Nano-sized manganese oxide–bovine serum albumin was synthesized and characterized. It is promising and biomimetic catalyst for water oxidation†
Mohammad Mahdi
Najafpour
*ab,
Davood Jafarian
Sedigh
a,
Cecil K.
King'ondu
c and
Steven L.
Suib
cd
aDepartment of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan 45137-66731, Iran. E-mail: mmnajafpour@iasbs.ac.ir; Tel: (+98) 241 415 3201
bCenter of Climate Change and Global Warming, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan 45137-66731, Iran
cDepartment of Chemistry, University of Connecticut, 55 N. Eagleville Rd., Unit 3060, Storrs, CT 06269-3060, USA
dInstitute of Materials Science, University of Connecticut, Storrs, CT 06269, USA
First published on 19th September 2012
Abstract
Nano-sized manganese oxide–bovine serum albumin was synthesized and characterized by scanning electron microscopy (SEM), atomic force microscopy (AFM), UV-vis, X-ray diffraction (XRD), infrared spectroscopy (FTIR), energy-dispersive X-ray spectroscopy (EDX), elemental mapping and transmission electron microscopy (TEM). The oxide shows water oxidizing activity in the presence of cerium(IV) ammonium nitrate as a non-oxo transfer oxidant. We used bovine serum albumin in the compound as a promising model for stabilizing proteins in photosystem II.
Replacing fossil fuels by an energy system that uses sustainable fuel is among the most critical issues of today's society.1 Water splitting into H2 and O2 is currently much discussed as a promising route for the conversion of solar, wind, ocean current, tidal or wave energy into hydrogen as a “solar fuel”.2 One of the great challenges to make such a plan is the development of efficient catalysts for the oxidation of water to molecular oxygen.2–4
Plants, algae and cyanobacteria use light energy to oxidize water efficiently.5 Water oxidation is catalysed by a Mn4CaO5 cluster known as a water oxidizing complex (WOC), or an oxygen-evolving complex (OEC), housed in photosystem II (PSII).6–8
Since the WOC consists of four manganese ions,7,8 particular attention has been given to the manganese compounds aimed at simulating the WOC of PSII.9 Few manganese complexes have been discovered so far that can act as homogeneous catalysts for the oxidation of water.9 However, manganese oxides have been reported as heterogeneous catalysts for water oxidation by Shilov and Shafirovich, Morita et al., Harriman et al. and other groups.10 Recently, in a different approach aimed at simulating the Mn4CaO5 cluster in PSII, we introduced layered manganese oxides as the closest structural and functional analogs to the WOC in PSII found so far.11 However, these synthetic models contain no amino acid groups to stabilize manganese oxide, proton transfer and/or decrease the activation energy for water oxidation as in PSII.7,8,12
There are at least six intrinsic proteins that appear to be required for water oxidation by the WOC in PSII. These are proteins CP 47, CP 43, the D1 protein, the D2 protein, and the α and β subunits of cytochrome b559.12 Thus, PSII consists of hundreds of amino acids (Fig. 1), but only a small fraction of the residues come in to direct contact with the manganese–calcium cluster (Fig. 1).12
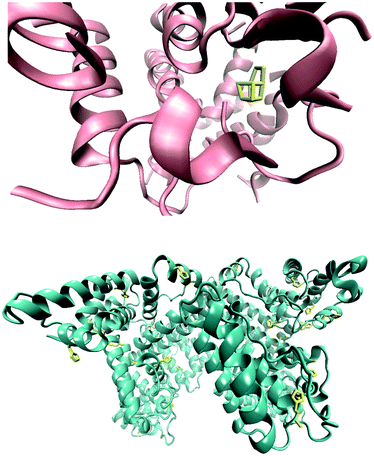 | ||
| Fig. 1 The Mn4CaO5 cluster and the surrounding amino acids. The whole structure of the Mn4CaO5 cluster resembles a distorted chair, with the asymmetric cubane. There is only a small fraction of the residues that come in to direct contact with the manganese–calcium cluster (top). A three dimensional image of the bovine serum albumin molecule. The imidazole groups as proposed sites for the interaction with manganese ions are shown in yellow (bottom). | ||
Many of the amino acids in PSII are involved in proton, water or oxygen transfer. The roles for the residues that come in contact directly with the manganese–calcium cluster include the regulation of charges and the electrochemistry of the Mn–Ca cluster, and helping coordinate water molecules at the appropriate metal sites and/or the stability of this cluster.12
The PSII manganese stabilizing protein is a highly conserved extrinsic component of the WOC and its deletion from the photosystem could cause a dramatic lowering of the rate of oxygen evolution.12 The PSII manganese stabilizing protein in addition to stabilizing manganese oxides and/or decreasing the activation energy for water oxidation, has also been suggested to be important in linking the active site of the WOC with the lumen and to be involved in the proton transfer network.12 Here, for the first time a nano-sized manganese oxide–bovine serum albumin (BSA) is studied as a structural and functional model for the WOC in PSII. BSA was used in the compound as a promising model for stabilizing proteins in PSII.
