Nonvolatile write-once read-many-times memory device based on an aromatic hyperbranched polyimide bearing triphenylamine moieties
Fei
Chen
,
Guofeng
Tian
,
Lei
Shi
,
Shengli
Qi
* and
Dezhen
Wu
State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: qisl@mail.buct.edu.cn; Fax: +86 10 6442 1693; Tel: +86 10 6442 4654
First published on 22nd October 2012
Abstract
An aromatic hyperbranched polyimide, poly(N,N,N′,N′-tetrakis(4-aminophenyl)benzidine-N,N- 4,4′-hexafluoroisopropylidene-diphthalimide) (6F-TEAPBD PI), was synthesized. Semiconductor parameter analysis on the sandwich devices using the synthesized polyimide as the active layer indicates that the polymer possesses distinct electrical bi-stable states with an ON/OFF current ratio of about 300 and a switching voltage at around 2.0 V, which could be applied as nonvolatile write-once read-many-times (WORM) memory. Mechanisms associated with the electrical switching effect are discussed on the basis of the experimental and quantum simulation results. It is suggested that the electric-field-induced charge transport from triphenylamine moieties to hexafluoropropylidene phthalimide units and the subsequent formation of charge-transfer complexes are responsible for the observed electrical memory effect.
1. Introduction
Polymer memory devices have received significant attention in recent years due to their unique advantages such as easy processability, good mechanical properties, flexibility, low-cost potential, and the three-dimensional (3D) multi-stack layer structures.1–8 And completely different from that of the traditional silicon-based memory cells, which store data by encoding each device as “0” or “1”, polymer memory achieves information storage based on the bistability of the materials, for instance, the high and low electrical conductivity response to an applied voltage. This feature makes polymer devices as one of the most promising candidates for the development of next-generation memory devices characterized by high storage density, fast write or read speed and multilevel designs. Various polymers, such as poly(N-vinylcarbazole) (PVK),6,9,10 poly(p-phenylene vinylene) (PPV)11 and poly(4,4′-diaminotriphenylamine-N,N-4,4′-hexafluoroisopropylidene-diphthalimide) (TP6F-PI),12 have been investigated and different types of memory effects were realized. Among them, aromatic polyimides have gained increasing attention in recent publications due to their superior thermal and mechanical properties, dimensional stability and high chemical resistance.2,12–14 By introducing various different electroactive moieties such as carbazole,9,14,15 ferrocene,16 triphenylamine,2,3,7,12,13 oxadiazole,4 pyrene9 and anthracene17 into the polyimide chain, different types of memory effects including write-once read-many-times (WORM), flash memory, dynamic random access memory (DRAM) and static random access memory (SRAM) have been realized. However, previous research works were mainly focused on linear polymers. Less attention has been paid to three-dimensional spatial polyimide structures.Hyperbranched polymers with three-dimensional dendritic architectures, especially for those with abundant functional groups, exhibit unique chemical and physical properties18 and have attracted widespread interests in various fields including drug delivery,19 gas separation,20 hydrogen storage,21 nonlinear optics,22 magnetics and optoelectronics.23,24 In addition, they can be readily synthesized in single-step polymerization procedures, making them promising materials for practical applications.18,25 However, the exploration of hyperbranched polymers as information storage materials such as electrical memory is rarely reported.
Herein, we report our work on the synthesis and electrical characterization of a hyperbranched polyimide derived from 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (6FDA) and N,N,N′,N′-tetrakis(4-aminophenyl)benzidine (TEAPBD) (a tetrafunctional monomer), the ideal structure of which is illustrated in Scheme 1. 6FDA and triphenylamine (TPA) units are intentionally combined into one macromolecule, since they are complementary electroactive moieties, in which TPA typically functions as an electron-donating group and 6FDA serves as electron-accepting part. Their combination is supposed to endow the material with strong potential to form electron donor–acceptor couples. Electrical characterization results suggest that the polymer possesses electrical bistability and the sandwich devices using the synthesized polyimide as the active layer (Au|polyimide|ITO) exhibit an irreversible electrical switching effect and could be applied as WORM memory in digital information storage.
