A study of the flavin response by Shewanella cultures in carbon-limited environments
Jared N.
Roy
a,
Heather R.
Luckarift
bc,
Carolin
Lau
a,
Akinbayowa
Falase
a,
Kristen E.
Garcia
a,
Linnea K.
Ista
a,
Privthiraj
Chellamuthu
d,
Ramaraja P.
Ramasamy
b,
Venkataramana
Gadhamshetty
b,
Greg
Wanger
e,
Yuri A.
Gorby
e,
Kenneth H.
Nealson
de,
Orianna
Bretschger
e,
Glenn R.
Johnson
b and
Plamen
Atanassov
*a
aDepartment of Chemical and Nuclear Engineering, Centre for Emerging Energy Technologies, The University of New Mexico, Albuquerque, 87131, NM. E-mail: plamen@unm.edu; Tel: +1 505 277-2640
bAirbase Sciences Branch, Air Force Research Laboratory, Tyndall Air Force Base, FL 32403
cUniversal Technology Corporation, Dayton, OH 45432
dDepartment of Earth Science, University of Southern California, Los Angeles, CA 90089
eThe J. Craig Venter Institute, San Diego, CA 92121
First published on 28th August 2012
Abstract
Mediated electron transfer has been implicated as a primary mechanism of extracellular electron transfer to insoluble electron acceptors in anaerobic cultures of the facultative anaerobe Shewanella oneidensis. In this work, planktonic and biofilm cultures of S. oneidensis exposed to carbon-limited environments trigger an electrochemical response thought to be the signature of an electrochemically active metabolite. This metabolite was detected via cyclic voltammetry for S. oneidensis MR-1 biofilms. The observed electrochemical potentials correspond to redox potentials of flavin-containing molecules. Chromatographic techniques were then used to quantify concentrations of riboflavin by the carbon-limited environmental response of planktonic S. oneidensis. Further evidence of flavin redox chemistry was associated with biofilm formation on multi-walled carbon nanotube-modified Toray paper under carbon-starved environments. By encapsulating one such electrode in silica, the encapsulated biofilm exhibits riboflavin redox activity earlier than a non-encapsulated system after media replacement. This work explores the electrochemical nature of riboflavin interaction with an electrode after secretion from S. oneidensis and in comparison to abiotic systems.
1. Introduction
Shewanella oneidensis MR-1 is a dissimilatory metal-reducing bacteria (DMRB) that is widely utilized as a model organism in microbial fuel cell (MFC) research.1–4S. oneidensis appears to use a combination of mechanisms for extracellular electron transfer (EET) to insoluble electron acceptors.5 These mechanisms include: (i) direct electron transfer (DET) through outer membrane cytochromes;6–9 (ii) direct electron conduction via extracellular appendages described as bacterial nanowires,10–12 and (iii) mediated electron transfer (MET) (or shuttling) through exogenous metabolites.13,14 Each of these mechanisms has been associated with electrochemical activity and, as such, exploited to produce power in MFCs.MFC power production is a fortuitous result of microbial metabolism in which the fuel cell performance is dictated by microbial EET processes, acting either individually or in concert. The ability to effectively transfer electrons is intrinsically linked to microbial metabolism and DMRB exhibit versatile EET mechanisms that allow the bacterium to respond to changes in physiological conditions.18 It is unclear, however, how EET mechanisms are controlled by physiological constraints or how environmental conditions may dictate each mode of EET.
Elucidating and understanding these relationships may lead to improved MFC systems by: 1) guiding the rational development of engineered surfaces to improve the physical association between microbes and products of metabolism; 2) determining microbial culture conditions that provide reproducible redox processes; and thereby 3) define optimal operational conditions for MFCs.
In this work we explore the phenomena of riboflavin production by MR-1 in electron donor-limited conditions,21 and investigate the influence of riboflavin redox chemistry within biofilms formed on the electrode. One inherent problem in the study of Shewanella spp. is the apparent rapid detachment of biomass from the biofilm under certain environmental stimuli.22 A method to artificially bind a culture to an electrode to mimic biofilm formation is investigated by immobilizing a defined population of MR-1 cells to an electrode by means of silica coating. This technique overcomes potential limitations of investigating riboflavin redox reactions within a S. oneidensis biofilm by preventing a loss of biomass during medium exchange. In either case, natural biofilm formation or silica immobilized biofilm formation, the subsequent adsorption of riboflavin onto electrode materials and the biofilm surface is predicted to dominate the electrochemical character of an MR-1 populated bio-anode under operating conditions defined within this study.23
The work described here further elucidates the role of riboflavin within Shewanella EET. In terms of MET, for example, studies originally reported by Marsili et al. and corroborated by others show that Shewanella spp. will use flavin compounds as a dominant redox mechanism in EET anaerobic respiration.14–17 Recently however, there is evidence to suggest the role of electron shuttles that mediate taxis of organisms towards insoluble electron acceptors; perhaps leading to biofilm formation on these surfaces once an environmental stimulus is present; for example, carbon or oxygen limitation, as described herein. A mechanism to hydrolyse cytoplasmic synthesized flavin mononucleotide (FMN) to flavin adenine dinucleotide (FAD) and riboflavin has also recently been described via the periplasmic protein UshA.16 This supports the idea of metabolic release of riboflavin into the environment where these environmentally bound flavins may serve in cell-to-cell signalling, sensing of redox gradients, or other non-respiratory functions.15 Furthermore, an idea of mediated energy taxis has been proposed in which S. oneidensis not only uses riboflavin as a mediator but as a signal to direct cell populations to insoluble electron acceptors.19 This idea becomes significant in the context of Geobacter biofilms, which have been shown to produce higher current densities compared to biofilms of Shewanella.20 As of yet, no such electrochemical endogenous metabolite has been identified for Geobacter biofilm formation.
