Photochemically active reduced graphene oxide with controllable oxidation level
Yan-Gu
Lin
*abc,
Chi-Kai
Lin
*b,
Jeffrey T.
Miller
b,
Yu-Kuei
Hsu
d,
Ying-Chu
Chen
c,
Li-Chyong
Chen
c and
Kuei-Hsien
Chen
ac
aInstitute of Atomic and Molecular Sciences, Academia Sinica, Taipei 10617, Taiwan
bChemical Sciences and Engineering Division, Argonne National Laboratory, IL 60439, USA. E-mail: yglin@anl.gov; linc@cmt.anl.gov
cCenter for Condensed Matter Sciences, National Taiwan University, Taipei 10617, Taiwan
dDepartment of Opto-Electronic Engineering, National Dong Hwa University, Hualien 97401, Taiwan
First published on 25th September 2012
Abstract
We have developed a novel one-step and effective electrochemical (EC) method to directly exfoliate graphite into thin reduced graphene oxide (RGO) nanosheets at room temperature. The oxidation degree of the RGOs depends on the switching potentials of the EC synthesis. The high switching potential can significantly increase the C/O ratio of the RGOs. The ability to control the light-absorption of the RGOs by simply adjusting the switching potentials can be further achieved. Additionally, we also construct a RGO–ZnO heterojunction and investigate its photoelectrochemical (PEC) properties. The results show that highly photoactive RGO as a photosensitizer can make H2 evolution easier and improve the photoconversion ability of ZnO under visible-light irradiation. This approach presents us with a possibility for the environmentally friendly, ultrafast, low-cost, and large-scale production of RGOs and great potential in solar-energy conversion applications of graphene-based materials.
Introduction
Graphene derivatives such as graphene oxide (GO) and reduced graphene oxide (RGO) have captured the attention of the scientific community in many energy-related applications.1,2 GO and RGO are believed to contain a large number of defects such as oxygen-containing functional groups in comparison with pure graphene.3 In many cases, these defects have a negative influence on the pristine properties of graphene,4 while in certain cases GO and RGO are more attractive than graphene itself.5 For instance, these oxygen-containing functional groups introduce sp3 defect sites to the nanosheets, distorting the intrinsic conjugated π-system and lowering overall strength and conductivity.6 Nevertheless, the chemical reactivity of these groups makes GO or RGO much more efficient as building blocks than graphene for fabricating hybrid nanomaterials.7 In addition, the reduction of GO is one of the general approaches to open the bandgap of graphene for semiconducting applications.8 Particularly, GO has many semiconducting π-conjugated sp2 domains of various sizes within an oxygenated sp3 domain.9 The GO bandgap increases with the oxidation level. Fully oxidized GO is an insulator, in contrast to RGO and graphene that are a semiconductor and a conductor, respectively.10 The photoresponse and photoreactivity, therefore, can be widely tuned by adjusting the oxidation degree of RGO. This inspired us to develop a facile and high-throughput strategy for preparing RGO with a highly tunable oxidation level.Traditionally, chemical exfoliation methods based on the Hummers' method, oxidation of graphite into GO, followed by chemical or thermal reduction, have drawn much attention due to the advantages of potentially low-cost and solution-processed fabrication.11,12 However, the initial oxidation and subsequent reduction processes typically involve strong oxidizing and reducing agents or high temperature treatment, respectively. These agents are corrosive, toxic, and even explosive, thus bringing in serious safety and environmental concerns. Meanwhile, high-temperature treatments are not only energy consuming but they may also led to a surface-area reduction of RGO. Furthermore, nowadays most methods of synthesizing RGO include a two-step process, wherein GO is prepared in the first step, followed by its reduction via chemical or thermal treatment in the second step. Recently, Su et al. first reported that high-quality graphene can be synthesized by the direct electrochemical (EC) exfoliation of graphite at room temperature.13 It should be noted that the EC method is an effective tool to modify electronic states by adjusting the external power source to change the Fermi energy level of electrode materials surface.14 To our best knowledge, investigations into the one-step synthesis of RGO without any oxidizing and reducing agents are still lacking to date. Herein, we demonstrate a novel one-step and ultrafast approach of obtaining colloidal dispersions of photochemically active RGO with tunable oxidation degree by simple EC exfoliation of graphite at room temperature. The C/O ratio of RGOs can be easily modulated by applying different switching potentials. This approach is environmentally friendly, ultrafast, low-cost, and easily scaled up for the mass production of RGOs. Additionally, we further build a 1D ZnO nanorod–2D RGO nanosheet heterojunction that highly enhances the photoconversion of ZnO under visible-light irradiation.