BSA is one of the most studied proteins that has a strong affinity for a variety of inorganic molecules binding to different sites.13 BSA is a soluble protein in the plasma of the circulatory system and acts in the transport and deposition of endogenous and exogenous substances. Albumin can readily undergo conformational changes, and is classed as a soft protein due to its relatively flexible structure. The three dimensional image of the serum albumin molecule is shown in Fig. 1.14
The charge, coulombic forces, van der Waals forces, hydrophobic interactions, sorbate conformational stability and surface area that the particle provides are important factors for the interaction of a protein and a compound.15
The nano-sized manganese oxide–BSA, reported here, was synthesized by a very simple method. In brief, manganese(II) chloride (80.0 mg, 0.62 mmol) was added to a solution of BSA (0.5 g) in water (40.0 mL) and the solution was stirred for four hours. Then to the solution, KMnO4 (44 mg in 8.0 mL water) was added under stirring. After evaporating water, the manganese oxide–BSA film was obtained. Nano-sized manganese oxide–BSA as both a solution and film shows water oxidizing activity.
The brown solution was characterized by UV-vis, SEM, TEM, IR, AFM, and XRD. The water oxidation activity of the compound was studied. Drying the solution in air and at room temperature produced a homogenous nano-sized manganese oxide–bovine serum albumin film (see the ESI†). In the IR spectrum of the compound, the broad band at ∼3200–3500 cm−1 related to the antisymmetric and symmetric O–H stretchings and the band at ∼1630 cm−1 related to the H–O–H bending (Fig. S7†). The intensities of these peaks reduced at higher temperatures. The absorption bands characteristic for a MnO6 core in the region 400–500 cm−1 assigned to the stretching vibrations of the Mn–O bonds in manganese oxide were also observed in the FTIR spectra of these compounds. The new peak at ∼400 nm in the UV-vis spectrum of the compound can also show manganese oxide formation in this compound (Fig. S8†). The XRD was of poor resolution but it shows the layered manganese oxide in the film (Fig. 2). The layered manganese oxide was also detected by TEM (Fig. 3). The layered structure of the manganese oxide is not surprising as the method is similar to the one used to synthesise birnessite manganese oxide.4
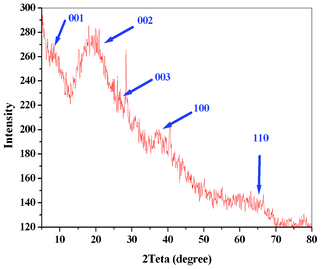 | ||
| Fig. 2 Powder X-ray diffraction patterns and related peaks for the layered manganese oxides in the manganese oxide–BSA film. | ||
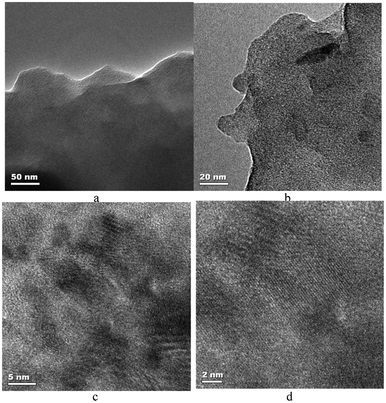 | ||
| Fig. 3 The TEM (a, b) and HRTEM images (c, d) of the manganese oxide–BSA film. | ||
The results show that BSA not only induces nucleation, but it could inhibit the further growth of manganese oxide as without BSA larger particles are formed. Moreover, the dispersion of manganese oxide in water is unique and may also increase the rate of reaction of particles with reactants, and could result in a high rate for many reactions.
