CuO nano particles and [bmim]BF4: an application towards the synthesis of chiral β-seleno amino derivatives via ring opening reaction of aziridines with diorganyl diselenides†
Syed M.
Salman
ab,
Senthil
Narayanaperumal
ac,
Ricardo S.
Schwab
*d,
Caroline R.
Bender
a,
Oscar E. D.
Rodrigues
*a and
Luciano
Dornelles
*a
aDepartamento de Química, Universidade Federal de Santa Maria (UFSM), Santa Maria, RS 97105-900, Brazil. E-mail: ldornel@gmail.com; Tel: +55 55 3220-8761
bDepartment of Chemistry, Abdul Wali Khan University (Palosa Campus), Mardan, Khyber Pakhtunkhwa, Pakistan
cDepartamento de Química, Universidade Federal de São Carlos (UFSCar), São Carlos, SP 13565-905, Brazil
dInstituto de Química, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS 91501-970, Brazil
First published on 13th August 2012
Abstract
A series of chiral β-seleno amino derivatives were synthesized via a ring opening reaction of different aziridines with diorganyl diselenides mediated by recyclable CuO nanopowder and an ionic liquid affording the corresponding products in good to excellent yields. The [bmim]BF4 ionic liquid acts as both promoter and reaction medium. Compared to the usual organic solvents the ionic liquid exhibited better performance with the advantage of its recyclability.
Organoselenium compounds have found wide utility because of their effects on an extraordinary number of very different reactions, including many C–C bond formations, under relatively mild reaction conditions.1 Moreover, chiral selenides and diselenides containing ligands offer an attractive and practical option for the development of asymmetric transformations.2 The biological and medicinal properties of organoselenium compounds are also increasingly appreciated, mainly due to their antioxidant, antitumor, antimicrobial, and antiviral properties.3
On the other hand, chiral aziridines are one of the most versatile three membered ring systems in synthetic chemistry and they are used as starting materials for the synthesis of nitrogen-containing compounds in organic transformations.4 They are also key intermediates for the stereo-controlled synthesis of nitrogen compounds (e.g., amino acids, heterocycles and peptides).5 However, the development of new methods for the introduction of selenium-containing groups into organic molecules, particularly in a stereocontrolled manner, in a short reaction time remains a significant challenge.6
Substantial number of reports have appeared in the literature describing the reductive cleavage of S–S/Se–Se bonds, employing NaBH4,7 Zn/HCl,8 Zn/InCl3,9 InI10 and others11 which generates selenium nucleophiles which upon further reaction with aziridines affords the corresponding chiral β-seleno amino derivatives. However, the synthesis of chiral β-seleno amines has been successfully accomplished, most procedures often require basic or acidic reaction conditions and the use of organic solvents which is undesirable, from an environmental point of view.12 In this context, our group has been working extensively towards the development of greener protocols for the synthesis of organoselenium compounds, we have recently reported the synthesis of chiral β-seleno amino derivatives under neutral conditions, employing a bench stable phenyl selenolate species (PhSeZnBr) and using an ionic liquid (IL) as an efficient and recyclable reaction medium.13 The most attractive features of ionic liquids are very low vapor pressure, nonflammability, ease of handling, reasonable thermal stability and the fact that they remain liquid within a wide range of temperatures.14 In addition, the use of ILs as a reaction media for the synthesis of organochalcogen compounds has appeared in a significant number of articles.13,15
On the other hand, nanoparticle-catalyzed reactions have made a great contribution to the recent growth in organoselenium chemistry. As the catalysis can be described by surface phenomena, the effective superficial area is an important factor. In this context, nanotechnology is an emerging trend towards synthetic organic chemistry. Nanomaterials with high surface area and reactive morphology have been studied widely as effective catalysts for organic synthesis.16 Recently, much emphasis has been placed on the improvement of CuO nanoparticle catalyzed transformations and significant progress has been made in this area.16a,b
In this context, we recently reported an eco-friendly cross-coupling reaction of diaryl diselenides with aryl and alkyl bromides15b and a cross-coupling of aryl and alkyl thiols with aryl iodides,15g catalyzed by CuO nanopowder in ionic liquid and the efficient synthesis of selenoesters from acyl chlorides, mediated by CuO nanopowder in an IL.15e Similarly CuO nanoparticles have been employed as an efficient recyclable catalyst as well as a mediator for cross-coupling reactions of organic diselenides with aryl boronic acids.17
In view of the above factors and our long standing interest in the synthesis and evaluation of [N,Se]-compounds as chiral ligands in asymmetric transformations18 and in biological screenings19 herein, we describe a simple, efficient, and versatile approach for the synthesis of chiral β-seleno amines. A series of chiral β-seleno amino derivatives were synthesized via a ring opening reaction of different protected and unprotected aziridines with diorganyl diselenides mediated by commercially available CuO nanopowder and an ionic liquid affording the corresponding products in good to excellent yields in a short reaction time under mild conditions, as depicted in Scheme 1.