2. Experimental
2.1 Bacterial strain and culture conditions
MR-1 was inoculated from frozen culture stocks onto tryptic soy (Sigma-Aldrich 22092) agar plates and incubated at 30 °C. Colonies were then re-cultured in Luria–Bertani (LB) (Sigma-Aldrich L3022) broth and incubated aerobically for approximately 18 h and then washed in 50 mM sodium phosphate buffer (pH 7). All glassware and media were autoclaved for 20 min at 15 psi and 121 °C prior to use. Experimental cultures of S. oneidensis MR-1 were grown in chemically defined medium (M1), from an original specification.24 This composition included 30 mM sodium fumarate for oxygen-limited cultures. Note: the vitamin solution used in the chemically defined medium contains no riboflavin. Washed (8000 rpm for 5 min, 3 repeats) LB cultures of MR-1 were sub-cultured into flasks containing chemically defined medium and were capped with a rubber stopper and incubated for approximately 48–60 h (30 °C, 150 rpm) until the optical density at 600 nm was ∼0.3. These planktonic cultures were then washed in 50 mM sodium phosphate buffer and re-suspended in the buffer to an optical density of 0.3 at 600 nm. Electrodes were incubated in this solution (exposed to filtered ambient air) for 24 h at 30 °C for biofilm formation. Note: the culture at this point is under carbon limitation.Cell counts were determined by conventional serial dilution, plating and colony counting to determine colony forming units per mL (CFU mL−1).
2.2 Electrode preparation and population
Toray® carbon paper TGP-H-060 (TP; Fuel Cell Earth, Stoneham, MA) was augmented with multi-walled carbon nanotubes (TP-CNT) using a modified technique described previously.25 Briefly, TP was plasma-treated for 12 s, and then placed in a standard 3-electrode configuration electrochemical cell with TP as the working electrode, a nickel mesh counter electrode and Ag/AgCl (saturated KCl) reference electrode (CH Instruments Inc.). The electrolyte contained an equimolar (14 mM) mixture of cobalt acetate, nickel acetate and boric acid in deionized (DI) water and metal nanoparticles were deposited on the TP by subjecting the cell to −1.3 V for 12 s. The paper was removed and sonicated in DI water, dried and placed in a tube furnace (Lindberg/Blue model TF55035A). TP samples were reduced in 5% hydrogen/95% nitrogen atmosphere and the temperature ramped from room temperature to 620 °C, at which point the H2 feed was replaced with 33% ethylene in nitrogen for the remainder of the temperature ramp to 750 °C, and held for 1 h to allow CNT growth.TP-CNT electrodes were again plasma treated for 10 s to sterilize the surface and to increase surface wettability. TP-CNT electrodes were incubated in 10 mL of an MR-1 cell suspension in sodium phosphate buffer overnight at 30 °C with no agitation. The culture is starved of a carbon source at this time. Following biofilm development, the electrodes were removed and used in electrochemical characterization experiments and visualized by scanning electron microscopy (SEM). Biofilm coated TP-CNT electrodes were further encapsulated in silica by chemical vapor deposition (CVD) of tetramethyl orthosilicate (TMOS) as described previously.26
2.3 Bioreactor cultures
M1 growth medium (pH 7.0) was used in all bioreactor studies. MR-1 plates were previously prepared and colonies were washed from the plate using M1 growth medium and used as the bioreactor inoculum.The bioreactor was a Bioflow 110 (New England Biosciences) operated in semi-batch mode at 30 °C, with stirring (600 rpm) and maintained at pH 7.0 (by addition of 1 N HCl). The gas flow into the bioreactor was controlled at 5% dissolved oxygen by mixing ultra high pure N2 and compressed air. Cell density was monitored by measuring optical density of the culture at 600 nm. To identify physiological conditions that affect riboflavin production, the bioreactor was also operated in semi-batch mode under anaerobic conditions; at which time 40 mM of sodium fumarate was added to the medium as the terminal electron acceptor.