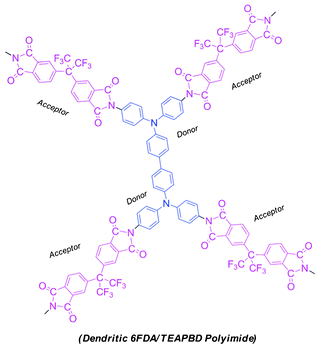 | ||
| Scheme 1 Illustrative chemical structures for the synthesized 6F-TEAPBD PI. | ||
2. Results and discussion
The electrical switching effect of the synthesized PI was demonstrated in the current–voltage (I–V) characteristics of the Au|polyimide|ITO sandwich devices, as shown in Fig. 1a. During the first positive scan from 0 to 5 V (ITO: cathode; Au: anode), a sharp increase in the current occurring at a threshold voltage of around 1.8 V was observed, indicating the transition of the device from the low conductivity state (the OFF state) to the high conductivity state (the ON state). After this transition, the device remained in the high-conductivity state, as observed during the following positive scan (the second sweep). The subsequent negative scan from 0 to −5 V (the third scan) did not turn the device from the ON state to the OFF state, and the device kept its high-conductivity state during the following forward scan (sweep 4 from 0 to 5 V) and reverse scan (sweep 5 from 0 to −5 V). This indicates that the OFF-to-ON transition is irreversible. Once the device is switched to its high conductivity state, it remains there and can not be retrieved to its initial low conductivity state. Subsequent measurement shows that the device further remained in the ON state after turning off the power, implying its nonvolatile features. As reported in literature,26 devices with such electrical characteristics can serve as write-once read-many-times (WORM) memory in digital information storage. The OFF-to-ON electrical switching process is equivalent to the “writing” process in a traditional memory cell. The distinct and accessible bistable electrical states in the voltage range from 0 to 1.8 V allow any constant voltage between 0 and 1.8 V (e.g. 1 V) to “read” the “OFF” or “0” signal (before switching on) and “ON” or “1” signal (after switching on) of the memory device. Further experiments on duplicated sandwich devices revealed that the observed I–V characteristic was repeatable, but with minor variations in the OFF-to-ON transition voltages (1.4–2.0 V). To this point, it is concluded that the synthesized 6F-TEAPBD PI possesses irreversible electrical bistabilites and the sandwich devices using the polyimide as the active layer could be applied as nonvolatile WORM memory in digital information storage. Also, the low OFF-to-ON electrical transition voltage promises low power-consuming characteristic of the fabricated WORM devices. However, it should be mentioned that, at a bias exceeding −10 V or +10 V, the device shows a sudden drop in the current to zero and the device could no longer be operated, which is suggested to be due to device failure at high electric field stress.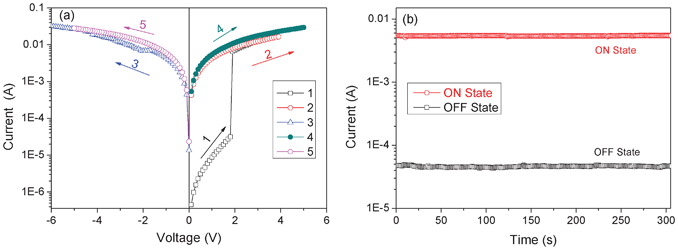 | ||
| Fig. 1 (a) Current–voltage (I–V) characteristics of the ITO|6F-TEAPBD PI|Au sandwich device; the sweep sequence and direction are indicated by the number and arrow, respectively (scans numbers 1, 2 and 4: 0 to +5 V; scans numbers 3 and 5: 0 to −5 V). (b) Effect of operation time on the currents of the sandwich device at the ON and OFF states tested at 1 V bias under ambient conditions. | ||
Besides, the I–V characteristics shown in Fig. 1a suggest that the sandwich device based on 6F-TEAPBD PI achieves an ON/OFF current ratio of about 300. To evaluate the stability of the observed electrical memory effect, the ON and OFF currents of the sandwich device were tested under ambient air conditions, the results of which are shown in Fig. 1b. Under a constant stress of 1 V, the device shows excellent operation stability and no degradation in current was observed during the measurement, demonstrating the nonvolatile features of the electrical switching effect. Besides, this nonvolatile irreversible electrical switching behavior was also observed when scanning the device first with negative bias (ITO: anode; Au: cathode), indicating its independence on the voltage polarity. However, the switching voltage is a little increased to around 2 V and the ON/OFF current ratio is reduced to about 100, for which we can not give a reasonable explanation so far. Besides, it should be mentioned that, although memory sandwich devices could be fabricated and an electrical switching effect could be observed by employing the 6F-TEAPBD polyimide as the active layer, the ON/OFF current ratio achieved in the current work is not so satisfactory, especially for practical applications. Our attempts to improve the ON/OFF current ratio by varying the thermal annealing temperature (80 and 100 °C), active layer thickness (40 and 54 nm) and the device measurement environment did not lead to any significant improvement, which is probably due to the essential characteristics of the synthesized polyimide.
Fig. 2a shows the UV/vis absorption spectrum for the synthesized 6F-TEAPBD polyimide. A considerably broad absorbance band centered at around 340 nm was observed on the spectrum, with the absorption edge extending to a wavelength (λedge) of 395 nm, based on which the optical energy band gap (Eg) of the material was calculated to be 3.15 eV according to the Plank equation (Eg = hc/λedge).