As shown in Fig. 3, very small particles of nano-sized manganese oxide attached to the BSA protein can be observed.
In the nano-sized manganese oxide–BSA compound, manganese oxide is amorphous, and a specific phase could not be identified.
The AFM and EDX elemental map images show that nucleation occurs at special locations most probably near imidazole and carboxylate groups (Fig. 4).
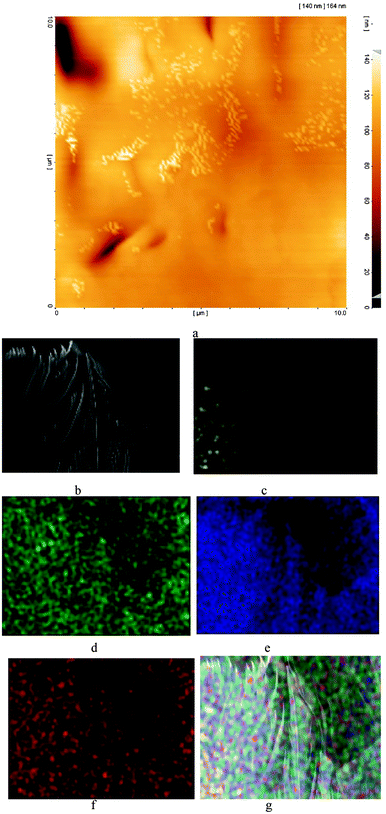 | ||
| Fig. 4 The AFM image shows that nucleation (white areas) occurs at special locations (a). The SEM image (b) and EDX elemental maps of the SEM image for Mn (c), N (d), C (e), O (f) and all of these elements in one image ((g) Mn: yellow, N: green, C: blue and O: red). | ||
(NH4)2Ce(NO3)6 is a strong non-oxo transfer oxidant and one-electron oxidant. This material is widely used as the primary oxidant in the oxidation of water to oxygen catalyzed by manganese compounds because of its solubility and stability in water.4,10 The nano-sized manganese oxide–BSA is a good catalyst for water oxidation in the presence of Ce(IV) (Fig. 5). The rate of oxygen evolution of this compound in the presence of Ce(IV) (0.1 M) is 270 μmol O2 per mol Mn per second.
![Water oxidation in an aqueous solution of 0.1 M Ce(iv) at 25.0 °C in the presence of nano-sized manganese oxide–BSA solution (top). Water oxidation in an aqueous solution of 0.2 mM [Ru(bpy)3]Cl2 at 25.0 °C in the presence of nano-sized manganese oxide–BSA film (bottom). In the presence of BSA, without manganese oxide, no oxygen was observed.](/image/article/2012/RA/c2ra21251j/c2ra21251j-f5.gif) | ||
| Fig. 5 Water oxidation in an aqueous solution of 0.1 M Ce(IV) at 25.0 °C in the presence of nano-sized manganese oxide–BSA solution (top). Water oxidation in an aqueous solution of 0.2 mM [Ru(bpy)3]Cl2 at 25.0 °C in the presence of nano-sized manganese oxide–BSA film (bottom). In the presence of BSA, without manganese oxide, no oxygen was observed. | ||
Ru(bpy)3+2 has been widely applied as a photosensitizer in artificial photosystems because of its superior photophysical properties with moderate pH.10 The photoinduced oxidation of manganese oxides in the presence of [Ru(bpy)3]+2 in aqueous solution needs the use of a sacrificial oxidant that is irreversibly reduced allowing the net conversion of [Ru(bpy)3]+2 to [Ru(bpy)3]+3 (Scheme 1). [Ru(bpy)3]+3 can then act as an oxidant toward compounds by an intermolecular electron transfer since the potential of the [Ru(bpy)3]+2/[Ru(bpy)3]+3 redox couple in aqueous solution is E1/2 = 1.1 V vs. Ag/AgCl/KCl (3.0 M).