2.4 Riboflavin quantification
Culture samples were periodically (and aseptically) removed from the bioreactor and clarified by filtration (0.2 μm). The concentration of riboflavin was measured using a 96 well fluorescent plate reader (FlexStation 3; Molecular Devices) with an excitation wavelength of 440 nm and observed fluorescence measured at 520 nm. Riboflavin concentrations are reported as the mean of 10 replicate samples and correlated to a standard curve (in the range 25 nM to 1 mM).2.5 HPLC analysis
Organic acid content of culture samples was determined using high pressure liquid chromatography (HPLC). Filter-clarified supernatant was acidified to a final concentration of 12.5 mM H2SO4 and analyzed using an Agilent HPLC (Agilent 1200, Agilent) with a reverse phase C18 column (Synergi-Hydro, Phenomenex). Chromatographs were generated using a multiwavelength detector (Agilent 1200, Agilent) set to 210 nm. A 20 μL sample of each electrolyte was injected into the sample loop and eluted using an isocratic sulfuric acid mobile phase (2.5 mM, pH 2.0) at a flow rate of 0.5 mL min−1 and at a column temperature of 25 °C. Peak areas were integrated and concentrations calculated by comparison to peak areas and retention times of known standards for lactate, pyruvate, and acetate.2.6 Electrochemical testing
All electrochemical measurements were conducted in a standard 3-electrode electrochemical cell containing a platinum wire as the counter electrode and Ag/AgCl (3 M KCl) reference electrode in 10 mL of sodium phosphate buffer (50 mM, pH 7.0), with 20 mM sodium lactate as the carbon source. TP-CNT electrodes populated with MR-1 biofilms were secured to glassy carbon (GC) rods (CH Instruments Inc., 3 mm diameter) as the working electrode. All electrochemical measurements were obtained using a Gamry 600 Potentiostat/Galvanostat/ZRA. The presence of riboflavin was determined by cyclic voltammetry (CV) (from −0.60 V to −0.30 V) at a 10 mV s−1 scan rate. The diffusive behaviour of riboflavin was characterized by varying the scan rate from 0.20 V s−1 to 0.001 V s−1. For the purposes of this study, peaks at approximately −0.45 V are of interest, as defined by the theoretical potential for riboflavin at pH 7.0.14,17 Measurements were taken in an electrochemical cell purged with N2; but exposed to ambient air. This system design creates a micro-aerobic environment since full removal of dissolved O2 is not required.2.7 Silica encapsulation
Washed cell suspensions were physically adsorbed to TP-CNT electrodes and exposed to 100 μL of TMOS in the vapour phase for 10 min. The TMOS precursor undergoes hydrolysis in aqueous solvent upon contact with high salt concentrations in the liquid medium and leads to sol–gel and silica particulate formation at the substrate surface.272.8 Scanning electron microscopy
For SEM analysis, cells were chemically fixed on the electrode in a 2.5% glutaraldehyde solution for approximately 12 h and then exposed to increasing concentrations of ethanol, culminating in three final washes at 100% ethanol. The samples were then subjected to critical point drying,11,24 using an automated critical point dryer, (Seevac Inc., Florida, USA) before coating with a 5 nm layer of gold–palladium using an Emitech K950X sputter coater (Emitech, USA). Fixed samples were viewed using an FEI Quanta 3D FEG microscope (FEI Company, Hillsboro, Oregon) at accelerating voltages ranging from 5–10 kV and the elemental composition was characterized by energy-dispersive X-ray spectroscopy (EDS) analysis.2.9 Electrochemical characteristics of riboflavin
The number of electrons involved in the redox processes observed for riboflavin was calculated using the following expression: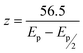 | (1) |
Where the peak potential (Ep) and half peak potential (Ep/2) are expressed in millivolts. Using the calculated value of z, the number of moles of oxidized or reduced electrochemical species was calculated using Faraday's Law:
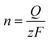 | (2) |
Where charge (Q) is obtained from analyzing the redox peaks from CV. Since the standard contains a known bulk concentration of riboflavin (Cb), the apparent diffusion coefficient (Dapp), of riboflavin was estimated using the expression:
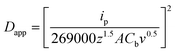 | (3) |
Where A is the porous area of the electrode, ν is the sweep rate in mV s−1, and ip is the peak current density. Finally the redox potential (Eredox) was obtained by midpoint evaluation.
The peak potential (Ep), peak current (Ip), half peak potential (Ep/2), and charge (Q) were obtained by analysis of the redox peaks, while the number of electrons (z), moles of riboflavin reacted (n), apparent diffusivity (Dapp) and redox potential (Eredox) were obtained by calculation.28
3. Results and discussion
3.1 Riboflavin production in cell cultures
During time course experiments, riboflavin production was observed to accumulate as a function of physiological state and population density (Fig. 1). In addition, results indicate that riboflavin production increases with respect to a defined cell density; particularly under micro-aerobic growth conditions (i.e. 5% dissolved oxygen).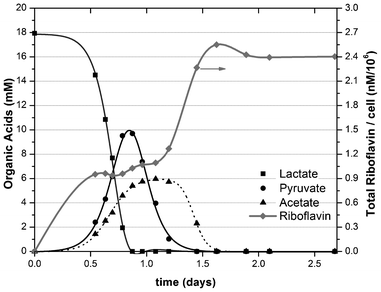 | ||
| Fig. 1 Conversion of lactate to oxidation products (acetate and pyruvate) and production of riboflavin during cell growth of S. oneidensis MR-1. | ||
The maximum concentration of riboflavin produced was 391 nM, which corresponded with the stationary phase of cell growth (cell density of 1.6 × 108 CFU mL−1). HPLC analysis of culture supernatants confirmed that volatile fatty acids were completely oxidized at this point and indicated that maximum levels of riboflavin production occurred immediately after all electron donors were consumed. This correlation may imply that riboflavin production in S. oneidensis is a response to intracellular carbon fluxes as has been proposed for other bacterial species.21 The onset of riboflavin production corresponds with depleted lactate at a point when acetate and pyruvate oxidation by-products begin to accumulate within the culture. A second kinetic increase in riboflavin production then occurs after pyruvate was consumed and acetate concentrations began to decrease (Fig. 1). Riboflavin production reaches a stable maxima (equivalent to 2.55 nM cell−1) once residual acetate has been consumed.
