A quinolinyl antipyrine based fluorescence sensor for Zn2+ and its application in bioimaging†
Qi-Hua
You
a,
Pui-Shan
Chan
b,
Wing-Hong
Chan
*a,
Sam C. K.
Hau
c,
Albert W. M.
Lee
a,
N. K.
Mak
b,
Thomas C. W.
Mak
c and
Ricky N. S.
Wong
b
aDepartment of Chemistry, Hong Kong Baptist University, Hong Kong, China. E-mail: whchan@hkbu.edu.hk; Fax: +852-3411-7348; Tel: +852-3411-7076
bDepartment of Biology, Hong Kong Baptist University, Hong Kong, China
cDepartment of Chemistry, The Chinese University of Hong Kong, Hong Kong, China
First published on 17th September 2012
Abstract
By incorporating 4-aminoantipyrine moiety onto 8-aminoquinoline with a suitable spacer, a highly selective and sensitive fluorescent Zn2+ sensor, QPA, was designed and constructed. In 25% ACN-HEPES buffer pH 7.0 solution, QPA exhibited 10.6-fold fluorescence enhancement at 500 nm upon addition of Zn2+. The limit of detection (LOD) was calculated to be 1.3 × 10−7 M according to fluorescence titration. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode of the metal complex was established by combined UV-vis, fluorescence and HRMS spectroscopic method. The membrane permeability of QPA to living cells and bioimaging of Zn2+ are demonstrated.
1 binding mode of the metal complex was established by combined UV-vis, fluorescence and HRMS spectroscopic method. The membrane permeability of QPA to living cells and bioimaging of Zn2+ are demonstrated.
Introduction
The development of highly selective and sensitive metal chemo-sensors is an active field in supramolecular chemistry.1–4 Zinc ion is the most abundant d-block transition metal essential in the human body with concentration ranging from sub-nM to 0.3 mM,5,6 playing a pivotal role in physiological processes such as enzyme regulation, gene expression, catalytic function of protein, apoptosis and so-forth.7–13 The disorder of zinc metabolism in biological systems is associated with a variety of diseases such as Alzheimer's disease, diabetes, epilepsy.14–20 Therefore, there is a huge demand and potential for exploring novel development of Zn2+ chemosensors.Since the first quinoline-based Zn2+ sensor TSQ (N-(6-methoxyl-8-quinolyl)-p-toluenesulfonamide) was reported,21 many fluorescent sensors based on 8-aminoquinoline structure have been developed and applied in in vitro and in vivo imaging Zn2+ in biological samples.22–26 Also, 8-aminoquinoline was tethered to silica nanoparticles, cyclodextrin or polymers to improve water solubility so that they can detect Zn2+ in aqueous solutions.27–30 The signal display mechanisms of these sensors were mainly based on the photo-induced electron transfer (PET) and internal charge transfer (ICT). However, some of these Zn2+ sensors have undesirable selectivity and are vulnerable to interference by other metal ions, particularly Cd2+.30–32 Therefore, the design and synthesis of a more versatile and improved performance Zn2+ fluorescent sensor is still a great challenge.
Herein, we synthesized 8-aminoquinoline derivative QPA (N-(quinolin-8-yl)-2-((1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)amino)acetamide) bearing 4-aminoantipyrine moiety as a new chemosensor for the detection of Zn2+, which showed high selectivity and sensitivity to Zn2+ over other competitive metal ions, operating on the basis of chelation-enhanced fluorescence (CHEF) mechanism. The introduction of 4-aminoantipyrine group provides two binding sites for coordinating Zn2+ ion and the enhanced PET effect from the lone pair of two nitrogen atoms in 4-aminoantipyrine group exerting onto quinoline moiety endowing the aptosensor with a low fluorescence off-state. The complementary ligating properties of the binding sites of QPA confer the sensor with high selectivity and sensitivity towards Zn2+ over other metal ions.