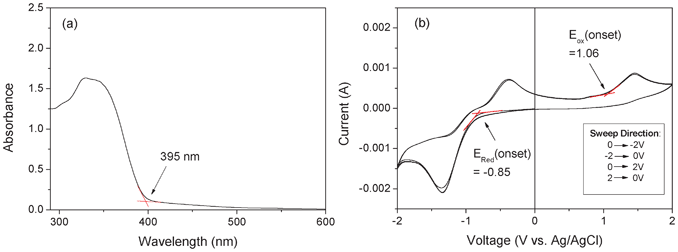 | ||
| Fig. 2 (a) UV/vis absorption spectrum for the synthesized 6F-TEAPBD polyimide; and (b) cyclic voltammetry sweeps of the 6F-TEAPBD polyimide measured in 0.1 M n-Bu4BF4/acetonitrile (scan rate: 0.05 V s−1). | ||
The electrochemical behavior of the synthesized polyimide was investigated by cyclic voltammetry (CyV) measurements conducted on thin electroactive films coated on a precleaned square platinum plate to elucidate the relative ionization and reduction potentials of our electroactive materials. Fig. 2b shows the CyV sweeps for both p- and n-doping processes of the synthesized 6F-TEAPBD PI film in the potential range of −2.0 to +2.0 V. The CyV curve suggests that the 6F-TEAPBD PI exhibits irreversible p-doping behavior during the anodic scan (0 → +2 V). A moderate but obvious ionization (oxidation) peak was observed at around 1.5 V vs. Ag/AgCl, and no reduction peak appears when scanning backward. The results imply that the triphenylamine (TPA) units in the 6F-TEAPBD polyimide are able to donate electrons and, as expected, readily function as hole-transporting sites in the material, and it has already been demonstrated that the introduction of triphenylamine species into electroactive polymer systems enhances the hole-injection properties of the resulting materials.27,28 It is suggested that the irreversible electron-donating behavior observed here might partially account for the irreversible electrical switching effect observed in the sandwich memory devices. When scanning cathodically (0 → −2 V), the polymer film exhibits a very strong reduction peak at about −1.3 V vs. Ag/AgCl, which should be related to the electrochemical reduction of the 6FDA moieties that have a high tendency to receive electrons. However, the CyV result indicates that this n-doping behavior is quasi-reversible, as verified by the observation of two oxidation peaks at −0.9 and −0.4 V when scanning backward. Nevertheless, the predominant peak at −1.3 V demonstrates the strong electron-withdrawing ability of the 6FDA units. Besides, the material exhibits good CyV reversibility during repeated cyclic scans between −2.0 and +2.0 V, indicating the excellent electrochemical stability of the synthesized polyimide and the good adhesion at the polymer/platinum interface. The first electron removal for the polyimide is assumed to occur at the nitrogen atoms of the triphenylamine groups in the polymer, since they are relatively more electron-rich.
Quantitatively, the energy levels of the highest occupied molecular orbital (HOMO, the top of the valence band) and the lowest unoccupied molecular orbital (LUMO, the bottom of the conduction band) of the synthesized polyimide can be estimated from the CyV onset oxidation potential (Eox(onset)) and the energy band gap obtained from the UV absorption spectrum (Eg) according to eqn (1) and (2):11,12,29
| HOMO = −[(Eox(onset) − Eferrocene) + 4.8)] (eV) | (1) |
| LUMO = Eg + HOMO | (2) |
From the CyV curve, Eox(onset) for the polyimide is at around 1.06 V vs. Ag/AgCl, and the energy gap (Eg) calculated from the UV absorption edge wavelength is 3.15 eV. Thus, the HOMO and LUMO energy levels of the 6F-TEAPBD PI are estimated to be −5.48 and −2.33 eV, respectively. In comparison, the onset reduction potential (Ered(onset)) of the polyimide determined from the CyV scans was −0.85 V, giving a CyV energy band gap at around 1.91 eV, which deviates considerably from the optical band gap (3.15 eV) calculated from the UV absorption spectrum, which is often found in many reports.