In contrast, when sufficient concentrations of an alternative electron acceptor are present in anaerobic cultures (e.g. sodium fumarate), no riboflavin is synthesized by S. oneidensis (data not shown). Under steady state anaerobic conditions, cell density decreased to ∼0.6 × 108 CFU mL−1 and riboflavin was no longer detected in the medium (considering a fluorescence detection limit of 25 nM). The correlation between riboflavin production and the presence of oxygen as an electron acceptor is in agreement with previous studies showing that aerobic conditions promote higher riboflavin and FMN production in MR-1.29 However, the results are incongruent with the idea that flavin molecules play a role in mediated electron transfer to insoluble electron acceptors under anaerobic conditions, e.g. in the MFC anode.
3.2 Abiotic riboflavin/electrode interaction
To understand the proposed biocatalytic interactions of microbial excreted riboflavin and the electrode, we must first consider any abiotic association between riboflavin and the electrode in an electrochemical system. The electrochemical characteristics of riboflavin were determined by CV studies and used to demonstrate the relationship between riboflavin concentration and redox activity in sterile electrolyte. The riboflavin concentrations selected for study were based on molar amounts typically reported in MFC studies.30 The symmetrical response of CV anodic and cathodic sweeps irrespective of riboflavin concentration indicates fully reversible redox behaviour (Fig. 2A). The redox potential (Eredox) for riboflavin was −0.45 V vs. Ag/AgCl for all concentrations investigated, in agreement with the standard thermodynamic potential of the flavin molecule.31 The amperometric response was proportional to riboflavin concentration and this behaviour is apparent once the non-Faradaic background capacitance of the graphite felt electrode is subtracted from the data set (Fig. 2A). The relationship between the reduced and oxidized species at the onset of electrode polarization (noxidized/nreduced) was consistent (∼1.26) and indicated that riboflavin in an abiotic system yields a reversible electrochemical response that can serve as a baseline for comparison to measurements in a microbial biofilm.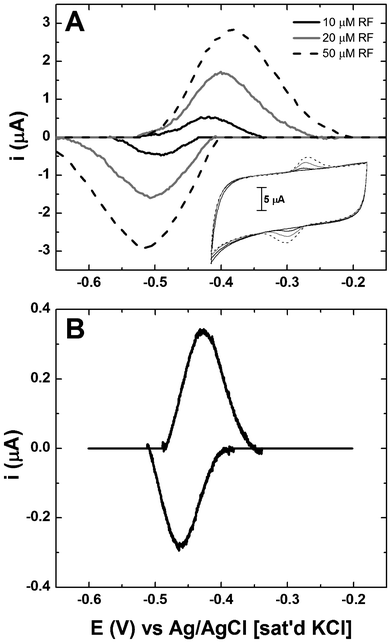 | ||
| Fig. 2 Riboflavin voltammetry associated with (A) abiotic systems containing known concentrations in the bulk and (B) associated with S. oneidensis MR-1 biofilms on TP-CNT. Data in panels A and B is shown with background subtraction to remove the effects of capacitance. Inset to panel A shows CV of riboflavin without background subtraction. | ||
These results confirm that CV can be used as a quantitative tool to analyse the electrochemical response of riboflavin. Parametric analysis was then performed on biofilms by extracting electrochemical parameters from CV and comparing the data to that of the flavin standards (Table 1). For consistency only the region between −0.60 V and −0.30 V was considered for peak analysis; the region above −0.30 V was discarded from analysis because of the mixed contribution from metabolite oxidation and other non-mediator related processes in this region. Although the riboflavin redox reaction theoretically involves two electrons, the actual redox response from measurement of standard solutions yielded values ranging from 0.73–1.38 and indicate a low Faradaic conversion of the compound. The empirical calculation of the number of electrons involved in the redox reaction of riboflavin appears to decrease with increasing riboflavin concentration. The apparent diffusivity (Dapp) was estimated to be between 0.6–4 × 10−8 cm2 s−1, which compares favourably with values reported in the literature.32 Since Dapp was empirically obtained based on peak current (ip), factors such as surface-bound riboflavin, ionic properties of the electrolyte, activation of the graphite felt, transfer coefficients and exchange current density may all indirectly influence Dapp but are not directly addressed by eqn (3). The analysis does confirm, however, that riboflavin is an electrochemically active species when interacting with carbonaceous materials and that in the presence of a biofilm is seen to accumulate at concentrations within the detectable range.33,34
| Extracted parameters | 10 μM Riboflavin | 20 μM Riboflavin | 50 μM Riboflavin | MR-1 on TP-CNT | ||||
|---|---|---|---|---|---|---|---|---|
| Oxidation | Reduction | Oxidation | Reduction | Oxidation | Reduction | Oxidation | Reduction | |