Experimental
General
1H-NMR and 13C-NMR spectra were recorded on a Bruker Advance-III 400 MHz Spectrometer (at 400 and 100 MHz, respectively) using trimethylsilane (TMS) as an internal standard. Low resolution mass spectra were recorded on a Finnigan MAT SSQ-710 mass spectrometer while high resolution mass spectra was performed on a Bruker Autoflex mass spectrometer (MALDI-TOF). Fluorescence emission spectra and UV-vis spectra were collected on a PE LS50B and a Cary UV-300 spectrometer, respectively. The melting point was determined with a MEL-TEMPII melting point apparatus (uncorrected). X-ray intensity data were measured at room temperature (298 K) on a Bruker AXS Kappa ApexII Duo diffractometer using frames of oscillation range 0.3°, with 2° < θ < 28°. The pH measurements were performed on a Orion 420A pH mV temperature meter with a combined glass-calomel electrode. Double-distilled water was used throughout. The excitation wavelength was 330 nm.All regents for synthesis were obtained commercially and were used without further purification. Solvents such as dimethylformamide (DMF), acetonitrile (ACN) and tetrahydrofuran (THF) were purchased from commercial sources and were the highest grade, dry dichloromethane (DCM) was distilled in calcium hydride. Silica gel (200–300 mesh, MACHEREY-NAGEL GmbH & Co. KG) was used for column chromatography. Analytical thin-layer chromatography was performed using TLC silica gel 60 F254 (aluminium sheets, Merck KGaA). In titration experiments, all the cations in the form of perchlorate or chloride and other substrates were purchased from Sigma-Aldrich, USA, and stored in a vacuum desiccator.
Sample preparation
The probe was dissolved in ACN as a stock solution (1 mM). Buffer solution was prepared by dissolving HEPES in water (100 mM). Slight variations in the pH of the solution were achieved by adding the minimum volumes of NaOH or HCl.Absorption and fluorescence analysis
Absorption spectra and fluorescence emission spectra were obtained with 1.0 cm quartz cells. The detection procedures were as follows: in 25% ACN-HEPES (100 mM, pH = 7.0) buffer solution containing 10 μM QPA, a Zn2+ sample was gradually titrated into the solution, the mixture was equilibrated for 2 min before measurement. The fluorescence intensity was measured simultaneously at λex/em = 330/550 nm. The excitation and emission slits were set to 5.0 nm and 5.0 nm, respectively.Cell culture
Human nasopharyngeal carcinoma cells (HK-1) were cultured in RPMI 1640 (Gibco) supplemented with 10% FBS (Gibco) and antibiotics (penicillin 50 U/mL; streptomycin 50 μg/ml). The cells were incubated at 37 °C in a humidified CO2 (5%) incubator.Confocal fluorescence imaging
HK-1 cells (1 × 105 cells) were grown onto a glass coverslip in 35 mm culture dish overnight. The Zinc sensor QPA and the zinc chelator N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylene diamine (TPEN) were dissolved in DMSO while the zinc perchlorate and the zinc carrier pyrithione were dissolved in H2O. All compounds were prepared at a stock concentration of 10 mM and then diluted in culture medium for incubation with cells. The cells were incubated with the zinc sensor QPA (10 μM) for 30 min at 37 °C. Additional zinc ions were introduced by incubating the cells with a mixture of zinc perchlorate (5 μM) and zinc ion carrier pyrithione (5 μM) for 15 min at room temperature. In order to remove the zinc ion, the cells were further incubated with the zinc chelator TPEN (20 μM) for 15 min at room temperature. The fluorescence images of the cells were observed under the confocal microscope (Olympus FV1000). QPA was excited at 405 nm with a diode laser and the emitted fluorescent signals at 500–600 nm were collected. Images were processed and analyzed using the FV10-ASW software (Olympus).Single-crystal structure determination
X-ray intensity data were measured at room temperature (298 K) on a Bruker AXS Kappa ApexII Duo diffractometer using frames of oscillation range 0.3°, with 2° < θ < 28°. The structures were solved by the direct method and refined by full-matrix least-squares on F2 using the SHELXTL program package.Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluant to afford 1 as a white solid (0.88 g, 96% yield). m.p.: >300 °C, 1H-NMR (400 MHz, CDCl3): 10.72 (1H, s), 8.86 (1H, dd, J = 4.0, 1.6 Hz), 8.75 (1H, dd, J = 5.6, 3.6 Hz), 8.18 (1H, dd, J = 8.0, 3.6 Hz), 7.57 (2H, m), 7.49 (1H, dd, J =8.4, 4.0Hz), 4.14 (2H, s); 13C-NMR(100 MHz, CDCl3): 164.0, 148.6, 138.7, 136.3, 133.8, 127.9, 127.2, 122.5, 121.8, 116.6, 29.7. HRMS(ESI): m/z calcd for C11H10N2OBr: [M + H+] 264.9976, found: 264.9957.