With the purpose of getting a clear understanding of the electronic structure and molecular orbitals of the current electroactive macromolecular system, molecular simulations were carried out. For the sake of model simplicity and practical availability for computations, the basic unit of 6F-TEAPBD polyimide with an ideal structure was selected as a model compound, with one TEAPBD tethered via imide rings to four 6FDA fragments, N,N,N′,N′-tetrakis(4-aminophenyl)benzidine-N,N-4,4′-trifluoroethylidene-phthalimide 1 (see Table 1). Different calculation methods ranging from semi-empirical (including AM1 and PM3) to ab initio (including HF) and density functional theory (including LSDA, B3LYP and B3PW91) were evaluated. The basis set was set as 6-31G(d), whenever necessary, in all the first-principle calculations.
| Components | TEAPBD | 6FDA | 6FDA-TEAPBD model compound 1b |
|---|---|---|---|
| a All the structures used for molecular orbital calculations were optimized first on AM1 level and then on HF/6-31G(d) level. b The structure of the 6FDA-TEAPBD model compound 1 was constructed from the optimized 6FDA and TEAPBD structure, and then optimized additionally on AM1 and HF/6-31G(d) level. | |||
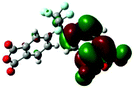
| HOMO level |
|
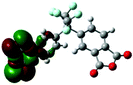
|
|
| (−4.25 eV) | (−8.28 eV) | (−5.16 eV) | |
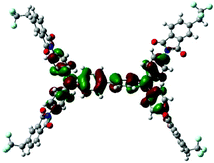
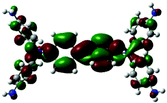
| LUMO level |
|
|
|
| (−0.17 eV) | (−2.98 eV) | (−2.24 eV) | |
| Energy gap | 4.08 eV | 5.30 eV | 2.92 eV |
| Dipole moment | 2.321 Debye | 5.237 Debye | 2.826 Debye |
For comparison purpose, Fig. 3 summarizes the calculated HOMO and LUMO energy levels, and the related energy band gaps, as well as the corresponding experimental results determined from cyclic voltammetry and the UV/vis absorption spectrum shown in Fig. 2. The results indicate that the worst results come from the semi-empirical-based computations (AM1 and PM3) and the ab initio (HF) calculations, with the observation of considerably large deviations from the experimental results either in energy levels or in band gaps. This is expected since semi-empirical calculation includes no diffuse basis functions and contains inaccuracies in its parameterization set. The ab initio method, HF, essentially takes no consideration of Coulomb electron correlation effects in its calculation. DFT methods, especially B3LYP and B3PW91, exhibit the best accuracy, in which the calculations based on the B3PW91/6-31G(d) theory level gave the most acceptable results. The HOMO, LUMO, and band gaps calculated from the B3PW91/6-31G(d) model chemistry are −5.16, −2.24 and 2.92 eV, respectively, which are almost identical with the experimentally determined results, −5.48, −2.33 and 3.15 eV. Therefore, the B3PW91/6-31G(d) model chemistry was eventually selected for all future simulations and discussions.
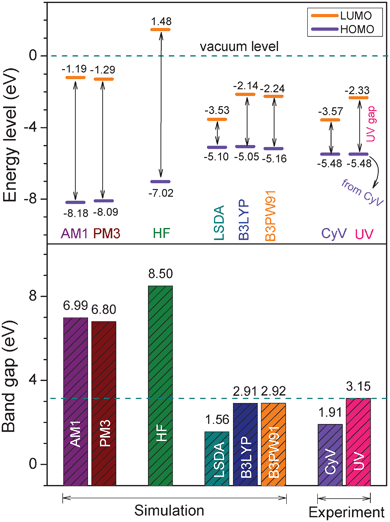 | ||
| Fig. 3 Comparison of the energy band gaps (bottom) and the HOMO, LUMO energy levels (top) of the 6FDA-TEAPBD model compound 1 calculated from model chemistry on different theory levels and those determined from the cyclic voltammetry (CyV) and UV/vis absorption spectrum of the polyimide. The basis set of 6-31G(d) was used in the ab initio and DFT calculations. | ||
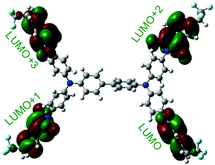
Table 1 shows the corresponding B3PW91/6-31G(d)-based simulation results for the 6FDA unit, the TEAPBD unit, and the 6FDA-TEAPBD model compound 1 used to represent the 6F-TEAPBD polyimide. The higher HOMO energy level of TEAPBD (−4.25 eV) indicates that the TEAPBD unit functions as the hole-accepting moiety, while the lower LUMO energy level of 6FDA (−2.98 eV) indicates the strong electron-withdrawing ability of 6FDA. The HOMO and LUMO energy levels of the model compound are at −5.16 and −2.24 eV, respectively, which are basically consistent with the results obtained from the CyV and UV characterization. These results indicate that when these two components are covalently connected, the HOMO of the 6F-TEAPBD unit is located on the TEAPBD side with a decreased HOMO energy level, whereas the LUMO of the 6F-TEAPBD unit is located on the 6FDA side with an increased LUMO energy level. The 6F-TEAPBD polyimide system is supposed to possess the same electronic structure, with TEAPBD functioning as the electron donor and 6FDA as the electron acceptor. The four 6FDA fragments attached with the TEAPBD core are supposed to serve as four effective electron-trapping sites since they correspond to the LUMO, LUMO+1, LUMO+2 and LUMO+3 orbitals, as illustrated in Table 1. Besides, the large dipole moment (2.826 Debye) observed in the model compound 1 suggests that the synthesized polyimide has a strong tendency for charge separation. These features suggest facile charge transfer from TEAPBD (HOMO) to 6FDA (LUMO), and the subsequent formation of charge-transfer complexes in the polyimide system.