| a Two values of Dapp were determined from each CV, one for the diffusion of reduced flavin from the electrolyte to the electrode surface (oxidation) and a second for the diffusion of oxidized metabolite away from the electrode surface into the electrolyte (reduction). | ||||||||
| E p (V) | −0.418 | −0.491 | −0.400 | −0.507 | −0.382 | −0.516 | −0.433 | −0.465 |
| I p (μA) | 0.59 | 0.62 | 1.70 | 1.76 | 2.89 | 3.09 | 0.48 | 0.344 |
| E p − Ep/2 (mV) | 41 | 46 | 47 | 55 | 65 | 75 | 24 | 32 |
| z | 1.38 | 1.23 | 1.20 | 1.03 | 0.87 | 0.75 | 2.35 | 1.76 |
| Q (μC) | 5.7 | 6.4 | 20.3 | 22.3 | 42.3 | 47.2 | 2.84 | 2.12 |
| n (mol) | 0.4 × 10−10 | 0.5 × 10−10 | 1.8 × 10−10 | 2.2 × 10−10 | 5.0 × 10−10 | 6.5 × 10−10 | 1.3 × 10−11 | 1.2 × 10−11 |
| D app (cm2 s−1)a | 0.6 × 10−8 | 0.9 × 10−8 | 1.9 × 10−8 | 3.2 × 10−8 | 2.3 × 10−8 | 4.0 × 10−8 | ||
| I p,c/ip,a | 1.05 | 1.04 | 1.07 | 0.72 | ||||
| n oxidized/nreduced | 1.25 | 1.22 | 1.30 | 0.92 | ||||
3.3 Microbial riboflavin production and electrode interaction
Riboflavin production in MR-1 biofilms (on TP-CNT) was confirmed by CV (typical example shown in Fig. 2B) where distinct oxidation and reduction peaks were observed at −0.45 V vs. Ag/AgCl in agreement with riboflavin standards (Table 1). As no exogenous riboflavin is added to the system, the redox-active flavin is clearly synthesized by the biofilm.35,36 Peak current was observed to correlate with riboflavin concentration, and when plotted and integrated using linear regression (oxidation current slope = 0.053 ± 0.014, r2 = 0.933; reduction current slope = 0.057 ± 0.013, r2 = 0.945) can be used to extrapolate an apparent concentration of riboflavin in the biofilm of ∼85 μM (79.6–90.9). The measurement can only be deemed “apparent” as no consideration is taken for surface-confined effects within the biofilm compared to bulk diffusion of riboflavin in standard solution measurements.When considering the relationship between reduced and oxidized species (noxidized/nreduced), values >1 indicate a higher concentration of the oxidized species, with the opposite being true of values less than 1. For the abiotic systems, noxidized/nreduced was greater than 1 and constant at ∼1.26 for all evaluated concentrations of riboflavin in the bulk media. In comparison, noxidized/nreduced for a biofilm-modified electrode was less than 1, indicating a higher concentration of the reduced species. This observation is consistent with the hypothesis that biologically secreted riboflavin leaves the cell in a reduced form either by interaction with terminal electron acceptor proteins or by some other unknown mechanism.36 Consequently the reduced riboflavin interacts with the electrode through indirect electron transfer.15,37
3.4 Silica encapsulated vs. natural biofilms
In previous work within our group, microbial fuel cell anodes were prepared by capturing biofilms in a silica matrix that provides enhanced operational longevity by preventing a loss of biomass. For this study, silica-encapsulated biofilms were again used and results indicated earlier electrochemical activity associated with riboflavin in comparison to non-encapsulated biofilms after media replacement. These silica-encapsulated biofilms displayed increased current density that was initially attributed to a high cell density. Subsequent studies, however, and further analysis indicated that the enhanced current density of the silica-encapsulated system may be attributed to accumulation of secreted riboflavin at the electrode surface and subsequent increased surface area due to the silica matrix allowing for increased riboflavin adsorption. Therefore to examine the diffusive and adsorptive behaviour of riboflavin, cyclic voltammetry was evaluated for biofilms on TP-CNT electrodes that were populated naturally and compared to biofilms artificially bound to the electrode via silica encapsulation.Physical and chemical characterization of the anodes identified distinctions between the two populations. When incubated with TP-CNT, MR-1 forms a biofilm on the material surface (Fig. 3A). The distribution of individual cells indicates that cells integrate with the CNT structures directly. Following silica encapsulation of the biofilm, EDS confirmed the introduction of silica and oxygen compared to a non-silica control (data not shown). Furthermore, SEM images of the silica-encapsulated biofilm revealed the preserved microbial cells and extracellular structures (Fig. 3B).