![Schematic of the photocatalytic water oxidation by the [Ru(bpy)3]2+ as a photosensitizer and the nano-sized manganese oxide–bovine serum albumin. A proposed mechanism for the water oxidation by nano manganese oxide–BSA. Similar to PSII, the hybrid may immobilize manganese oxides in a hydrophilic and highly oxidation stable environment (bottom).](/image/article/2012/RA/c2ra21251j/c2ra21251j-s1.gif) | ||
| Scheme 1 Schematic of the photocatalytic water oxidation by the [Ru(bpy)3]2+ as a photosensitizer and the nano-sized manganese oxide–bovine serum albumin. A proposed mechanism for the water oxidation by nano manganese oxide–BSA. Similar to PSII, the hybrid may immobilize manganese oxides in a hydrophilic and highly oxidation stable environment (bottom). | ||
In this study, K2S2O8 was used as the electron acceptor for water oxidation during irradiation with visible light (λ > 400 nm), oxygen evolution was observed upon adding the nano-sized manganese oxide–BSA compound to an aqueous solution containing tris(2,2′-bipyridyl)ruthenium(II) chloride and K2S2O8. The intensity of light was less than one-tenth of bright sunlight (on a bright sunny day illumination can be more than 100,000 lux) (Fig. 5). The rate of oxygen evolution of this compound in these conditions is 140 μmol O2 per mol Mn per second.
Compared with the water oxidation activity of other manganese oxides nano-sized manganese oxide–BSA materials are good catalysts for water oxidation thus showing that the incorporation of manganese oxides into BSA can improve the water oxidation activity of these compounds (Table S1, ESI†). At least, BSA could help in the dispersion of these nanoparticles in solution, and the dispersivity of the particles in water could increase the rate of the reaction of the particles with oxidants (Ce(IV) or [Ru(bpy)3]+3).16
As reported in previous publications,19 we propose that the O–O bond may be formed by the attack of an outer-sphere water on an OH attached to a highly valent manganese ion in the oxide structure. The attack of the water molecule on the OH group may occur by a nucleophilic, radical or radical–nucleophilic mechanism (Scheme 1).
In conclusion, we introduced BSA as a promising structural and functional model for stabilizing proteins in PSII. Both BSA and the stabilizing protein in PSII are proteins that form hydrophilic and highly oxidation stable environments for manganese oxides. It is clear that the sequence of amino acids is different in the stabilizing protein in PSII. In other words, the 3 billion year evolutionary experiments by nature have provided a better sequence of amino acids around manganese ions to form an efficient catalyst.
MMN and DJS are grateful to the Institute for Advanced Studies in Basic Sciences for financial support. CKK and SLS acknowledge the support of the US Department of Energy, Office of Basic Energy Sciences, Division of Chemical, Geological and Biological Sciences. Fig. 1 was made with VMD and the images are owned by the Theoretical and Computational Biophysics Group, NIH Resource for Macromolecular Modeling and Bioinformatics, at the Beckman Institute, University of Illinois at Urbana-Champaign. The original data for Fig. 1 is from ref. 7 (PDB: 3ARC).
References
- (a) M. Z. Jacobson, Energy Environ. Sci., 2009, 2, 148 RSC; (b) J. Barber, Chem. Soc. Rev., 2009, 38, 185 RSC; (c) R. M. Navarro, M. C. Alvarez-Galvan, J. A. Villoria de la Mano, S. M. Al-Zahrani and J. L. G. Fierro, Energy Environ. Sci., 2010, 3, 1865 RSC; (d) M. Pagliaro, A. G. Konstandopoulos, R. Ciriminnaa and G. Palmisano, Energy Environ. Sci., 2010, 3, 279 RSC.