1) as the eluant to afford 1 as a white solid (0.88 g, 96% yield). m.p.: >300 °C, 1H-NMR (400 MHz, CDCl3): 10.72 (1H, s), 8.86 (1H, dd, J = 4.0, 1.6 Hz), 8.75 (1H, dd, J = 5.6, 3.6 Hz), 8.18 (1H, dd, J = 8.0, 3.6 Hz), 7.57 (2H, m), 7.49 (1H, dd, J =8.4, 4.0Hz), 4.14 (2H, s); 13C-NMR(100 MHz, CDCl3): 164.0, 148.6, 138.7, 136.3, 133.8, 127.9, 127.2, 122.5, 121.8, 116.6, 29.7. HRMS(ESI): m/z calcd for C11H10N2OBr: [M + H+] 264.9976, found: 264.9957.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 1
ethyl acetate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as the eluant to afford QPA as a yellow solid (0.28 g, 72% yield). m.p.: 180–181°C, 1H-NMR (400 MHz, ACN-d3): 11.19 (1H, br. s), 8.88 (1H, dd, J = 4.2 Hz, J = 1.6 Hz), 8.82 (1H, dd, J = 7.4 Hz, J = 1.5 Hz), 8.33 (1H, dd, J = 8.3 Hz, J = 1.6 Hz), 7.67–7.56 (4H, m), 7.51–7.43 (4H, m), 7.33–7.28 (1H, m), 3.98 (2H, s), 3.75 (1H, br. s), 2.90 (3H, s), 2.29 (3H, s); 13C-NMR (100 MHz, CDCl3): 170.30, 162.53, 148.52, 142.65, 138.91, 136.21, 135.16, 134.15, 129.14, 128.06, 127.32, 126.21, 123.15, 121.92, 121.63, 120.86, 116.67, 52.79, 37.35, 10.73. HRMS(ESI): m/z calcd for C22H21N5O2: [M+H+] = 388.1773, found: 388.4032, [M+Na+] = 410.1593, found: 410.3490.
4) as the eluant to afford QPA as a yellow solid (0.28 g, 72% yield). m.p.: 180–181°C, 1H-NMR (400 MHz, ACN-d3): 11.19 (1H, br. s), 8.88 (1H, dd, J = 4.2 Hz, J = 1.6 Hz), 8.82 (1H, dd, J = 7.4 Hz, J = 1.5 Hz), 8.33 (1H, dd, J = 8.3 Hz, J = 1.6 Hz), 7.67–7.56 (4H, m), 7.51–7.43 (4H, m), 7.33–7.28 (1H, m), 3.98 (2H, s), 3.75 (1H, br. s), 2.90 (3H, s), 2.29 (3H, s); 13C-NMR (100 MHz, CDCl3): 170.30, 162.53, 148.52, 142.65, 138.91, 136.21, 135.16, 134.15, 129.14, 128.06, 127.32, 126.21, 123.15, 121.92, 121.63, 120.86, 116.67, 52.79, 37.35, 10.73. HRMS(ESI): m/z calcd for C22H21N5O2: [M+H+] = 388.1773, found: 388.4032, [M+Na+] = 410.1593, found: 410.3490.
Results and discussion
Fluorescent Zn2+ sensors bearing 2-amino-N-(quinolin-8-yl)acetamide (AQA) moiety have been reported.22–32,34–35 The preliminary work showed that the fluorescence of AQA was only turn-on upon either addition of Zn2+ or Cd2+ (Fig. S1, ESI†). To eliminate the interference of Cd2+ on Zn2+ detection, we called for the appendage of 4-aminoantipyrine moiety to AQA to form a new probe, QPA. The binding experiments revealed that the fluorescence of QPA can be turned on upon addition of Zn2+ and in contrast negligible fluorescent enhancement was observed in the presence of Cd2+ (Fig. S2, ESI†). Therefore, a highly selective Zn2+ chemosensor has emerged.As described in Scheme 1, QPA was synthesized from substitution reaction of 8-aminoquinoline to 2-bromacetyl bromide affording compound 1 in 96% yield. Then 1 was reacted with 4-aminoantipyrine in DMF to give rise to QPA in 72% yield. The control compound AQA was synthesized from 1 with NaN3, followed by reduction by PPh3 obtained in 65% overall yield.34 The structure of QPA and AQA was confirmed by 1H NMR, 13C NMR, HRMS (Supporting information†) and X-ray diffraction method (Fig. 1). Both AQA and QPA showed good solubility in 25% ACN aqueous solution.