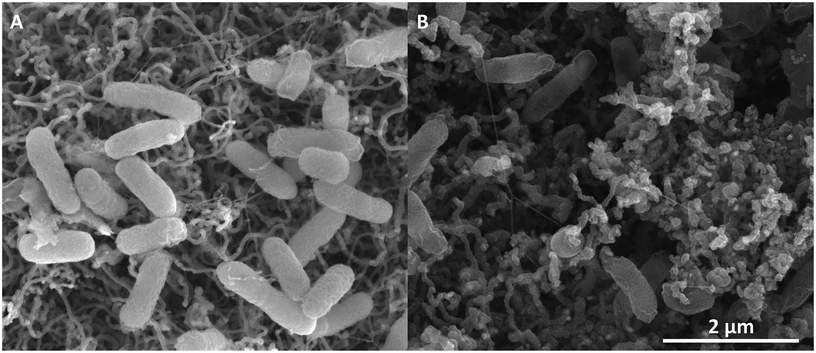 | ||
| Fig. 3 SEM image of carbon source limited S. oneidensis MR-1 natural biofilms on TP-CNT (A) and silica-encapsulated cells (B). | ||
Both natural and silica-encapsulated biofilms demonstrate reversible peaks at a midpoint potential of −0.45 V irrespective of scan rate (Fig. 4). Peak currents plotted against scan rate yield a linear dependence (Fig. 4B), from which we conclude that a significant concentration of riboflavin must be adsorbed to the electrode surface in agreement with the Laviron model.38
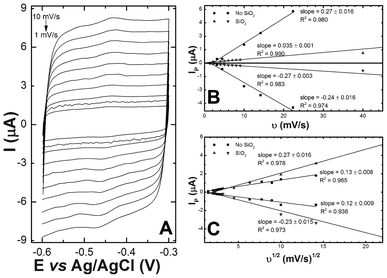 | ||
| Fig. 4 (A) Cyclic voltammetry of riboflavin associated with S. oneidensis MR-1 with varying scan rates. Peak currents for both natural biofilm and silica-encapsulated biofilms plotted against (B) the scan rate and (C) the square root of the scan rate. | ||
Additionally, the peak currents of each oxidative and reductive peak were plotted against the square root of the scan rate, yielding linear relationships in both electrode systems and indicating diffusion-based limitation (Fig. 4C). While the two observations appear to be contradictory, it is possible that the redox process could be controlled by the diffusion of counter ions to maintain electro-neutrality on the electrode surface.34,39
Observations from previous studies outside of this group provide information suggesting that the removal of surrounding medium reduces electrocatalytic activity of the anode by removal of the mediator, riboflavin. Electrodes housing naturally formed biofilms and silica-encapsulated biofilms underwent medium replacement with carbon substrate-free electrolyte. CVs of the natural biofilm did not yield any redox peaks at the onset. After 24 h of residence time in the electrochemical cell containing no carbon source, however, oxidation and reduction peaks appear near a midpoint potential of −0.45 V indicating the accumulation of riboflavin at the electrode surface (Fig. 5A). Additionally, the capacitive current of the system increased despite the lack of essential compounds required for cell proliferation. This increase in electrode capacitance was attributed to the microbial secretion of riboflavin and the associated redox behaviour of that metabolite with the electrode under carbon-limiting conditions. In comparison, MR-1 populated electrodes that had been encapsulated in silica exhibited a fully reversible redox peak at approximately −0.45 V vs. Ag/AgCl (Fig. 5B) from the onset. After 24 h residence time, the capacitive current of the system increased as observed for the natural biofilm. In effect, the natural biofilm must reach a cell density sufficient to act as a binding matrix for the riboflavin, with the density of the natural biofilm being compromised during media replacement. When the biofilm is artificially prepared by silica immobilization, the cell density is high from the onset and any riboflavin production is readily captured by the dense cell and silica matrix. Once the biofilm population stabilizes, electrocatalytic performance is comparable for both systems.
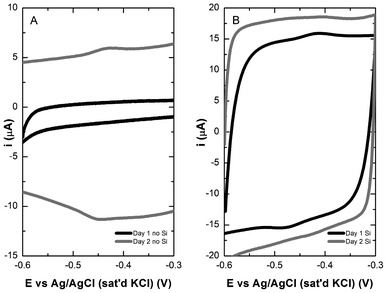 | ||
| Fig. 5 Cyclic voltammetry of S. oneidensis MR-1 biofilm (A) and silica-encapsulated biofilm (B) on TP-CNT after 1 (black) and 2 (grey) days in a carbon limited environment. | ||
3.5 Electrode reduction performance influenced by riboflavin
Silica-encapsulated and natural biofilm populated electrodes were analysed by galvanostatic polarization to evaluate the performance in terms of current density of the electrode systems over time (Fig. 6). At the onset of medium replacement (M1 medium), the natural biofilm anode exhibits an open circuit potential (OCP) of approximately −0.15 V vs. Ag/AgCl that increases dramatically over the following 24 h to a final OCP approaching −0.35 V, consistent with the establishment of a stable, electrochemically active biofilm. Prolonged incubation results in a slight decrease of the OCP (−0.32 V) that may be attributed to diffusion and mass transfer limitations across such a dense cellular matrix. The silica-encapsulated biofilm electrode exhibits an OCP of approximately −0.37 V immediately after medium replacement. The galvanostatic polarization curves vary little over the following 2 days, but again on day 4, a slight decrease in anodic performance is observed. These results indicate that the silica film was able to retain concentrations of biomass and potentially riboflavin that affect the performance of the electrode. In summary, when comparing the two systems, the natural biofilm requires ∼24 h after media replacement to re-establish critical biomass on the electrode for measurable performance difference. At that point, riboflavin is present but the relationship between EET processes is vague. The silica-encapsulated electrode, however, shows superior electrocatalytic activity at the onset due to the preservation of the biomass on the surface. In fact, silica encapsulation produced current output at the onset that surpassed that of the natural biofilm. Furthermore, the initial OCP for the silica-encapsulated electrode indicates that riboflavin may be retained on or near the electrode surface, as the OCP measurements approached the midpoint potential of riboflavin.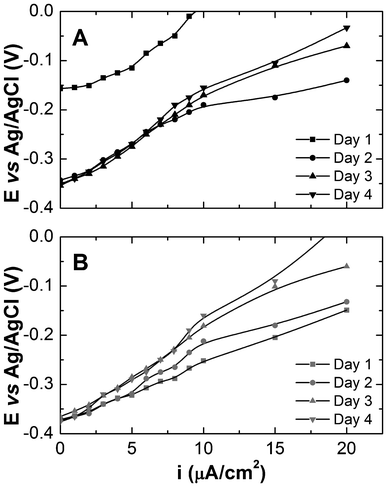 | ||
| Fig. 6 Polarization curves for S. oneidensis MR-1 biofilms without (A) and with silica encapsulation (B). | ||
Silica-encapsulation may also induce changes in the micro-environment relative to electron donor availability, pH, biofilm life-cycle, and oxygen diffusion that will unequivocally influence physiology and in turn, likely affect electrochemical response. Future explorations should address these factors as they relate to improved electrochemical performance of encapsulated biofilms. Nonetheless, the data collected here suggest that silica encapsulation may inhibit electrochemically active metabolite diffusion from the surface, and provide increased surface area for riboflavin adsorption.