 | ||
| Scheme 1 General synthesis of chiral β-seleno amino derivatives. | ||
In order to optimize the protocol and to understand the influence of different variables on this reaction, several components were studied. We chose Ts protected aziridine derived from L-phenylalanine 1a and diphenyl diselenide as model substrates to optimize the reaction conditions (Table 1). To promote the reaction, standard conditions were employed: Ts protected aziridine 1a (1.0 eq) was treated with diphenyl diselenide (0.5 equiv) in the presence of 10 mol% of CuO nanopowder and KOH (2 equiv) in ionic liquid (0.5 mL) for 60 min, at 80 °C. In a first set of experiments, we studied the influence of different ionic liquids to promote the reaction (Fig. 1).
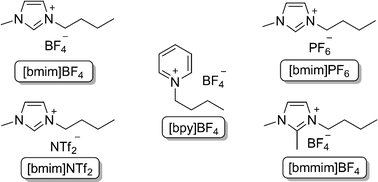 | ||
| Fig. 1 Room temperature ionic liquids. | ||
| Entry | Solventa | Yield (%)b |
|---|---|---|
| a Ionic liquids were subjected to vacuum before use. b Yield for isolated pure products. | ||
| 1 | [bmim]BF4 | 94 |
| 2 | [bmim]PF6 | 85 |
| 3 | [bmim]NTf2 | 72 |
| 4 | [bpy]BF4 | 64 |
| 5 | [bmmim]BF4 | 45 |
| 6 | THF | 14 |
In all these experiments the desired product was obtained, ranging from 45 to 94% (Table 1 entries 1–5). The result for [bmim]BF4 was found to be better than the other ionic liquids (Table 1, entry 1). Also, we investigated the influence of a common organic solvent for the reaction course. Surprisingly, when the reaction was carried with THF, the expected product was obtained in 14% yield (Table 1, entry 6).
Although the improved capability of ionic liquids to accelerate many organic reactions compared to other organic solvents has been extensively reported, the origin of its behavior is still an intriguing subject of study.20 Properties such as strong dipolar and dispersion forces, hydrogen bond acidity (related to the cationic portion), and hydrogen bond basicity (related to the anionic portion) would account for the complex solvent interactions exhibited by ILs.21 Our experimental results (Table 1, entry 1 vs. 5) suggest that due to the acidity of the C-2 proton of the imidazolium cation22 of [bmim]BF4, the hydrogen bond interaction of imidazolium cation with the aziridine facilitate the ring opening reaction with the selenium nucleophile. Moreover, if the extended hydrogen bond interactions really accounts for an effective formation of products, reactions carried out in [bpy]BF4 and [bmmim]BF4 which have much lower hydrogen bond donor values compared to the above mentioned ionic liquids would result in the formation of products in lower yields, this implied that the [bmim] moiety play a key role to this reaction.23 With these results in hands, [bmim]BF4 was chosen as a solvent for the subsequent reactions.
Continuing the optimization, we performed another set of experiments to evaluate the amount of CuO nanopowder required to promote the reaction as depicted in Fig. 2.