Fig. 4 shows the energy level diagram for sandwich devices using the 6F-TEAPBD polyimide as the active layer. The HOMO and LUMO energy levels of the polyimide determined from CyV and UV spectra are at −5.48 and −2.33 eV, respectively, which are associated with the triphenylamine moieties and the 6FDA parts, respectively, based on quantum calculations. In our current sandwich device, the distinctly lower energy barrier between the metal electrodes (work function: ITO = −4.8 eV and Au = −5.1 eV) and the HOMO level (−5.48 eV) suggests that the 6F-TEAPBD polyimide most likely functions as a p-type material and hole injection from metal electrodes into the HOMO is more favorable during the charge injection process. Theoretically, an applied voltage exceeding 0.38 V (the barrier between Au and HOMO) will provide enough energy for the holes on Au (or electrons on HOMO) to overcome the energy barrier between Au and the HOMO to inject into (for electrons, extract from) the active layer of the sandwich device. Thus, during the voltage scanning process, holes are readily loaded into the material under a low bias. Accordingly, the device exhibits an instant increase in the current at the initial stage of voltage sweep, as observed in Fig. 1a (first scan). However, it is supposed that hole injection (or electron extraction) at the initial stage (under low voltages) will be rather limited since simulation results in Table 1 suggest that the 6FDA moieties possess considerable ability to block the mobility of the injected holes due to their considerably low HOMO energy level (−8.28 eV). Concomitantly, the holes injected will induce a countering space-charge field in the polymer matrix, which will screen the applied external electric field, thereby inhibiting further charge injection into the active layer.30 Thus, the device exhibits a restricted increase in the current under low bias (0 → +2 V), and the material remains at low-conductivity level (OFF state), as observed in Fig. 1a.
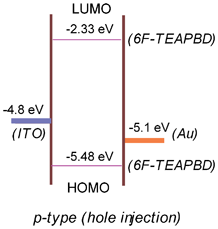 | ||
| Fig. 4 Energy level diagram for the ITO|6F-TEAPBD PI|Au sandwich device. | ||
With the increase of the applied external electric field, and the continuous injection of holes into the HOMO located at the TEAPBD moieties and electron injection into the LUMO located at the 6FDA parts, percolation pathways for charge carriers are finally generated between the charged HOMO and charged LUMO through the charge-transfer interactions and the subsequent formation of charge-transfer complexes. Consequently, the polymer soon becomes fully p-doped under the induction of the applied electric field, resulting in the switch of the device to its high-conductivity state (ON state) at a critical bias (2 V in the present case). The device further remains in its high-conductivity state during the subsequent voltage sweeps, most likely due to the strong electron affinity of the 6FDA units, which could effectively hold the electrons trapped in the 6FDA moieties, and inhibit their escape and further neutralization with the holes located at the triphenylamine sites in the polyimide. Besides, the considerably large dipole moment (2.826 Debye) of the polyimide basic unit (6FDA-TEAPBD, see Table 1), which will be further enhanced after charge separation, could also help to promote the generation and the subsequent stabilization of the formed charge separation structures. Therefore, the polymer could not be retrieved to its initial state and the device exhibits irreversible and nonvolatile electrical switching characteristics.