4. Conclusions
The results within this study provide insight into the phenomenon of extracellular riboflavin associated with S. onidensis MR-1 metabolism and subsequent electrochemical interaction on the electrode. Riboflavin production appears at a maximum for S. oneidensis, under micro-aerobic and electron donor-limiting conditions. Riboflavin production clearly varies in response to specific growth condition (e.g. aerobic vs. anaerobic conditions, electron donor versus electron acceptor limitations) suggesting that riboflavin synthesis or release is regulated and responsive to the physiological state of the organism. The redox behaviour of riboflavin under abiotic conditions was essentially identical to the populated anodes.The electrochemical response after the MFC medium (electrolyte) was removed and replaced was consistent with the presence of a soluble redox mediator. In biotic systems with an established biofilm on an electrode, removal of the surrounding medium results in a significant decrease in electrochemical activity. This could be attributed to the loss of riboflavin near the surface of the electrode, the perturbation of the biofilm from the act of media replacement, or both. After subsequent incubation in fresh electrolyte that is free of carbon substrate, the capacitance of the electrode is increased and riboflavin redox chemistry is observed after a refractory period. The refractory time is somewhat overcome with silica-encapsulated cells on the electrode surface. The silica likely limits perturbation of the biofilm by media replacement and preserves electrochemical activity. For both encapsulated and non-encapsulated biofilms there is no exogenous carbon source in solution during the 24 hour retention time. Despite this, the capacitive currents of the voltammograms increases, and the concentration of riboflavin appears to increase, further suggesting that S. oneidensis riboflavin production is stimulated by carbon limitation. Based on these observations we speculate that riboflavin secretion is a response to changes in local environment other than electron acceptor availability. Accordingly, the organism has not adapted a regulatory mechanism that promotes riboflavin-mediated electron transfer dominating in anaerobic environments. Instead, the special environmental circumstances of the MFC allow S. onedensis to take advantage of riboflavin as a redox shuttle for indirect extra-cellular electron transfer to insoluble electron acceptors.
Acknowledgements
This study was funded by the Material Science Directorate of the U.S. Air Force Research Laboratory and the Air Force Office of Scientific Research. Venkataramana Gadhamshetty was supported through a postdoctoral fellowship from Oak Ridge Institute for Science and Education. Jared Roy was supported through a pre-doctoral fellowship from Oak Ridge Institute for Science and Education.References
- B. E. Logan, Nat. Rev. Microbiol., 2009, 7, 375 CrossRef CAS PubMed.
- D. R. Lovley, Nat. Rev. Microbiol., 2006, 4, 497 CrossRef CAS PubMed.
- C. Myers and K. Nealson, Science, 1988, 240, 1319 CAS.
- V. J. Watson and B. E. Logan, Biotechnol. Bioeng., 2010, 105, 489 CrossRef CAS PubMed.
- D. R. Lovley, Curr. Opin. Biotechnol., 2008, 19, 564 CrossRef CAS PubMed.
- R. A. Bouhenni, G. J. Vora, J. C. Biffinger, S. Shirodkar, K. Brockman, R. Ray, P. Wu, B. J. Johnson, E. M. Biddle, M. J. Marshall, L. A. Fitzgerald, B. J. Little, J. K. Fredrickson, A. S. Beliaev, B. R. Ringeisen and D. A. Saffarini, Electroanalysis, 2010, 22, 856 CrossRef CAS.
- B. E. Logan and J. M. Regan, Trends Microbiol., 2006, 14, 512 CrossRef CAS PubMed.
- C. R. Myers and J. M. Myers, J. Bacteriol., 1992, 174, 3429 CAS.
- C. R. Myers and J. M. Myers, Lett. Appl. Microbiol., 2003, 37, 254 CrossRef CAS PubMed.
- M. Y. El-Naggar, G. Wanger, K. M. Leung, T. D. Yuzvinsky, G. Southam, J. Yang, W. M. Lau, K. H. Nealson and Y. A. Gorby, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 18127 CrossRef CAS PubMed.
- Y. A. Gorby, S. Yanina, J. S. McLean, K. M. Rosso, D. Moyles, A. Dohnalkova, T. J. Beveridge, I. S. Chang, B. H. Kim, K. S. Kim, D. E. Culley, S. B. Reed, M. F. Romine, D. A. Saffarini, E. A. Hill, L. Shi, D. A. Elias, D. W. Kennedy, G. Pinchuk, K. Watanabe, S. a. i. Ishii, B. Logan, K. H. Nealson and J. K. Fredrickson, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 11358 CrossRef CAS PubMed.