- J. O. M. Bockris, Energy—The Solar Hydrogen Alternative, Wiley & Sons, New York, 1977 Search PubMed.
- (a) S. Y. Reece, J. A. Hamel, K. Sung, T. D. Jarvi, A. J. Esswein, J. J. H. Pijpers and D. G. Nocera, Science, 2011, 334, 645 CrossRef CAS; (b) W. M. W. Kanan and D. G. Nocera, Science, 2008, 321, 1072 CrossRef CAS.
- M. M. Najafpour, T. Ehrenberg, M. Wiechen and P. Kurz, Angew. Chem., Int. Ed., 2010, 49, 2233 CrossRef CAS.
- (a) J. Kern and G. Renger, Photosynth. Res., 2007, 94, 183–202 CrossRef CAS; (b) T. Wydrzynski, S. Satoh and Govindjee, PSII: The Light-Driven Water: Plastoquinone Oxidoreductase, Advances in Photosynthesis and Respiration Series, Springer, Dordrecht, 2005, vol. 22 Search PubMed; (c) L. X. Shi, M. Hall, C. Funk and W. P. Schroder, Biochim. Biophys. Acta, Bioenerg., 2012, 1817, 13 CrossRef CAS; (d) S. Styring, J. Sjöholm and F. Mamedov, Biochim. Biophys. Acta, Bioenerg., 2012, 1817, 76 CrossRef CAS; (e) M. M. Najafpour, A. Nemati Moghaddam, Y. N. Yang, E. Aro, R. Carpentier, J. J. Eaton-Rye, C. Lee and S. I. Allakhverdiev, Photosynth. Res., 2012, 114, 1 Search PubMed.
- (a) V. K. Yachandra, V. J. DeRose, M. J. Latimer, I. Mukerji, K. Sauer and M. P. Klein, Science, 1993, 260, 675 CrossRef CAS; (b) J. E. Penner-Hahn, Structural characterization of the Mn site in the photosynthetic oxygen-evolving complex, in Metal Sites in Proteins and Models: Redox Centres, ed. H. A. O. Hill, P. J. Sadler and A. J. Thomson, Springer, Berlin/Heidelberg, Germany, 1998, vol. 90, pp. 1–36 Search PubMed; (c) H. Dau, L. Iuzzolino and J. Dittmer, Biochim. Biophys. Acta, Bioenerg., 2001, 1503, 24 CrossRef CAS; (d) K. Sauer, J. Yano and V. K. Yachandra, Coord. Chem. Rev., 2008, 252, 318 CrossRef CAS; (e) A. Zouni, H. T. Witt, J. Kern, P. Fromme, N. Krauss, W. Saenger and P. Orth, Nature, 2001, 409, 739 CrossRef CAS; (f) N. Kamiya and J.-R. Shen, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 98 CrossRef CAS; (g) K. N. Ferreira, T. M. Iverson, K. Maghlaoui, J. Barber and S. Iwata, Science, 2004, 303, 1831 CrossRef CAS; (h) B. Loll, J. Kern, W. Saenger, A. Zouni and J. Biesiadka, Nature, 2005, 438, 1040 CrossRef CAS; (i) J. Yano, J. Kern, K. Sauer, M. J. Latimer, Y. Pushkar, J. Biesiadka, B. Loll, W. Saenger, J. Messinger and A. Zouni, Science, 2006, 314, 821 CrossRef CAS; (j) J. W. Murray, K. Maghlaoui, J. Kargul, N. Ishida, T.-L. Lai, A. W. Rutherford, M. Sugiura, A. Boussac and J. Barber, Energy Environ. Sci., 2008, 1, 161 RSC; (k) K. Kawakami, Y. Umena, N. Kamiya and J. R. Shen, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 8567 CrossRef CAS; (l) A. Guskov, J. Kern, A. Gabdulkhakov, M. Broser, A. Zouni and W. Saenger, Nat. Struct. Mol. Biol., 2009, 16, 334 CrossRef CAS; (m) M. Broser, C. Glöckner, A. Gabdulkhakov, A. Guskov, J. Buchta, J. Kern, F. Müh, H. Dau, W. Saenger and A. Zouni, J. Biol. Chem., 2011, 286, 15964 CrossRef CAS; (n) E. M. Sproviero, J. A. Gascón, J. P. McEvoy, G. W. Brudvig and V. S. Batista, J. Am. Chem. Soc., 2008, 130, 3428–3442 CrossRef CAS; (o) M. M. Najafpour, Plant Biosyst., 2006, 140, 163 Search PubMed.