3. Experimental section
3.1. Characterization
Fourier transform infrared (FT-IR) spectra were recorded under ambient conditions on a Tensor 27 Fourier transform spectrophotometer (Bruker, Germany). KBr was used as a nonabsorbent medium. Proton nuclear magnetic resonance (1H NMR, 400 MHz) spectra and carbon-13 nuclear magnetic resonance (13C NMR, 100 MHz) spectra were recorded on a Bruker AV600 spectrometer using DMSO-d6 as the solvent. Differential scanning calorimetry (DSC) measurements were performed on a TA Q20 system at a heating rate of 5 K min−1. Mass spectra (MS) were collected on a Waters Quattro Premier XE system using acetonitrile as the solvent. Elemental analyses were carried out on a varioELcube elemental analyzer. Thermogravimetric analysis (TGA) was undertaken under both air and nitrogen atmospheres using a TA Q50 instrument at a heating rate of 10 K min−1. Gel permeation chromatography (GPC) analysis was carried out on a Waters 515–2410 system using polystyrene standards as molecular weight references and tetrahydrofuran (THF) as the eluent. The thickness of the polyimide films coated on the ITO substrate was measured using atomic force microscopy (Digital Instruments Nanoscope IIIa, Veeco Instrument) under contact mode. The current–voltage (I–V) characteristics of the sandwich devices were recorded by a Keithley 4200 SCS semiconductor parameter analyzer (Keithley, Cleveland, OH) equipped with a Micromanipulator 6150 probe station in a clean and metallically shielded box in ambient environment at room temperature. Ultraviolet/visible (UV/vis) absorption spectra were recorded on a Shimadzu UV-2550 spectrophotometer. Cyclic voltammetry (CyV) measurement was performed on a CHI660D Electrochemical Workstation (Shanghai Chenhua Instruments Inc, China) using a three-electrode cell under a nitrogen environment. The polyimide films coated on a platinum square electrode (working electrode) was scanned anodically and cathodically (scan rate: 50 mV s−1) in a solution of tetrabutylammonium tetrafluoroborate (n-Bu4BF4) in acetonitrile (0.1 M) with Ag/AgCl and a platinum as the reference and counter electrodes, respectively. Ferrocene was used as an external reference for calibration (0.38 V vs. Ag/AgCl). Molecular simulations of the basic unit of 6F-TEAPBD PI were carried out using the Gaussian 03 software package. The molecular orbital and electronic properties were calculated on theory levels including semi-empirical, ab initio and density functional theory (DFT).3.2 Reagents
4,4′-(Hexafluoroisopropylidene)diphthalic anhydride (6FDA) was purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd. and used as received. Dimethylacetamide (DMAc) (analytically pure, ≤0.1% water) was produced by Tianjin Fu Chen Chemicals Reagent Factory and used after distillation over calcium hydride under reduced pressure. N,N,N′,N′-Tetrakis(4-aminophenyl)benzidine (TEAPBD, C36H32N6) was synthesized in our lab by using benzidine and 1-fluoro-4-nitrobenzene as the starting materials following the procedure described in literature developed for the synthesis of N,N-bis(4-aminophenyl)-N′,N′-bis(4-tert-butylphenyl)-1,4-phenylenediamine.31 The TEAPBD precursor, N,N,N′,N′-tetrakis(4-nitrophenyl)benzidine (TENPBD, C36H24N6O8) was synthesized with a yield of 42%. TENPBD, FT-IR (KBr, cm−1): 1583, 1340 (nitro group). 1H NMR (DMSO-d6, 400 MHz, J/Hz), δ (ppm): 8.22 (d, 8H, J = 8.7), 7.84 (d, 4H, J = 8.2), 7.36 (d, 4H, J = 8.2), 7.27 (d, 8H, J = 8.6). 13C NMR (DMSO-d6, 100 MHz, J/Hz), δ (ppm): 122.7, 125.6, 127.5, 128.6, 142.2, 151.4. Elemental analysis: calc. for C36H24N6O8, C 64.67, H 3.62, N 12.57, O 19.14; found, C 65.14, H 3.50, N 12.46, O 18.90. Mp (DSC, °C): 369. TEAPBD (C36H32N6) was synthesized with a final yield of 18%. TEAPBD, FT-IR (KBr, cm−1): 3419 (amino group). UV-vis (λmax/nm) (DMAc): 302 and 364. 1H NMR (DMSO-d6, 400 MHz), δ (ppm): 7.26 (d, 4H, J = 8.2), 6.80 (d, 8H, J = 7.8), 6.62 (d, 4H, J = 8.0), 6.53 (d, 8H, J = 8.1), 4.95 (s, 8H). 13C NMR (DMSO-d6, 100 MHz), δ (ppm): 114.7, 117.1, 125.9, 127.1, 129.8, 131.4, 135.9, 145.4. MS (m/z): [M]+ calc. for C36H32N6, 548.7; found, 548.7. Elemental analysis: calc. for C36H32N6, C 78.80, H 5.88, N 15.32; found, C 78.95, H 6.57, N 14.48. Mp (DSC, °C): 306.3.3. Polyimide synthesis