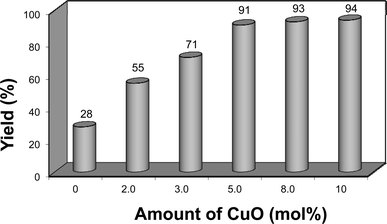 | ||
| Fig. 2 Optimization of CuO nanopowder. Yield is the isolated product. | ||
Without CuO, the reaction proceeds very slowly and the product was obtained in low yield. We found that varying the amount of CuO nanopowder had an positive effect on the reaction course. When the amount of CuO nanopowder was increased from 2.0 to 3.0 mol%, the yield of compound 2a was significantly raised from 55% to 71%. By using 5.0 mol% of CuO nanopowder, the yield was further improved to 91%. Nonetheless, raising the amount of CuO nanopowder up to 8.0 or 10 mol% did not affect the yield, affording a similar range of 93 and 94% respectively, as shown in Fig. 2.
To further optimize the protocol, it was necessary to examine the effect of the different bases, temperature and the reaction time (Table 2). Unfortunately, a significant decrease in the yield was observed when decreasing the reaction temperature from 80 to 40 or 60 °C (Table 2, entries 1, 2 and 3). When the temperature was increased up to 100 °C, the yield of compound 2a was not significantly modified (entry 4). The variation in the reaction time was also studied. Decreasing the reaction time to 40 min had a significant impact in the product formation, reducing to 78% (Table 2, entry 5). Longer reaction times did not have any influence on the yield (Table 2, entries 6 and 7).
The influence of the different bases were also studied in order to cleave the Se–Se bond affording the nucleophilic selenolate species. In this context, a number of inorganic bases were used to afford the β-seleno amines in good yields (Table 2 entries 1, 8–10). Notably, KOH gave the best performance among the screened bases, furnishing the desired product in a better yield (Table 2, entry 1), whereas other bases such as K2CO3 and Cs2CO3 gave good yields (Table 2, entries 8 and 9). On the other hand, when Na2CO3 was used the product formation was observed in moderate yield (entry 10). In the absence of base, the product formation was not observed (Table 2, entry 11).
With the optimal conditions in hands, and in order to evaluate the scope and limitations of the current methodology, the present protocol was extended to a broader range of protected aziridines 1a–f as well as different diorganyl diselenides using [bmim]BF4 at 80 °C for 60 min, Table 3.24
| Entry | R | R1 | R2 | Product | Yield (%)a |
|---|---|---|---|---|---|
| a Yield for pure isolated products. b Diphenyl disulfide was used instead of diphenyl diselenide. | |||||
| 1 | Bn | Ts, 1a |
|
2a | 91 |
| 2 | i-Pr | Ts, 1b |
|
2b | 93 |
| 3 | Bn | Ts, 1a |
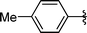
|
2c | 75 |
| 4 | i-Bu | Ts, 1c |
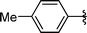
|
2d | 72 |
| 5 | Bn | Boc, 1d |
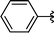
|
2e | 93 |
| 6 | i-Pr | Boc, 1e |
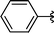
|
2f | 95 |
| 7 | Bn | Boc, 1d |
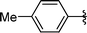
|
2g | 85 |
| 8 | i-Pr | Boc, 1e |
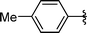
|
2h | 80 |
| 9 | Bn | Boc, 1d |
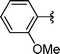
|
2i | 72 |
| 10 | i-Pr | Boc, 1e |
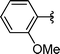
|
2j | 74 |
| 11 | Bn | Boc, 1d |
|
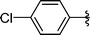
2k | >99 |
| 12 | Bn | Boc, 1d |
|
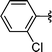
2l | 76 |
| 13 | Bn | Boc, 1d |

|
2m | 82 |
| 14 | Bn | Boc, 1d |
|
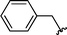
2n | 75 |
| 15b | Bn | Boc, 1d |
|
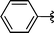
2o | 73 |
| 16 | Bn | H, 1f |
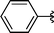
|
2p | 62 |
Analysing Table 3, all the chiral β-seleno amines were obtained in good to excellent yields from different protected aziridines. The ring-opening process was performed in the presence of aziridines bearing a t-butoxycarbonyl group (R1 = Boc) instead of a tosyl group (R1 = Ts) as well as different substituents (R = Bn and i-Pr). In these cases, the corresponding products 2e–g were obtained with 93, 95 and 85% yields respectively (Table 3, entries 5–7), whereas the products 2a–c were obtained with 91, 93 and 75% yields respectively (Table 3, entries 1–3).