- R. Ray, S. Lizewski, L. A. Fitzgerald, B. Little and B. R. Ringeisen, J. Microbiol. Methods, 2010, 82, 187 CrossRef CAS PubMed.
- Y. Gorby, J. Mclean, A. Korenevsky, K. Rosso, M. El-Naggar and T. Beveridge, Geobiology, 2008, 6, 232 CrossRef CAS PubMed.
- E. Marsili, D. B. Baron, I. D. Shikhare, D. Coursolle, J. A. Gralnick and D. R. Bond, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 3968 CrossRef CAS PubMed.
- D. Coursolle, D. B. Baron, D. R. Bond and J. A. Gralnick, J. Bacteriol., 2009, 192, 467 CrossRef PubMed.
- E. D. Covington, C. B. Gelbmann, N. J. Kotloski and J. A. Gralnick, Mol. Microbiol., 2010, 78, 519 CrossRef CAS PubMed.
- H. von Canstein, J. Ogawa, S. Shimizu and J. R. Lloyd, Appl. Environ. Microbiol., 2008, 74, 615 CrossRef CAS PubMed.
- R. P. Ramasamy, V. Gadhamshetty, L. J. Nadeau and G. R. Johnson, Biotechnol. Bioeng., 2009, 104, 882 CrossRef CAS PubMed.
- R. Li, J. M. Tiedje, C. Chiu and R. M. Worden, Environ. Sci. Technol., 2012, 46, 2813 CrossRef CAS PubMed.
- R. Saravanan, P. Kavanagh, V. O'Flaherty, D. Leech and K. P. Katrui, Langmuir, 2012, 28, 7904 CrossRef PubMed.
- M. Dauner, M. Sonderegger, M. Hochuli, T. Szyperski, K. Wuthrich, H. Hohmann, U. Sauer and J. Bailey, Appl. Environ. Microbiol., 2002, 68, 1760 CrossRef CAS PubMed.
- K. Thormann, R. Saville, S. Shukla and A. Spormann, J. Bacteriol., 2005, 187, 1014 CrossRef CAS PubMed.
- S. Berchmans and R. Vijayavalli, Langmuir, 1995, 11, 286 CrossRef CAS.
- O. Bretschger, A. Obraztsova, C. A. Sturm, I. S. Chang, Y. A. Gorby, S. B. Reed, D. E. Culley, C. L. Reardon, S. Barua, M. F. Romine, J. Zhou, A. S. Beliaev, R. Bouhenni, D. Saffarini, F. Mansfeld, B. H. Kim, J. K. Fredrickson and K. H. Nealson, Appl. Environ. Microbiol., 2007, 73, 7003 CrossRef CAS PubMed.
- C. Wang, M. Waje, X. Wang, J. M. Tang, R. C. Haddon and Y. S. Yan, Nano Lett., 2004, 4, 345 CrossRef CAS.
- G. Gupta, S. B. Rathod, K. W. Staggs, L. K. Ista, K. Abbou Oucherif, P. B. Atanassov, M. S. Tartis, G. A. Montaño and G. P. López, Langmuir, 2009, 25, 13322 CrossRef CAS PubMed.
- H. R. Luckarift, S. R. Sizemore, J. Roy, C. Lau, G. Gupta, P. Atanassov and G. R. Johnson, Chem. Commun., 2010, 46, 6048 RSC.
- A. Bard and L. Faulkner (ed.), John Wiley and Sons, 2001, 833.
- H. Wang, K. Hollywood, R. M. Jarvis, J. R. Lloyd and R. Goodacre, Appl. Environ. Microbiol., 2010, 76, 6266 CrossRef CAS PubMed.
- S. B. Velasquez-Orta, I. M. Head, T. P. Curtis, K. Scott, J. R. Lloyd and H. von Canstein, Appl. Microbiol. Biotechnol., 2010, 85, 1373 CrossRef CAS PubMed.
- C. Walsh, Acc. Chem. Res., 1980, 13, 148 CrossRef CAS.
- C. Bonazzola and E. Calvo, J. Electroanal. Chem., 1998, 449, 111 CrossRef CAS.
- A. Albert, Biochem. J, 1950, 47 Search PubMed , xxv.
- A. Pereira, A. d. S. Santos and L. T. Kubota, J. Colloid Interface Sci., 2003, 265, 351 CrossRef CAS PubMed.
- R. P. Ramasamy, Z. Ren, M. M. Mench and J. M. Regan, Biotechnol. Bioeng., 2008, 101, 101 CrossRef CAS PubMed.
- L. Shi, D. J. Richardson, Z. Wang, S. N. Kerisit, K. M. Rosso, J. M. Zachara and J. K. Fredrickson, Environ. Microbiol. Rep., 2009, 1, 220 CrossRef CAS PubMed.
- D. Baron, E. LaBelle, D. Coursolle, J. A. Gralnick and D. R. Bond, J. Biol. Chem., 2009, 284, 28865 CrossRef CAS PubMed.
- E. Laviron, J. Electroanal. Chem., 1979, 101, 19 CrossRef CAS.
- M. Yamashita, S. Rosatto and L. Kubota, J. Braz. Chem. Soc., 2002, 13, 635 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