Polymerization of the polyimide was carried out by first mixing 6FDA and TEAPBD in DMAc at room temperature to give the hyperbranched poly(amic acid) precursor followed by chemical cyclo-imidization at relatively higher temperature conditions to form the final hyperbranched polyimide. Due to the existence of the four identical amino groups in TEAPBD, a gel was readily formed during the synthesis of hyperbranched poly(amic acid).21 Thus, monomer addition manner and monomer molar ratio must be strictly controlled20 and the addition of TEAPBD into 6FDA must be slow enough to avoid the occurring of gelation. Further, it is necessary that all the glassware used for polymerization is prebaked on a vacuum-purge line, and then cooled under vacuum and purged with N2. As a typical example, 0.25 g of 6FDA was dissolved in 4 ml of DMAc under N2 followed by dropwise addition of 0.1 g of TEAPBD (dissolved in 6 ml of DMAc) into the solution over 2 h. The reaction mixture was then vigorously stirred at 30 °C for 3 h. Then 1.0 g triethylamine and 3.0 g of acetic anhydride were added, and the reaction mixture was further stirred at 30 °C for 3 h, and then at 60 °C for 8 h. Then, the resulting solution was poured into an excess amount of diethyl ether under vigorous stirring to give the polyimide as a precipitate. The crude product was collected and further purified by re-precipitation twice in diethyl ether, and then washed and subsequently dried under vacuum for over 24 h to give the final polyimide in powder form. FT-IR (KBr, cm−1): 1782, 1730, 1380, 723 (imide ring). 1H NMR (DMSO-d6, δ, ppm): 7.21 (8H), 7.40 (8H), 7.67 (4H), 7.77 (4H), 7.85–8.17 (aromatic H of 6FDA). UV-vis (λmax/nm) (DMAc): 331. Glass transition temperature (Tg) (DSC, °C): not detected. 5%-weight-loss temperature (by TGA, °C): 508 (under N2), 450 (in air). GPC (polystyrene standard): Mn = 8696, Mw/Mn = 1.3.3.4. Sandwich device fabrication
The sandwich memory devices using the synthesized polyimide as the active layer were fabricated by spreading a 30 mg ml−1 polyimide solution in DMAc onto a pre-cleaned indium–tin oxide (ITO) substrate using a spin coater set at 1600 rpm. The ITO conductive glass was precleaned sequentially with water, acetone, chloroform and isopropanol in an ultrasonic bath for 15 min and then dried by a N2 gun. After keeping in a clean box programmed at constant temperature and humidity for ca. 4 h, the spin-coated films were thermally treated under vacuum at 80 °C for 8 h to remove the remaining solvent. The thickness of the polyimide films on ITO was about 40 nm, as determined by atomic force microscopy measurement. Finally, the sandwich devices (ITO|polyimide|Au) were fabricated by vacuum evaporation of a thin Au layer (ca. 300 nm) through a shadow mask (the holes are of cylinder shape with diameters of 4, 3, 2 and 1 mm) onto the polymer surface as the top electrode, the structure of which is shown in Scheme 2.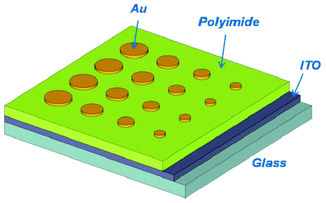 | ||
| Scheme 2 Illustrative structures for the ITO|6F-TEAPBD polyimide|Au sandwich device. | ||
4. Conclusions
To sum up, nonvolatile write-once read-many-times (WORM) electrical memory effect was observed in our present work, based on the aromatic hyperbranched polyimide poly(N,N,N′,N′-tetrakis(4-aminophenyl)benzidine-N,N-4,4′-hexafluoroisopropylidene-diphthalimide) (6F-TEAPBD PI), in which triphenylamine moieties function as electron-donating groups and hexafluoroisopropylidene phthalimide units serve as electron-accepting species. The fabricated memory device in a simple ITO|polymer active layer|Au sandwich structure exhibits two distinct but irreversible conductivity states, and displays a threshold transition voltage at around 2.0 V and an ON/OFF current ratio of about 300 with good operation stability. The nonvolatile and irreversible electrical memory effect observed in the current system was ascribed to the electric-field-induced charge-transfer interactions between the hexafluoroisopropylidene diphthalimide and the triphenylamine groups, and the strong electron affinity of the hexafluoro-phthalimide units which supposedly possess the ability to retain the charge-separation state of the generated charge-transfer complexes.Acknowledgements
The authors thank the financial support from the National Natural Science Foundation of China (project No. 50903006) and the Specialized Research Fund for the Doctoral Program of Higher Education (project No. 20090010120008).References
- S. G. Hahm, S. Choi, S.-H. Hong, T. J. Lee, S. Park, D. M. Kim, W.-S. Kwon, K. Kim, O. Kim and M. Ree, Adv. Funct. Mater., 2008, 18, 3276 CrossRef CAS.
- D. M. Kim, S. Park, T. J. Lee, S. G. Hahm, K. Kim, J. C. Kim, W. Kwon and M. Ree, Langmuir, 2009, 25, 11713 CrossRef CAS.
- K. Kim, S. Park, S. G. Hahm, T. J. Lee, D. M. Kim, J. C. Kim, W. Kwon, Y.-G. Ko and M. Ree, J. Phys. Chem. B, 2009, 113, 9143 CrossRef CAS.
- Y.-L. Liu, K.-L. Wang, G.-S. Huang, C.-X. Zhu, E.-S. Tok, K.-G. Neoh and E.-T. Kang, Chem. Mater., 2009, 21, 3391 CrossRef CAS.