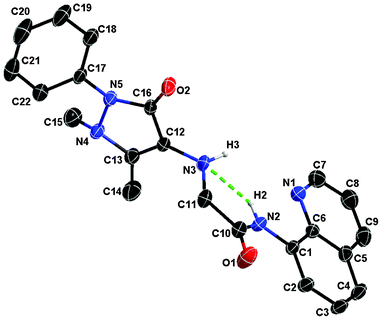 | ||
| Fig. 1 X-ray crystal structure of QPA. All hydrogen atoms are omitted for clarity (30% probability level for the thermal ellipsoids). The green dashed line indicates the internal H-bond between H2 and N3 atoms. | ||
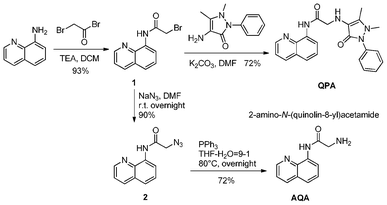 | ||
| Scheme 1 Facile synthesis of AQA and QPA. | ||
Initially the binding behavior of QPA towards Zn2+ was investigated by UV-vis spectroscopy. The absorption spectrum of QPA exhibited a strong band at 238 nm and also a weak broad band at about 300 nm in 25% ACN-HEPES buffer (100 mM, pH = 7.0) solution. Upon addition of Zn2+, the absorbance at 238 and 300 nm decreased with concomitant formation of new peaks at 252 and 350 nm (Fig. 2). Clear isosbestic points at 245, 275 and 328 nm are apparent, which indicates the formation of only one active zinc complex with the probe.
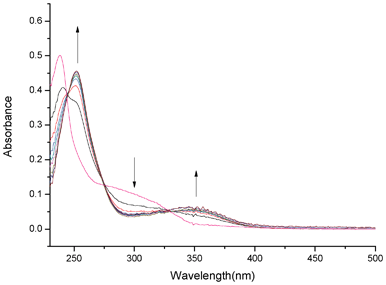 | ||
| Fig. 2 UV-vis spectra of QPA (10 μM) in 25% ACN-HEPES (100 mM, pH = 7.0) upon addition of Zn2+ (1-8 equiv.). | ||
The fluorescence emission spectrum from 350 to 600 nm was obtained by exciting QPA at 330 nm. Operating on PET mechanism, QPA exhibited very weak fluorescence at 500 nm in 25% ACN-HEPES buffer (100 mM, pH = 7.0). Addition of Zn2+ (0∼10 equiv.) produces a new emission band centered at 500 nm with a 10.6-fold increase in fluorescence (Fig. 3a). A greenish fluorescence emission of the solution was observed under UV lamp irradiation (Fig. 3a, inset). These results may be due to the breakage of the intramolecular hydrogen bond and electron transfer from the nitrogen atom of the quinoline moiety to metal ion after binding of QPA with Zn2+.33 Another reason for fluorescence enhancement could be that the binding of QPA and Zn2+ formed a more rigid chelating ring.24,35 On the basis of non-linear fitting of the titration curve of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model, the association constant of QPA-Zn2+ was computed to be (5.85 ± 0.54) × 104 M−1 (R2 = 0.986) using Benesi-Hildebrand equation (Fig. 3b).36–38 This binding model is also supported by Job's plot (Fig. 4). In low Zn2+ concentration (0∼9 μM), the fluorescence of QPA increased linearly upon addition of Zn2+, and the limit of detection (LOD) for Zn2+ is 1.3 × 10−7 M (3σ/slope, Fig. S3, ESI†).
1 binding model, the association constant of QPA-Zn2+ was computed to be (5.85 ± 0.54) × 104 M−1 (R2 = 0.986) using Benesi-Hildebrand equation (Fig. 3b).36–38 This binding model is also supported by Job's plot (Fig. 4). In low Zn2+ concentration (0∼9 μM), the fluorescence of QPA increased linearly upon addition of Zn2+, and the limit of detection (LOD) for Zn2+ is 1.3 × 10−7 M (3σ/slope, Fig. S3, ESI†).