Concerning the R2 group from diorganyl diselenide, the influence of an electron-donating or an electron-withdrawing group, such as -chloro, -methyl and -methoxy in the aromatic ring of the diselenide was also investigated. For instance, electron-withdrawing groups, such as 4-ClPh and 2-ClPh affords better yields (Table 3, entries 11 and 12) as compared with electron-donating groups, 4-MePh and 2-MeOPh (Table 3, entries 7 and 9). We also employed other diselenide sources in this reaction, e.g. benzylic and alkyl moieties. For instance, dibenzyl diselenide and diethyl diselenide were reacted with 1d, allowing the preparation of the desired products 2m–n in good yields (Table 3, entries 13 and 14).
Due to the success obtained with the preparation of the β-seleno amines, we decided to extend our studies to the sulfur analogue, in order to prepare chiral β-thio amino derivatives. By using our standard protocol, diphenyl disulfide was used instead of diphenyl diselenide providing the corresponding β-thio amino derivative 2o in good yield (Table 3, entry 15).
The successful ring opening procedure with CuO and diphenyl diselenide was also evaluated in the presence of unprotected aziridine with R = Bn. A good result was obtained for unprotected aziridine (Table 3, entry 16) affording the desired β-seleno amine 2p in 62% yield. These aziridines commonly require severe reaction conditions to undergo ring opening (e.g., use of Lewis acid and reflux for several hours) affording the products in low yields and with poor regioselectivity.5b
Continuing our efforts to provide synthetic methods for the use of the industrial community, we attempted to reuse the ionic liquid, which was one of the prime objectives in our project. In this regard, we performed a set of experiments aiming to reuse the reaction media, Fig. 3. After the first reaction with 4-chloro diphenyl diselenide and aziridine 1d in [bmim]BF4 the ionic liquid was recovered and subjected to another run, affording the product in 98% yield. This process was repeated three more times, affording the desired product in excellent yields.25
![Recycling of [bmim]BF4. aYield for pure isolated products.](/image/article/2012/RA/c2ra21488a/c2ra21488a-f3.gif) | ||
| Fig. 3 Recycling of [bmim]BF4. aYield for pure isolated products. | ||
Similarly CuO nanopowder was also recycled and further reused for four runs and no loss of activity was observed providing the β-seleno amine in very good yields Table 4.25 A slight decrease in yield was observed for the last two runs (Table 4, entries 3 and 4).
In summary, we have described a simple and efficient protocol for the synthesis of structurally diverse chiral β-chalcogen amino derivatives via a ring opening reaction of protected and unprotected aziridines with diorganyl dichalcogenide in ionic liquid in a short reaction time promoted by CuO nanopowder. The corresponding products were obtained in good to excellent yields. Other noteworthy features of this methodology are: (i) the solvent/ionic liquid [bmim]BF4 acts as both promoter and recyclable reaction medium which offers better performance as compared to common organic solvents; (ii) use of low-loading CuO catalyst; (iii) recyclable CuO nanopowder and (iv) mild reaction conditions. Further investigations into the utility of this novel methodology are underway in our laboratory aiming at the synthesis of new chiral-chalcogen derivatives.
