, NH2), along with the disappearance of band at 1228 cm−1 (γ, NH2) in FTIR spectra suggest that H3PO4 does not protonate NH2 to NH3+ in PAM but interacts with it by O–H⋯O
O), 1473 to 1430 cm−1 (υ, C–N), and 994 to 951 cm−1 (ω, NH2), along with the disappearance of band at 1228 cm−1 (γ, NH2) in FTIR spectra suggest that H3PO4 does not protonate NH2 to NH3+ in PAM but interacts with it by O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, O–H⋯N–C, O–H⋯NH2 to form hydrogen bonds.13 The formation of these hydrogen bonds plays an important role in the conductivity behavior of the membranes.
C, O–H⋯N–C, O–H⋯NH2 to form hydrogen bonds.13 The formation of these hydrogen bonds plays an important role in the conductivity behavior of the membranes.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–N, or NH2 in the PAM/PAM IPN. These hydrogen bonds can serve as the pathways for proton transfer as illustrated in Fig. 7. The low Ea also seems to suggest that such a proton transfer process is energetically favorable. The low conductivity mainly results from the low concentration of protons or hydrogen bonds formed. As H3PO4 loading increases, H3PO4 forms its own percolative network to transfer protons via Grotthuss mechanism by successive proton transfer and reorientation steps. This is also the reason why Ea at 68.7 wt% H3PO4 loading is closer to that of pure H3PO4. To further confirm the percolating effect, the room-temperature proton conductivity was measured and shown as a function of H3PO4 loading in Fig. 6b. A fitting of the experimental data with percolation theory35 yields a threshold of H3PO4fc = 58.9 wt% and critical exponent β = 2, suggesting that a 3D percolation network has been formed.
O, C–N, or NH2 in the PAM/PAM IPN. These hydrogen bonds can serve as the pathways for proton transfer as illustrated in Fig. 7. The low Ea also seems to suggest that such a proton transfer process is energetically favorable. The low conductivity mainly results from the low concentration of protons or hydrogen bonds formed. As H3PO4 loading increases, H3PO4 forms its own percolative network to transfer protons via Grotthuss mechanism by successive proton transfer and reorientation steps. This is also the reason why Ea at 68.7 wt% H3PO4 loading is closer to that of pure H3PO4. To further confirm the percolating effect, the room-temperature proton conductivity was measured and shown as a function of H3PO4 loading in Fig. 6b. A fitting of the experimental data with percolation theory35 yields a threshold of H3PO4fc = 58.9 wt% and critical exponent β = 2, suggesting that a 3D percolation network has been formed.




![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–N and NH2 in PAM/PAM IPN at lower H3PO4 loading, and hydrogen bonds in H3PO4 itself at higher H3PO4 loading. The highest proton conductivity of the membrane reaches 0.0833 S cm−1 at 183 °C in dry air and remains relatively stable over an 80 h period. The membranes also exhibit excellent acid retention ability. These profound advantages along with low-cost synthesis and easy film castability promise the new membrane to be a strong candidate as high-temperature PEM.
O, C–N and NH2 in PAM/PAM IPN at lower H3PO4 loading, and hydrogen bonds in H3PO4 itself at higher H3PO4 loading. The highest proton conductivity of the membrane reaches 0.0833 S cm−1 at 183 °C in dry air and remains relatively stable over an 80 h period. The membranes also exhibit excellent acid retention ability. These profound advantages along with low-cost synthesis and easy film castability promise the new membrane to be a strong candidate as high-temperature PEM.