Experimental section
EC exfoliation of RGO from graphite
A natural graphite flake (average size 5–20 mm from NGS, Germany) was employed as an electrode and source of RGO for EC exfoliation. The graphite flake was adhered to a tungsten wire by a silver pad and then was inserted as the anode into a diluted (0.5–1 M) H2SO4 solution. A ground Pt wire was placed parallel to the graphite flake with a separation of 3–5 cm. The EC exfoliation process was carried out by applying a DC switching bias on the graphite electrode. To prepare the RGO nanosheet suspension, the exfoliated RGO nanosheets were collected with a 100 nm porous filter and washed with DI water by vacuum filter. After drying, they were dispersed in dimethylformamide (DMF) solution by gentle water-bath sonication for 5 min, and then the suspension was subjected to centrifugation at 2500 rpm. All of these EC exfoliation experiments were performed at room temperature.Preparation of 1D ZnO nanorod–2D RGO nanosheet hybrid by electrophoresis technique
The ZnO nanorod arrays were firstly grown on the Indium Tin Oxide (ITO) substrate by the typical electro-deposition method. The details for the nanorod array growth process are described following the procedure proposed in the work by Hsu et al.18 A simplified attachment route for the RGO–ZnO nanohybrids was then used by the electrophoresis technique. Typical concentrations of RGO (synthesized by a +8V/−8V switching bias) and applied direct current (DC) voltage were 1.5 mg mL−1 and 10 V, respectively. The deposition time was in the range 1–10 min.Characterizations
SEM measurements were made on a JEOL 6700 field-emission SEM. To obtain TEM images, the nanostructure of the exfoliated RGO nanosheets was analyzed using a JEOL JEM-2100 TEM system. The atomic force microscopy (AFM) analyses were performed in a Veeco Dimension-Icon system. The X-ray diffraction (XRD) analyses were performed on a Bruker D8 Advance diffractometer with Cu (40 kV, 40 mA) radiation. X-Ray photoelectron spectroscopy (XPS) spectra were obtained using a Microlab 350 system. Micro-Raman analyses were performed on a Jobin Yivon Labram HR800 spectrometer. UV-Visible absorption spectra were carried out with a JASCO V-670 instrument. In the PEC water-splitting reaction, a RGO–ZnO photoanode was used as the working electrode with a surface area of 0.5–1 cm2 and a platinum plate as the counter electrode (2-electrode system). All the photoelectrochemical (PEC) studies were conducted in a 0.1 M Na2SO4 solution as the supporting electrolyte medium using an Electrochemical Multichannel Solartron Analytical System. The water-splitting photoelectrode was illuminated with AM 1.5G simulated solar light at 100 mW cm−2. Incident photon to current conversion efficiency (IPCE) measurements were performed by a 2-electrode system under monochromatic irradiation, emitted from a 150 W Xe lamp equipped with bandpass filters and a variable neutral density filter. Evolved H2 gas was detected by an on-line gas chromatograph.Results and discussion
Fig. 1a schematically illustrates our experimental setup, where a natural graphite flake was employed as the electrode and source of RGO for EC exfoliation. A Pt wire was chosen as the grounded electrode. When a diluted H2SO4 solution was used as an electrolyte, a static bias of +2.7 V was applied to the graphite for 1 min, and then high switching voltages were applied until one obtained the desired amounts of exfoliated sheets. The initial low bias helps to wet the sample and likely causes gentle intercalation of SO42− ions to the grain boundary of graphite.13 Note that the high positive potential activated the exfoliation and oxidized