Acknowledgements
The authors thank CNPq, CAPES and FAPERGS (PRONEM-11/2080-9) for financial support. Syed M. Salman is grateful to TWAS-CNPq for the PhD fellowship. We are also obliged to CNPq (INCT Catálise, INCT_NANOBIOSIMES).References
- (a) K. B. Sharpless and R. F. Lauer, J. Am. Chem. Soc., 1972, 94, 7154 CrossRef CAS; (b) D. Seebach and N. Peleties, Chem. Ber., 1972, 105, 511 CrossRef CAS; (c) D. Seebach and A. K. Beck, Angew. Chem., Int. Ed. Engl., 1974, 13, 806 CrossRef; (d) H. J. J. Reich, J. Org. Chem., 1975, 40, 2570 CrossRef CAS; (e) M. Sevrin, D. Vanende and A. Krief, Tetrahedron Lett., 1976, 30, 2643 CrossRef; (f) M. Sevrin, W. Dumont, L. D. Hevesi and A. Krief, Tetrahedron Lett., 1976, 30, 2647 CrossRef; (g) A. L. Braga, A. Reckziegel, C. C. Silveira and J. V. Comasseto, Synth. Commun., 1994, 24, 1165 CrossRef CAS; (h) A. L. Braga, G. Zeni, L. H. Andrade and C. C. Silveira, Synlett, 1997, 595 CrossRef CAS; (i) C. C. Silveira, A. L. Braga, A. S. Vieira and G. Zeni, J. Org. Chem., 2003, 68, 662 CrossRef CAS; (j) M. R. Detty and M. A. Goodman, Synlett, 2006, 1100 CrossRef.
- (a) For a comprehensive review about the use of chiral organoselenium in asymmetric catalysis see: T. Wirth, Tetrahedron, 1999, 55, 1 CrossRef CAS; (b) T. Wirth, Angew. Chem., Int. Ed., 2000, 39, 3741 Search PubMed; (c) A. L. Braga, D. S. Lüdtke, F. Vargas and R. C. Braga, Synlett, 2006, 1453 CrossRef CAS.
- (a) G. Mugesh and H. Singh, Chem. Soc. Rev., 2000, 29, 347 RSC; (b) G. Mugesh, W. W. Du Mont and H. Sies, Chem. Rev., 2001, 101, 2125 CrossRef CAS; (c) C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255 CrossRef CAS; (d) B. K. Sarma and G. Mugesh, Org. Biomol. Chem., 2008, 6, 965 RSC; (e) E. E. Alberto, V. do Nascimento and A. L. Braga, J. Braz. Chem. Soc., 2010, 21, 2032 CrossRef CAS; (f) V. Nascimento, E. E. Alberto, D. W. Tondo, D. Dambrowski, M. R. Detty, F. Nome and A. L. Braga, J. Am. Chem. Soc., 2012, 134, 138 CrossRef CAS.
- Aziridines and Epoxides in Organic Synthesis; ed. A. K. Yudin, Wiley-VCH, Weinheim, Germany, 2006 Search PubMed.
- (a) W. H. Pearson, B. W. Lian and S. C. Bergmeier, In Aziridines and Azirines Monocyclic; Comprehensive Heterocyclic Chemistry II, ed. A. Padwa, Pergamon Press, Oxford, 1996, Vol. 1A, pp 1–60 Search PubMed; (b) X. E. Hu, Tetrahedron, 2004, 60, 2701 CrossRef CAS; (c) A. L. Braga, D. S. Lüdtke, M. W. Paixão, E. E. Alberto, H. A. Stefani and L. Juliano, Eur. J. Org. Chem., 2005, 4260 CrossRef CAS.
- (a) C. Mukherjee, P. Tiwari and A. K. Misra, Tetrahedron Lett., 2006, 47, 441 CrossRef CAS; (b) P. Tiwari and A. K. Misra, Tetrahedron Lett., 2006, 47, 2345 CrossRef CAS; (c) R. Caputo, S. Capone, M. D. Greca, L. Longobardo and G. Pinto, Tetrahedron Lett., 2007, 48, 1425 CrossRef CAS; (d) M. Abdo and S. Knapp, J. Am. Chem. Soc., 2008, 130, 9234 CrossRef CAS; (e) L. H. Andrade and A. V. Silva, Tetrahedron: Asymmetry, 2008, 19, 1175 CrossRef CAS; (f) Z. Rafinski and J. Scianowski, Tetrahedron: Asymmetry, 2008, 19, 1237 CrossRef CAS.