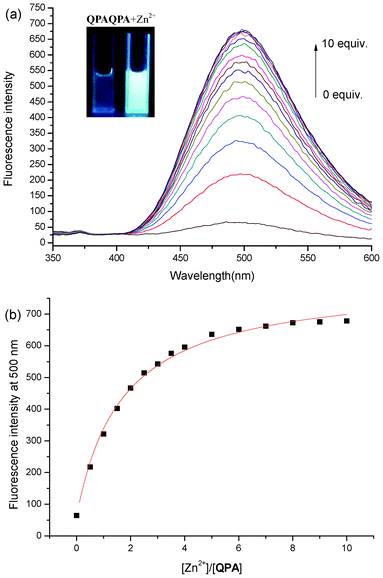 | ||
| Fig. 3 (a) Fluorescence titration of QPA (10 μM) in 25% ACN-HEPES (100 mM, pH = 7.0) with addition of Zn2+. Inset: visible emission (irradiated by 365 nm light) observed from QPA in the absence and presence of Zn2+ (10 equiv.); (b) fluorescence intensity at 500 nm of QPA (10 μM) in 25% ACN-HEPES buffer (100 mM, pH = 7.0) as a function of concentration of free Zn2+ and the probe. | ||
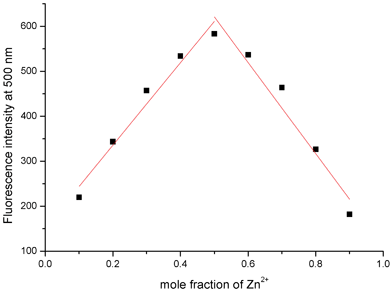 | ||
| Fig. 4 Job's plot by fluorescence method of the complex between QPA and Zn2+ in 25% ACN-HEPES (100 mM, pH = 7.0). Total concentration of QPA and Zn2+ is 50 μM. | ||
For practical applications, the sensing ability of QPA towards Zn2+ at different pH values was investigated (Fig. 5). As shown in Fig. 5, the fluorescence intensity of the QPA-Zn2+ complex increases gradually from pH 4.0 to 6.5, reaching a steady high reading at around pH 6.5. Apparently, in acidic conditions, protonation of the amino group in the 4-aminoantipyrine moiety would reduce its binding ability to Zn2+ and suppress the formation of the complex.26,35 On the other hand, due to the formation of the less soluble Zn(OH)+ or Zn(OH)2 in alkali conditions, fewer QPA-Zn2+ complexes could be formed at high pH. Thus, fluorescence of the complex decreases with the increase of pH from 8.0 to 10.0.32 A plateau of stable reading from pH 6.5 to 8.0, covering the physiological pH window, was observed. Therefore, subsequent metal binding studies were carried out in HEPES buffer solution at pH = 7.0.
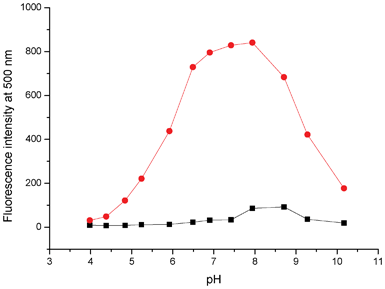 | ||
| Fig. 5 Fluorescence intensity at 500 nm of QPA (10 μM) in 25% ACN-HEPES (100 mM) with (red) and without (black) addition of Zn2+ (10 equiv.) as a function of pH. | ||
We also investigated the time course of QPA to different equivalents of Zn2+ in 25% ACN-HEPES buffer (20 mM, pH = 7.0) (Fig. S4, ESI†). We found that the fluorescence of the probe became steady within 1 min after addition of Zn2+. Therefore, this system could be used to detect Zn2+ in real-time.
The selectivity of QPA to various metal ions was examined systematically. Among the 14 metal ions being studied, upon excitation at 330 nm, only Zn2+ induced a dramatic fluorescence enhancement of QPA. On the other hand, Cd2+ and Fe2+ induced a slight enhancement of the probe, and Cu2+ quenched the fluorescence completely because of its paramagnetic property (Fig. 6a). When 10 equiv. of these competing metal ions were added to a mixture of QPA with 10 equiv. of Zn2+, only Fe3+, Hg2+ and Co2+ can quench the fluorescence to a small extent and Cu2+ quenched completely the fluorescence (Fig. 6b). Furthermore, to establish the reversibility binding of Zn2+ by QPA, the fluorescence enhancement of the probe at 500 nm triggered by 10 equiv. of Zn2+ can be completely switched-off by the addition of 10 equiv. of EDTA. The same extent of fluorescence enhancement can be recovered when an additional 10 equiv. of Zn2+ was introduced and this complexation/decomplexation cycle can be repeated 5 times (Fig. S5, ESI†). These results show that QPA is suitable to be used as a reversible fluorescent chemosensor to detect Zn2+.