the graphene sheets. The produced functional groups are reduced when the bias is switched to a high negative potential. These exfoliated RGO sheets were collected using filtration and redispersed in DMF solution. Fig. 1b shows the photograph of the dispersed RGO sheets in DMF solution. The representative SEM image of the RGO sheets is presented in Fig. 1c. It clearly exhibits an aggregated sheet-structure with some wrinkles. The prepared RGO had a thickness of ∼3.8 nm with a lateral size of several hundred nanometres measured by AFM, which confirms that the RGOs are composed of few-layer carbon sheets. Fig. 1d displays the TEM image typically seen for an exfoliated RGO nanosheet. Our TEM observation reveals that the layer number of these EC exfoliated RGOs is about 4 (few-layer RGO). The XRD patterns of the pristine graphite and exfoliated RGO are also recorded. Compared with the pristine graphite (26.6°), a broad peak of RGO centered at 23.36° is visible, confirming a random packing of graphene sheets in the RGO.15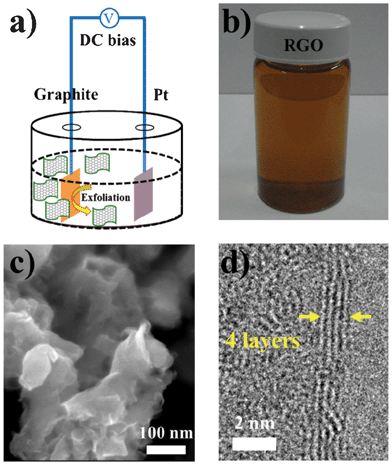 | ||
| Fig. 1 (a) Schematic illustration for the EC exfoliation of graphite. (b) Photograph of the dispersed RGO sheets in a DMF solution. (c) SEM image of the exfoliated RGO sheets. (d) Typical TEM image for an exfoliated few-layer RGO. | ||
To intuitively evaluate the oxidation level and determine the composition of the as-prepared RGO sheets, XPS was utilized. The C1s spectrum of the RGO sheets (Fig. 2) could be deconvoluted into several peaks: C–C (284.6 eV), C–O (286.7 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.5 eV) and O
O (288.5 eV) and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH (289.3 eV). With the increase of switching bias during the EC exfoliation process, the intensities of the carbon atoms bonded to oxygen were reduced remarkably, while the peak of the sp2 carbon increased significantly. Such change indicated that effective deoxygenation could be achieved at much higher switching bias. The reason is the high negative bias can overcome the energy barriers for the reduction of the oxygen functionalities, thus, the exfoliated GO induced by the positive bias can be efficiently reduced.14 In addition, the relative C/O atomic ratios of the RGOs were calculated from the XPS spectra (by using a ratio of the area of the C(1s) peak to the area of the O(1s) peak) for a better comparison (Fig. 2d). Notably, the high switching bias can significantly increase the C/O ratio of RGOs, showing low oxidation degree. Our results offer a facile means by which the C/O ratio of RGOs can be easily tuned between that of GO (C/O ratio ∼2) and highly reduced GO (C/O ratio ∼10).
C–OH (289.3 eV). With the increase of switching bias during the EC exfoliation process, the intensities of the carbon atoms bonded to oxygen were reduced remarkably, while the peak of the sp2 carbon increased significantly. Such change indicated that effective deoxygenation could be achieved at much higher switching bias. The reason is the high negative bias can overcome the energy barriers for the reduction of the oxygen functionalities, thus, the exfoliated GO induced by the positive bias can be efficiently reduced.14 In addition, the relative C/O atomic ratios of the RGOs were calculated from the XPS spectra (by using a ratio of the area of the C(1s) peak to the area of the O(1s) peak) for a better comparison (Fig. 2d). Notably, the high switching bias can significantly increase the C/O ratio of RGOs, showing low oxidation degree. Our results offer a facile means by which the C/O ratio of RGOs can be easily tuned between that of GO (C/O ratio ∼2) and highly reduced GO (C/O ratio ∼10).