- (a) M. Miyashita, M. Hoshino and A. Yoshikoshi, Tetrahedron Lett., 1988, 29, 347 CrossRef CAS; (b) I. Andreadou, W. M. P. B. Menge, J. N. M. Commandeur, E. A. Worthington and N. P. E. Vermeulen, J. Med. Chem., 1996, 39, 2040 CrossRef CAS; (c) M. A. Goodman and M. R. Detty, Organometallics, 2004, 23, 3016 CrossRef CAS.
- (a) L. W. Bieber, A. C. P. F. Sá, P. H. Menezes and S. M. C. Gonçalves, Tetrahedron Lett., 2001, 42, 4597 CrossRef CAS; (b) F. M. Andrade, W. Massa, C. Peppe and W. Uhl, J. Organomet. Chem., 2005, 690, 1294 CrossRef; (c) B. Movassagh and M. Shamsipoor, Synlett, 2005, 121 CrossRef CAS; (d) C. Santi, S. Santoro, L. Testaferri and M. Tiecco, Synlett, 2008, 1471 CrossRef CAS.
- A. L. Braga, P. H. Schneider, M. W. Paixão and A. M. Deobald, Tetrahedron Lett., 2006, 47, 7195 CrossRef CAS.
- (a) B. C. Ranu, T. Mandal and S. Samanta, Org. Lett., 2003, 5, 1439 CrossRef CAS; (b) B. C. Ranu and T. Mandal, J. Org. Chem., 2004, 69, 5793 CrossRef CAS.
- (a) J. V. Comasseto, E. S. Lang, J. Tercio, B. Ferreira, F. Simonelli and V. R. Correia, J. Organomet. Chem., 1987, 334, 329 CrossRef CAS; (b) M. Yoshimatsu, T. Sato, H. Shimizu, M. Hori and T. Kataoka, J. Org. Chem., 1994, 59, 1011 CrossRef CAS; (c) R. K. Gujadhur and D. Venkataraman, Tetrahedron Lett., 2003, 44, 81 CrossRef CAS; (d) D. Sureshkumar, V. Ganesh, R. S. Vidyarini and S. Chandrasekaran, J. Org. Chem., 2009, 74, 7958 CrossRef CAS; (e) B. C. Ranu, A. Saha and T. Mandal, Tetrahedron, 2009, 65, 2072 CrossRef CAS; (f) A. L. Braga, P. H. Schneider, M. W. Paixão, A. D. Deobald, C. Peppe and D. P. Bottega, J. Org. Chem., 2006, 71, 4305 CrossRef CAS.
- (a) A. L. Braga, D. S. Lüdtke, M. W. Paixão and O. E. D. Rodrigues, Org. Lett., 2003, 5, 2635 CrossRef CAS; (b) A. L. Braga, M. W. Paixão and G. Marin, Synlett, 2005, 1675 CrossRef CAS; (c) A. L. Braga, R. S. Schwab, E. E. Alberto, S. M. Salman, J. Vargas and J. B. Azeredo, Tetrahedron Lett., 2009, 50, 2309 CrossRef CAS; (d) V. Ganesh and S. Chandrasekaran, Synthesis, 2009, 3267 CAS.
- S. M. Salman, R. S. Schwab, E. E. Alberto, J. Vargas, L. Dornelles, O. E. D. Rodrigues and A. L. Braga, Synlett, 2011, 69 CAS.
- (a) J. Dupont, R. F. Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667 CrossRef CAS; (b) C. C. Cassol, G. Ebeling, B. Ferrera and J. Dupont, Adv. Synth. Catal., 2006, 348, 243 CrossRef CAS; (c) J. Ranke, S. Stolte, R. Störmann, J. Arning and B. Jastorff, Chem. Rev., 2007, 107, 2183 CrossRef CAS; (d) P. Hapiot and C. Lagrost, Chem. Rev., 2008, 108, 2238 CrossRef CAS.