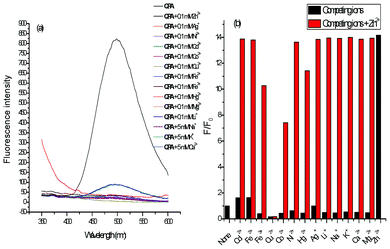 | ||
| Fig. 6 (a) Fluorescence spectra of QPA (10 μM) in 25% ACN-HEPES (100 mM, pH = 7.0) upon addition of various metal ions; (b) The fluorescence response of QPA (10 μM) in 25% ACN-HEPES (100 mM, pH = 7.0) in the absence (black bars) and presence (red bars) of Zn2+ (10 equiv.) upon addition of various metal ions (0.1 mM of Cd2+, Fe2+, Fe3+, Cu2+, Co2+, Ni2+, Pb2+, Hg2+, Ag+, Li+, Mg2+, and 5 mM of Na+, Ca2+, K+). The response is normalized to the integrated fluorescence intensity of free dye (F0). | ||
The binding mode of the complex was studied by MALDI-TOF HRMS and 1H NMR spectroscopic method. When the complex was subjected to mass spectral measurement, a clear peak of m/z 450.0459 corresponding to [QPA+Zn2+-H+]+ was observed (Fig. S7, ESI†). However, attempts to prepare a single crystal of QPA-Zn2+ complex were unsuccessful.
To evaluate the binding mode of QPA-Zn2+ complex, detailed 1H NMR titration was carried out (Fig. 7). Upon gradual addition of Zn2+ to the ACN-d3 solution of QPA, with the addition of 0.5 equiv. of Zn2+, a large upfield shift of H7 (Δδ = 0.64) and H6 (Δδ = 0.24), ascribed to the amide proton and the ortho- aromatic proton to the carboxamido group in the quinoline ring, respectively, was observed. At the same time, H1 (Δδ = 0.06) and H3 (Δδ = 0.06), which are ascribed to the ortho- and para-aromatic protons to the quinoline nitrogen, showed negligible downfield shift. These results confirm that Zn2+ coordinates to the nitrogen atom of the carboxamido group and seemingly has no interaction with the nitrogen atom of quinoline moiety. Also, a large downfield shift of NCH3 (Δδ = 0.38), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3 (Δδ = 0.16) and H8 (Δδ = 0.26) adjacent to the carboxamido group were also observed. These observations suggest that the metal ion has strong coordination with nitrogen atom in NCH3 and oxygen of carbonyl in the antipyrine moiety.39 In contrast to other 8-aminoquinoline based Zn2+ sensors which coordinate with nitrogen atom in quinoline moiety,23–28,30–35 the contribution of strong ligating groups by the antipyrine moiety of QPA deprived of the metal binding affinity of the quinoline nitrogen atom. To our best knowledge, this is the first 8-aminoquinoline based Zn2+ sensor which did not involve quinoline nitrogen atom as a ligating group. Therefore, a binding mode between QPA and Zn2+ is proposed (Fig. 8).
CCH3 (Δδ = 0.16) and H8 (Δδ = 0.26) adjacent to the carboxamido group were also observed. These observations suggest that the metal ion has strong coordination with nitrogen atom in NCH3 and oxygen of carbonyl in the antipyrine moiety.39 In contrast to other 8-aminoquinoline based Zn2+ sensors which coordinate with nitrogen atom in quinoline moiety,23–28,30–35 the contribution of strong ligating groups by the antipyrine moiety of QPA deprived of the metal binding affinity of the quinoline nitrogen atom. To our best knowledge, this is the first 8-aminoquinoline based Zn2+ sensor which did not involve quinoline nitrogen atom as a ligating group. Therefore, a binding mode between QPA and Zn2+ is proposed (Fig. 8).
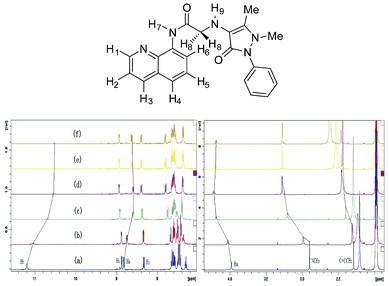 | ||
| Fig. 7 Partial 1H NMR spectra (400 MHz) of QPA (20 mM) in ACN-d3. (a) Free QPA; (b) QPA + 0.1 equiv. of Zn2+; (c) QPA + 0.3 equiv. of Zn2+; (d) QPA + 0.5 equiv. of Zn2+; (e) QPA + 0.8 equiv. of Zn2+; (f) QPA + 1.0 equiv. of Zn2+. | ||
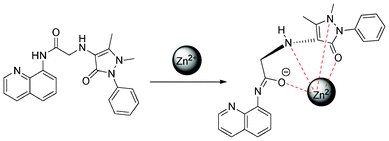 | ||
| Fig. 8 Proposed binding mechanism of QPA and Zn2+. | ||
A preliminary study on the Zn2+-sensing behaviors of QPA in the biological system was carried out by fluorescence microscopy using HK-1 cells40 as model cells. The results show that QPA-stained HK-1 cells exhibited good fluorescence responses for Zn2+. After incubation with QPA at 37 °C for 1 hour, the cells displayed very weak fluorescence (Fig. 9a). Presumably, the native Zn2+ concentration in the cell is too low to turn on the probe. When exogenous Zn2+ is introduced by incubation with 5 μM Zn(ClO4)2/pyrithione (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the cells displayed bright green fluorescence (Fig. 9b). This fluorescence signal can be quenched by subsequent incubation with cell permeable chelator TPEN (Fig. 9c). Being a strong Zn2+ chleator, TPEN can remove Zn2+ completely from its binding to QPA. This finding indicates that the fluorescence emission is a result of reversible Zn binding to QPA in the cell environment.