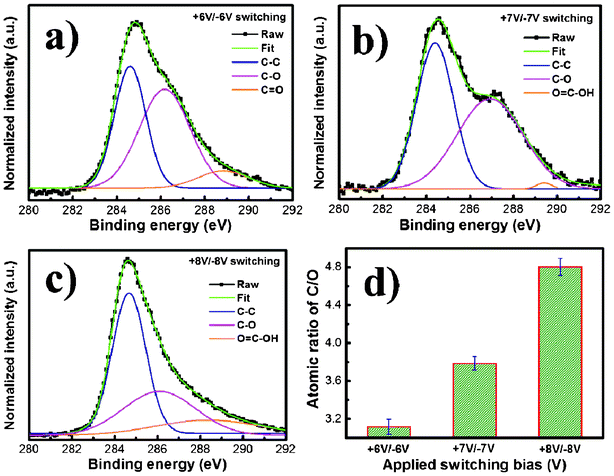 | ||
| Fig. 2 XPS spectra (C1s binding energy) for the EC exfoliated RGO nanosheets synthesized by (a) +6V/−6V, (b) +7V/−7V, and (c) +8V/−8V switching bias. (d) The values of C1s/O1s (atomic ratios) obtained by XPS survey spectra. | ||
Raman spectroscopy is a powerful nondestructive tool to distinguish ordered and disordered crystal structures of carbon. Fig. 3a shows the Raman spectra (excited by 532 nm laser) of RGOs with different switching potentials during the EC exfoliation process. It is clearly seen that a substantially increased degree of defect density (D/G) intensity ratio of RGOs with the increase of the switching bias is observed, indicating that the fast exfoliation during the switching process altered the structure of the RGO with a high quality of structural defects. Furthermore, the 2D bands of all the RGOs were centered at 2689 cm−1 with a shoulder at higher wavenumbers, indicating the RGOs constituted of multi-layer (2–4 layers) graphene sheets,16 consistent with the TEM observations. Fig. 3b displays the UV-visible absorption spectra of RGOs with different switching potentials during the EC exfoliation process. The absorption spectra show an increase in absorbance and a red-shift with the enlarged switching potential. It had been reported that the bandgap of graphene is opened by oxygen functionalization, and the bandgap value of RGO increases with the increase of oxygen coverage density and O/C ratio.17 Surprisingly, our result manifests the ability to control the bandgap by simply adjusting the switching potentials.
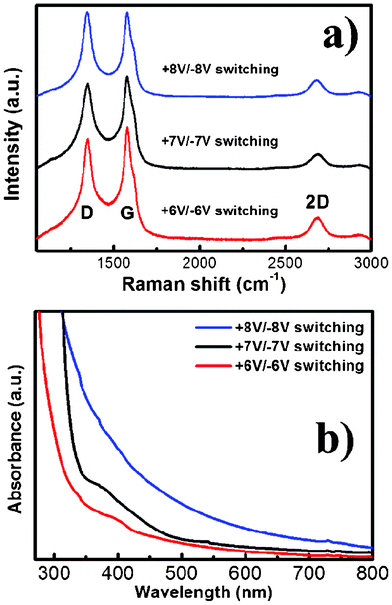 | ||
| Fig. 3 (a) Raman spectra and (b) light absorption spectra for the EC exfoliated RGO nanosheets synthesized by different switching bias (+6V/−6V, +7V/−7V, and +8V/−8V). | ||
For the further applicability of RGOs in solar-to-fuel conversion, we established a 1D ZnO nanorod–2D RGO nanosheet hybrid (the inset of Fig. 4a) by the electrophoresis technique. To quantify the photoconversion property, measurements of the incident photon to current conversion efficiency (IPCE) were made to study the photoresponse of pristine ZnO and RGO–ZnO nanocomposites as a function of incident light wavelength (Fig. 4a). Pristine ZnO has a strong photoresponse only in the near-UV region. Surprisingly, the nanocomposite samples display substantial photoactivity in the visible-light region from 400 to 450 nm in addition to the strong photoresponse in the near-UV region. Particularly, the IPCE of the RGO–ZnO photoanodes at the monochromatic wavelength of 400 nm is up to 24%. It should be noted that RGO strikingly improves the light absorption of ZnO in the visible region for the hybrid photoanode, and RGO may be regarded as the photosensitizer material. The photoresponse characteristics of pristine ZnO, RGO–ZnO, and Pt–RGO–ZnO photoelectrodes were evaluated by inserting them in a photoelectrochemical (PEC) cell under UV-visible light irradiation with 1 V bias potential (vs. Pt counter electrode) (Fig. 4b). Clearly, the RGO-photosensitized ZnO nanoarchitectures manifest a pronounced photocurrent, which increases to 1.6 mA cm−2 under illumination. In comparison to pristine ZnO, the photocurrent density of the RGO–ZnO hybrid nanoelectrodes is about 3.3 times greater than that of pristine ZnO, suggesting that ZnO loaded with RGO can