- (a) G. W. Kabalka and B. Venkataiah, Tetrahedron Lett., 2002, 43, 3703 CrossRef CAS; (b) D. Singh, E. E. Alberto, O. E. D. Rodrigues and A. L. Braga, Green Chem., 2009, 11, 1521 RSC; (c) S. Narayanaperumal, E. E. Alberto, F. M. Andrade, E. J. Lenardão, P. S. Taube and A. L. Braga, Org. Biomol. Chem., 2009, 7, 4647 RSC; (d) S. Narayanaperumal, E. E. Alberto, K. Gul, O. E. D. Rodrigues and A. L. Braga, J. Org. Chem., 2010, 75, 3886 CrossRef CAS; (e) D. Singh, S. Narayanaperumal, K. Gul, M. Godoi, O. E. D. Rodrigues and A. L. Braga, Green Chem., 2010, 12, 957 RSC; (f) G. Tabarelli, E. E. Alberto, A. M. Deobald, G. Marin, O. E. D. Rodrigues, L. Dornelles and A. L. Braga, Tetrahedron Lett., 2010, 51, 5728 CrossRef CAS; (g) R. S. Schwab, D. Singh, E. E. Alberto, P. Piquini, O. E. D. Rodrigues and A. L. Braga, Catal. Sci. Technol., 2011, 1, 569 RSC; (h) C. S. Freitas, A. M. Barcellos, V. G. Ricordi, J. M. Pena, G. Perin, R. G. Jacob, E. J. Lenardão and D. Alves, Green Chem., 2011, 13, 2931 RSC; (i) S. Narayanaperumal, E. E. Alberto, K. Gul, C. Y. Kawasoko, L. Dornelles, O. E. D. Rodrigues and A. L. Braga, Tetrahedron, 2011, 67, 4723 CrossRef CAS; (j) K. Gul, S. Narayanaperumal, L. Dornelles, O. E. D. Rodrigues and A. L. Braga, Tetrahedron Lett., 2011, 52, 3592 CrossRef CAS.
- (a) L. Rout, S. Jammi and T. Punniyamurthy, Org. Lett., 2007, 9, 3397 CrossRef CAS; (b) L. Rout, T. K. Sen and T. Punniyamurthy, Angew. Chem., Int. Ed., 2007, 46, 5583 CrossRef CAS; (c) M. Shibasaki and M. Kanai, Chem. Rev., 2008, 108, 2853 CrossRef CAS; (d) M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952 CrossRef CAS; (e) V. P. Reddy, A. V. K. K. Swapna and K. R. Rao, Org. Lett., 2009, 11, 951 CrossRef CAS; (f) A. Saha, D. Saha and B. C. Ranu, Org. Biomol. Chem., 2009, 7, 1652 RSC; (g) V. P. Reddy, A. V. Kumar, K. Swapna and K. R. Rao, Org. Lett., 2009, 11, 1697 CrossRef CAS; (h) S. Narayanaperumal, K. Gul, C. Y. Kawasoko, D. Singh, L. Dornelles, O. E. D. Rodrigues and A. L. Braga, J. Braz. Chem. Soc., 2010, 21, 2079 CrossRef CAS; (i) V. P. Reddy, A. V. Kumar and K. R. Rao, Chem. Lett., 2010, 39, 212 CrossRef CAS.
- D. Alves, C. G. Santos, M. W. Paixão, L. C. Soares, D. de Souza, O. E. D. Rodrigues and A. L. Braga, Tetrahedron Lett., 2009, 50, 6635 CrossRef CAS.
- (a) For selected examples see: P. H. Schneider, H. S. Schrekker, C. C. Silveira, L. A. Wessjohann and A. L. Braga, Eur. J. Org. Chem., 2004, 2715 CrossRef CAS; (b) A. L. Braga, D. S. Lüdtke, F. Vargas and M. W. Paixão, Chem. Commun., 2005, 2512 RSC; (c) A. L. Braga, J. A. Sehnem, F. Vargas and R. C. Braga, J. Org. Chem., 2005, 70, 9021 CrossRef CAS; (d) R. S. Schwab, F. Z. Galetto, J. B. Azeredo, A. L. Braga, D. S. Lüdtke and M. W. Paixão, Tetrahedron Lett., 2008, 49, 5094 CrossRef CAS; (e) R. S. Schwab, L. C. Soares, L. Dornelles, O. E. D. Rodrigues, M. W. Paixão, M. Godoi and A. L. Braga, Eur. J. Org. Chem., 2010, 3574 CrossRef CAS; (f) L. C. Soares, E. E. Alberto, R. S. Schwab, P. S. Taube, V. Nascimento, O. E. D. Rodrigues and A. L. Braga, Org. Biomol. Chem., 2012, 10, 6595 RSC.