1), the cells displayed bright green fluorescence (Fig. 9b). This fluorescence signal can be quenched by subsequent incubation with cell permeable chelator TPEN (Fig. 9c). Being a strong Zn2+ chleator, TPEN can remove Zn2+ completely from its binding to QPA. This finding indicates that the fluorescence emission is a result of reversible Zn binding to QPA in the cell environment.
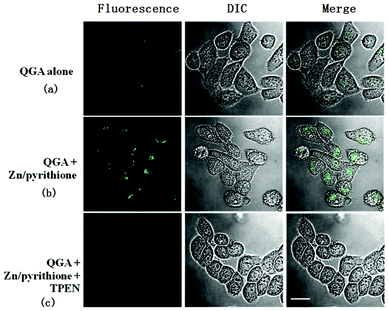 | ||
Fig. 9 HK-1 cells were incubated with QPA (10 μM) (a), followed by 5 μM Zn(ClO4)2/pyrithione (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (b), followed by further incubation with TPEN (20 μM) (c). Green dots represent the fluorescence from zinc ions interacting with the QPA. Scale bar: 20 μm. 1) (b), followed by further incubation with TPEN (20 μM) (c). Green dots represent the fluorescence from zinc ions interacting with the QPA. Scale bar: 20 μm. | ||
Conclusions
By tethering 4-aminoantipyrine moiety onto the molecular platform comprising 8-aminoquinoline as the fluorescent signal display unit, a highly selective and sensitive fluorescent Zn2+ sensor, QPA, was designed and constructed. Under physiological pH conditions, in vitro detection of Zn2+ at subnanomolar concentrations can be achieved in aqueous ACN solution. The binding mode of the metal complex was established by combined UV-vis, fluorescence and 1H NMR spectroscopic method. Moreover, QPA can be used as an imaging reagent of Zn2+ in living cells.Acknowledgements
The work was supported by a grant from the Research Committee of Hong Kong Baptist University (FRG2/11-12/121).References
- A. W. Czarnik, Fluorescent Chemosensors for Ion and Molecular Recognition, American Chemical Society: Washington, DC, 1993 Search PubMed.
- A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, T. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef.
- R. Martinez-Manez and F. Sancanon, Chem. Rev., 2003, 103, 4419 CrossRef CAS.
- T. Gunnlaugsson, M. Glynn, G. M. Tocci, P. E. Kruger and F. M. Pfeffer, Coord. Chem. Rev., 2006, 250, 3094 CrossRef CAS.
- S. J. Lippard and J. M. Berg, Principle of Bioinogranic Chemistry; University Science Book: CA, 1994, 10, 14pp. 78–183 Search PubMed.
- E. M. Nolan and S. J. Lippard, Acc. Chem. Res., 2009, 42, 193 CrossRef CAS.
- T. V. O'Halloran, Science, 1993, 261, 715 CAS.
- K. H. Falchuk, Mol. Cell. Biochem., 1998, 188, 41 CrossRef CAS.
- P. Jiang, Coord. Chem. Rev., 2004, 248, 205 CrossRef CAS.
- W. Maret, Biometals, 2001, 14, 187 Search PubMed.
- S. C. Burdette and S. J. Lippard, Proc. Natl. Acad. Sci., U. S. A., 2003, 100, 3605 CrossRef CAS.
- J. M. Berg and Y. Shi, Science, 1996, 271, 1081 CrossRef CAS.
- B. L. Vallee and K. H. Falchuk, Psychol. Rep., 1993, 73, 79 Search PubMed.
- A. I. Bush, Trends Neurosci., 2003, 26, 207 CrossRef CAS.
- C. J. Frederickson, J. Y. Koh and A. I. Bush, Nat. Rev. Neurosci., 2005, 6, 449 CrossRef CAS.