effectively improve PEC water-splitting. The maximum photocurrent (2.5 mA cm−2) generated from the RGO–ZnO hybrid nanoelectrodes by depositing Pt is realized. As RGO is visible-light sensitive, we further evaluated the PEC properties of all the photoelectrodes under visible-light irradiation (λ > 400 nm), which is presented in Fig. 4c. In this case, no photocurrent is recorded for bare ZnO. In contrast, noteworthy photocurrent responses are observed for each switch-on/off event in the case of the RGO–ZnO and Pt–RGO–ZnO photoelectrodes, which confirms the high photo-activity of the RGO–ZnO hybrid nanoelectrodes under visible light. Nevertheless, the visible-light photocurrent of the RGO-coated ZnO photoelectrode cannot be kept as high as the UV-visible photocurrent, implying an unique electron–hole transport process occurred in the interface between RGO and ZnO. Compared to the UV-visible light photoexcited process, the main difference for RGO photosensitized ZnO is that ZnO is not photoexcited under only visible-light irradiation. In this case, the increased photocurrent is due to the synergetic effects of RGO and ZnO, and the photoexcited electrons–holes from ZnO must be involved in this transport. In the RGO sensitized ZnO photoanode excited by UV-visible light, a part of the photoexcited electrons on the conduction band of RGO can be left by recombining the holes on the valence band of RGO with the electrons on the conduction band of ZnO (Fig. 5). The left electrons on the conduction band of RGO then tunnel into the current collector ITO substrate. While under visible-light irradiation, the ZnO nanorod is not excited, so the direct Z-scheme mechanism does not occur well in the RGO sensitized ZnO photoanode. To evaluate the photoreaction that produces H2, a photoelectrolysis reaction was tested under a bias of 1 V between the RGO modified ZnO working electrode and the Pt counter electrode (with no reference electrode). Fig. 4d shows the rates of H2 evolution in 0.1 M Na2SO4 solution over different photoelectrodes under UV-visible and only visible light irradiation (λ > 400 nm), respectively. Under UV-visible light irradiation, as compared to bare ZnO, a significantly larger rate of H2 evolution is obtained over RGO modified ZnO. The rate of H2 evolution calculated for the Pt–RGO–ZnO photoelectrode is 55 μmol h−1, which is even 5 times of magnitude higher than that for bare ZnO. Furthermore, in the case of bare ZnO, it is difficult to detect H2 under only visible light irradiation. In contrast, distinct H2 evolution is observed over RGO–ZnO and Pt–RGO–ZnO photoelectrodes under visible light irradiation, which further demonstrates the important promoting effect of RGO in H2 evolution photocatalysis. These photocatalytic results are consistent with the IPCE and PEC data.
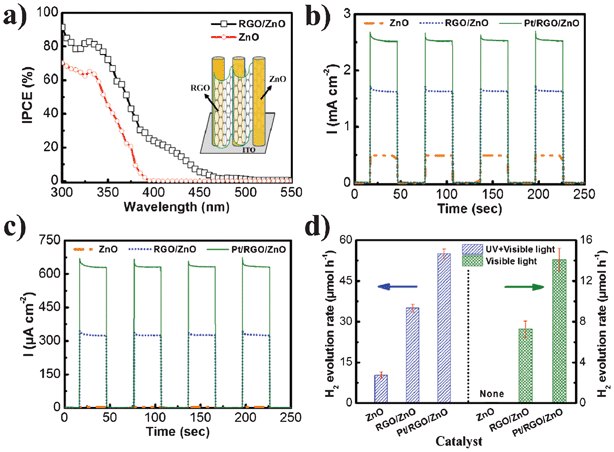 | ||
| Fig. 4 (a) The measured IPCE spectra of pristine ZnO nanorods and RGO–ZnO nanohybrids in the region 300–550 nm at a potential of 0.4 V (vs. Pt) in the two-electrode system. Inset: schematic illustration for the 1D ZnO nanorod–2D RGO nanosheet hybrid. (b) Photocurrent responses of bare ZnO, RGO–ZnO, and Pt–RGO–ZnO photoelectrodes under UV-visible light irradiation (λ > 300 nm). (c) Photocurrent responses of bare ZnO, RGO–ZnO, and Pt–RGO–ZnO photoelectrodes under visible-light irradiation (λ > 400 nm). (d) Comparison of photocatalytic performance over bare ZnO, RGO–ZnO, and Pt–RGO–ZnO photoelectrodes under UV-visible light (λ > 300 nm) and visible-light (λ > 400 nm) irradiation. | ||