- (a) Selected examples: R. M. Rosa, R. B. de Oliveira, J. Saffi, A. L. Braga, R. Roesler, F. D. Pizzol, J. C. F. Moreira, M. Brendel and J. A. P. Henriques, Life Sci., 2005, 77, 2398 CrossRef; (b) A. L. Braga, E. E. Alberto, L. C. Soares, J. B. T. Rocha, J. H. Sudati and D. H. Ross, Org. Biomol. Chem., 2009, 7, 43 RSC; (c) D. B. Santos, V. P. P. Schiar, M. C. P. Ribeiro, R. S. Schwab, D. F. Meinerz, J. Allebrandt, J. B. T. Rocha, C. W. Nogueira, M. Aschner and N. B. V. Barbosa, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2009, 676, 21 CrossRef CAS; (d) E. E. Alberto, L. L. Rossato, S. H. Alves, D. Alves and A. L. Braga, Org. Biomol. Chem., 2011, 9, 1001 RSC; (e) A. de S. Prestes, S. T. Stefanello, S. M. Salman, A. M. Pazini, R. S. Schwab, A. L. Braga, N. B. de V. Barbosa and J. B. T. Rocha, Mol. Cell. Biochem., 2012, 365, 85 CrossRef.
- M. A. P. Martins, C. P. Frizzo, D. N. Moreira, N. Zanatta and H. G. Bonacorso, Chem. Rev., 2008, 108, 2015 CrossRef CAS.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508 CrossRef CAS.
- S. T. Handy and M. Okello, J. Org. Chem., 2005, 70, 1915 CrossRef CAS.
- M.-H. Yang, G.-B. Yan and Y.-F. Zheng, Tetrahedron Lett., 2008, 49, 6471 CrossRef CAS.
- Typical procedure for the synthesis of chiral β-seleno amine 2a: In a Schlenk tube under Argon atmosphere at room temperature, CuO nanopowder (0.008 mmol, 5.0 mol%) followed by diphenyl diselenide (156 mg, 0.5 mmol) and KOH (1.0 mmol, 2.0 equiv) were added to a solution of aziridine 1a (287 mg, 1.0 mmol) in [bmim]BF4 (1.0 mL) and the temperature of the reaction was raised up to 80 °C. After completion of the reaction (monitored by TLC), the product was extracted by successive washing with diethyl ether (5 × 6 mL) and dried over MgSO4. The solvents and volatiles were completely removed under vacuum to give the crude product. The compound was purified by column chromatography over silica gel (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate, 80
ethyl acetate, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) furnishing the pure β-seleno amine (404 mg, 91%).
20) furnishing the pure β-seleno amine (404 mg, 91%). - Recycling of [bmim]BF4 and CuO nanopowder. The solvent [bmim]BF4 and CuO nanopowder can be recycled without loss of activity (Fig. 3 and Table 4). After completion of the reaction workup, the reaction mixture was treated with ethanol, and filtered through a Teflon membrane. The CuO nanopowder was recovered from the membrane by washing with water and collected by further centrifugation and drying under vacuum. It was reused for the next reaction, and no loss of activity was observed, providing the product in high yields. The ionic liquid was recovered from the ethanol (10 mL) after filtration, evaporation of the solvent and drying the [bmim]BF4 (1 mL) under vacuum for reuse in subsequent reactions.
Footnote |
| † Electronic Supplementary Information (ESI) available: Synthetic procedures and compound characterization data. See DOI: 10.1039/c2ra21488a |
| This journal is © The Royal Society of Chemistry 2012 |