- A. B. Chausmer, J. Am. Coll. Nutr., 1998, 17, 109 CAS.
- J. L. Smith, S. Xiong, W. R. Markesbery and M. A. Lovell, Neuroscience, 2006, 140, 879 Search PubMed.
- E. J. Ho, Nutr. Biochem., 2004, 15, 572 CrossRef CAS.
- R. Sladek, Nature, 2007, 445, 881 CrossRef CAS.
- M. Lu and D. Fu, Science, 2007, 317, 1746 CrossRef CAS.
- C. J. Frederickson, E. J. Kasarskis, D. Ringo and R. E. Frederickson, J. Neurosci. Methods, 1987, 20, 91 CrossRef CAS.
- E. M. Nolan, J. Jaworski, K.-I. Okamoto, Y. Hayashi, M. Sheng and S. J. Lippard, J. Am. Chem. Soc., 2005, 127, 16812 CrossRef CAS.
- X. Zhou, Y. Lu, J.-F. Zhu, W.-H. Chan, A. W. M. Lee, P.-S. Chan, R. N. S. Wong and N. K. Mak, Tetrahedron, 2011, 67, 3412 CrossRef CAS.
- G. Xie, Y. Shi, F. Hou, H. Liu, L. Huang, P. Xi, F. Chen and Z. Zeng, Eur. J. Inorg. Chem., 2012, 2012, 327 Search PubMed.
- C. Zhang, Y. Zhang, Y. Chen, Z. Xie, Z. Liu, X. Dong, W. He, C. Shen and Z. Guo, Inorg. Chem. Commun., 2011, 14, 304 Search PubMed.
- Y. Zhang, X. Guo, W. Si, L. Jia and X. Qian, Org. Lett., 2008, 10, 473 CrossRef CAS.
- P. Pal, S. K. Rastogi, C. M. Gibson, D. E. Aston, A. L. Branen and T. E. Bitterwolf, Appl. Mater. Interfaces, 2011, 3, 279 Search PubMed.
- S. K. Rastogi, P. Pal, D. E. Aston, T. E. Bitterwolf and A. L. Branen, Appl. Mater. Interfaces, 2011, 3, 1731 Search PubMed.
- Y. Li, Z. Yang, Z. Liu, B. Wang and S. Li, Sensors and Actuators B, 2011, 160, 1504 CrossRef CAS.
- Y. Liu, N. Zhang, Y. Chen and L.-H. Wang, Org. Lett., 2007, 9, 315 CrossRef CAS.
- C. Zhao, Y. Zhang, P. Feng and J. Cao, Dalton Trans., 2012, 41, 831 RSC.
- X. Zhou, B. Yu, Y. Guo, X. Tang, H. Zhang and W. Liu, Inorg. Chem., 2010, 49, 4002 CrossRef CAS.
- T. Yang, C. Tu, J. Y. Zhang, L. P. Lin, X. M. Zhang, Q. Liu, J. Ding, Q. Xu and Z. J. Guo, Dalton Trans., 2003, 3419 RSC.
- J.-F. Zhu, H. Yuan, W.-H. Chan and A. W. M. Lee, Org. Biomol. Chem., 2010, 8, 3957 RSC.
- G. Xie, P. Xi, X. Wang, X. Zhao, L. Huang, F. Chen, Y. Wu, X. Yao and Z. Zeng, Eur. J. Inorg. Chem., 2011, 2011, 2927 Search PubMed.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS.
- C. Yang, L. Liu, T.-W. Mu and Q.-X. Guo, Anal. Sci., 2000, 16, 537 CrossRef CAS.
- M. I. Rodíguez-Cáceres, R. A. Agbaria and I. M. Warner, J. Fluoresc., 2005, 15, 185 CrossRef CAS.
- V. N. Krishnamurthy and S. Soundararajan, Proceedings Math. Sci., 1967, 65, 148 Search PubMed.
- D. P. Huang, J. H. Ho, Y. F. Poon, E. C. Chew, D. Saw, M. Lui, C. L. Li, L. S. Mak, S. H. Lai and W. H. Lau, Int. J. Cancer, 1980, 26, 127 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary data contain fluorescence spectra of QPA and 8-NQ-NH2 upon addtion of Zn2+ and Cd2+, measurement of detection limit, time course of QPA/Zn2+ complex formation, stepwise complexation/decomplexation cycle of QPA to Zn2+, MALDI-TOF HRMS of QPA and QPA/Zn2+, 1H, 13C NMR spectrum of QPA. CCDC reference numbers 892765. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra21736h |
| This journal is © The Royal Society of Chemistry 2012 |