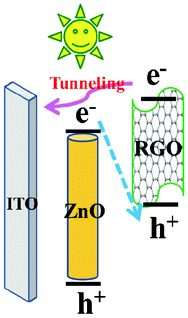 | ||
| Fig. 5 The possible schematic process of photoexcited electron–hole transport in the RGO photosensitized ZnO nanoelectrode. | ||
In summary, a novel one-step and ultrafast approach to the synthesis of highly photoactive RGOs is reported by the simple EC exfoliation of graphite at room temperature. The oxidation degree of RGOs can be easily controlled by applying different switching potentials. The effective deoxygenation could be achieved at a much higher switching bias. A red-shift of light absorption over RGOs with the enlarged switching potential can be also observed. Furthermore, by constructing a RGO–ZnO heterojunction and investigating its PEC properties, two potential functions of RGO have been explored. Firstly, RGO as an effective promoter can make H2 evolution easier. Secondly, highly photoactive RGO can improve the photoconversion ability of ZnO under visible-light irradiation. Our green and efficient process has provided a straightforward approach to generate photochemically active RGO with tunable oxidation degree, among other applications, as high-performance photoelectrode sensitizer in energy conversion devices.
Acknowledgements
This work was supported by the National Science Council, Dragon's Gate Program, Taiwan. We gratefully thank NSC, IAMS, NTU and Argonne National Laboratory for financial support for this project.References
- X. Huang, X. Qi, F. Boey and H. Zhang, Chem. Soc. Rev., 2012, 41, 666 RSC.
- Q. Xiang, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2012, 41, 782 RSC.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228 RSC.
- G. Eda and M. Chhowalla, Adv. Mater., 2010, 22, 2392 CrossRef CAS.
- H. Bai, C. Li and G. Q. Shi, Adv. Mater., 2011, 23, 1089 CrossRef CAS.
- O. C. Compton and S. T. Nguyen, Small, 2010, 6, 711 CrossRef CAS.
- S. B. Yang, X. L. Feng, S. Ivanovici and K. Müllen, Angew. Chem., Int. Ed., 2010, 49, 8408 CrossRef CAS.
- C. Gomez-Navarro, R. T. Weitz, A. M. Bittner, M. Scolari, A. Mews, M. Burghard and K. Kern, Nano Lett., 2007, 7, 3499 CrossRef CAS.
- Y. Matsumoto, M. Koinuma, S. Ida, S. Hayami, T. Taniguchi, K. Hatakeyama, H. Tateishi, Y. Watanabe and S. Amano, J. Phys. Chem. C, 2011, 115, 19280 CAS.
- R. J. W. E. Lahaye, H. K. Jeong, C. Y. Park and Y. H. Lee, Phys. Rev. B, 2009, 79, 125435 CrossRef.
- S. Park and R. S. Ruoff, Nat. Nanotechnol., 2009, 4, 217 CrossRef CAS.
- W. Gao, L. B. Alemany, L. Ci and P. M. Ajayan, Nat. Chem., 2009, 1, 403 CrossRef CAS.
- C. Su, A. Lu, Y. Xu, F. Chen, A. N. Khlobystov and L. Li, ACS Nano, 2011, 5, 2332 CrossRef CAS.
- H. L. Guo, X. F. Wang, Q. Y. Qian, F. B. Wang and X. H. Xia, ACS Nano, 2009, 3, 2653 CrossRef CAS.
- H. J. Shin, K. K. Kim, A. Benayad, S. M. Yoon, H. K. Park, I. S. Jung, M. H. Jin, H. K. Jeong, J. M. Kim, J. Y. Choi and Y. H. Lee, Adv. Funct. Mater., 2009, 19, 1987 CrossRef CAS.
- D. Graf, F. Molitor, K. Ensslin, C. Stampfer, A. Jungen, C. Hierold and L. Wirtz, Nano Lett., 2007, 7, 238 CrossRef CAS.
- H. Huang, Z. Li, J. She and W. Wang, J. Appl. Phys., 2012, 111, 054317 CrossRef.
- Y. K. Hsu, Y. G. Lin and Y. C. Chen, Electrochem. Commun., 2011, 13, 1383 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