- C.-U. Pinnow and T. Mikolajick, J. Electrochem. Soc., 2004, 151, K13 CrossRef CAS.
- E. Y. H. Teo, Q. D. Ling, Y. Song, Y. P. Tan, W. Wang, E. T. Kang, D. S. H. Chan and C. Zhu, Org. Electron., 2006, 7, 173 CrossRef CAS.
- K.-L. Wang, Y.-L. Liu, J.-W. Lee, K.-G. Neoh and E.-T. Kang, Macromolecules, 2010, 43, 7159 CrossRef CAS.
- K.-L. Wang, Y.-L. Liu, I. H. Shih, K.-G. Neoh and E.-T. Kang, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5790 CrossRef CAS.
- Q.-D. Ling, D.-J. Liaw, E. Y.-H. Teo, C. Zhu, D. S.-H. Chan, E.-T. Kang and K.-G. Neoh, Polymer, 2007, 48, 5182 CrossRef CAS.
- P. Y. Lai and J. S. Chen, IEEE Electron Device Lett., 2011, 32, 387 Search PubMed.
- Y.-Z. Lee, X. Chen, S.-A. Chen, P.-K. Wei and W.-S. Fann, J. Am. Chem. Soc., 2001, 123, 2296 CrossRef CAS.
- Q.-D. Ling, F.-C. Chang, Y. Song, C.-X. Zhu, D.-J. Liaw, D. S.-H. Chan, E.-T. Kang and K.-G. Neoh, J. Am. Chem. Soc., 2006, 128, 8732 CrossRef CAS.
- T. J. Lee, C.-W. Chang, S. G. Hahm, K. Kim, S. Park, D. M. Kim, J. Kim, W.-S. Kwon, G.-S. Liou and M. Ree, Nanotechnology, 2009, 20, 135204 CrossRef.
- G. Tian, D. Wu, S. Qi, Z. Wu and X. Wang, Macromol. Rapid Commun., 2011, 32, 384 CrossRef CAS.
- B. Hu, F. Zhuge, X. Zhu, S. Peng, X. Chen, L. Pan, Q. Yan and R.-W. Li, J. Mater. Chem., 2012, 22, 520 RSC.
- G. Tian, S. Qi, F. Chen, L. Shi, W. Hu and D. Wu, Appl. Phys. Lett., 2011, 98, 203302 CrossRef.
- S. Park, K. Kim, D. M. Kim, W. Kwon, J. Choi and M. Ree, ACS Appl. Mater. Interfaces, 2011, 3, 765 CrossRef CAS.
- C. Gao and D. Yan, Prog. Polym. Sci., 2004, 29, 183 CrossRef CAS.
- X. Hu and J. Ji, Biomacromolecules, 2011, 12, 4264 CrossRef CAS.
- J. Fang, H. Kita and K.-i. Okamoto, Macromolecules, 2000, 33, 4639 CrossRef CAS.
- Z. Wang, B. Zhang, H. Yu, L. Sun, C. Jiao and W. Liu, Chem. Commun., 2010, 46, 7730 RSC.
- A. Scarpaci, E. Blart, V. r. Montembault, L. Fontaine, V. Rodriguez and F. Odobel, ACS Appl. Mater. Interfaces, 2009, 1, 1799 CrossRef CAS.
- K.-L. Wang, S.-T. Huang, L.-G. Hsieh and G.-S. Huang, Polymer, 2008, 49, 4087 CrossRef CAS.
- T. Sakano, F. Ito, T. Ono, O. Hirata, M. Ozawa and T. Nagamura, Thin Solid Films, 2010, 519, 1458 Search PubMed.
- H. Chen and J. Yin, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 3804 CrossRef CAS.
- S. Möller, C. Perlov, W. Jackson, C. Taussig and S. R. Forrest, Nature, 2003, 426, 166 CrossRef.
- F. Liang, Y.-J. Pu, T. Kurata, J. Kido and H. Nishide, Polymer, 2005, 46, 3767 CrossRef CAS.
- Q. H. Zhao, Y. H. Kim, T. T. M. Dang, D. C. Shin, H. You and S. K. Kwon, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 341 Search PubMed.
- P. I. Djurovich, E. I. Mayo, S. R. Forrest and M. E. Thompson, Org. Electron., 2009, 10, 515 CrossRef CAS.
- G. Liu, Q. Ling, E.-T. Kang, K.-G. Neoh, D.-J. Liaw, F.-C. Chang, C.-X. Zhu and D. S.-H. Chan, J. Appl. Phys., 2007, 102, 024502 CrossRef.
- H.-M. Wang and S.-H. Hsiao, Polymer, 2009, 50, 1692 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