- Y. Umena, K. Kawakami, J. R. Shen and N. Kamiya, Nature, 2011, 473, 55 CrossRef CAS.
- Y. Umena, N. Kamiya and J. R. Shen, J. Photochem. Photobiol., B, 2011, 104, 9 CrossRef CAS.
- (a) M. M. Najafpour, A. Nemati Moghaddam and S. I. Allakhverdiev Govindjee, Biochim. Biophys. Acta, Bioenerg., 2012, 1817, 1110 CrossRef CAS; (b) R. Pace, An Integrated Artificial Photosynthesis Model, in Artificial photosynthesis: From basic biology to industrial application, ed. A. F. Collings and C. Critchley, Wiley-VCH, Weinheim, 1st edn, 2005, pp 13–34 Search PubMed; (c) H. Dau and I. Zaharieva, Acc. Chem. Res., 2009, 42, 1861 CrossRef CAS; (d) W. Lubitz, E. J. Reijerse and J. Messinger, Energy Environ. Sci., 2008, 1, 15 RSC; (e) C. W. Cady, R. H. Crabtree and G. W. Brudvig, Coord. Chem. Rev., 2008, 252, 444 CrossRef CAS; (f) M. M. Najafpour and S. I. Allakhverdiev, Int. J. Hydrogen Energy, 2012, 37, 8753 CrossRef CAS; (g) K. Beckmann, H. Uchtenhagen, G. Berggren, M. F. Anderlund, A. Thapper, J. Messinger, S. Styring and P. Kurz, Energy Environ. Sci., 2008, 1, 668 RSC.
- (a) V. Y. Shafirovich and A. E. Shilov, Kinetika i Kataliz (translated from Russian), 1979, 20, 1156 Search PubMed; (b) M. Morita, C. Iwakura and H. Tamura, Electrochim. Acta, 1977, 22, 325 CrossRef CAS; (c) A. Harriman, I. J. Pickering, J. M. Thomas and P. A. Christensen, J. Chem. Soc., Faraday Trans. 1, 1988, 84, 2795 RSC; (d) F. Jiao and H. Frei, Chem. Commun., 2010, 46, 2920 RSC; (e) M. M. Najafpour, Origins Life Evol. Biosphere, 2011, 41, 237 CrossRef CAS; (f) M. M. Najafpour, Dalton Trans., 2011, 40, 3805 RSC; (g) M. M. Najafpour, Geomicrobiol. J., 2011, 28, 714 CrossRef CAS; (h) M. M. Najafpour, Dalton Trans., 2011, 40, 3793 RSC; (i) M. M. Najafpour, S. Nayeri and B. Pashaei, Dalton Trans., 2011, 40, 9374 RSC; (j) A. Iyer, J. Del-Pilar, C. K. King'ondu, E. Kissel, H. Fabian Garces, H. Huang, A. M. El-Sawy, P. K. Dutta and S. L. Suib, J. Phys. Chem. C, 2012, 116, 6474 CrossRef CAS; (k) M. M. Najafpour and B. Pashaei, Dalton Trans., 2012, 41, 10156 RSC; (l) M. M. Najafpour, F. Rahimi, E. Aro, C. Lee and S. I. Allakhverdiev, J. R. Soc. Interface, 2012, 9, 2383 Search PubMed; (m) M. M. Najafpour and A. Nemati Moghaddam, Dalton Trans., 2012, 41, 10292 RSC; (n) M. M. Najafpour, B. Pashaei and S. Nayeri, Dalton Trans., 2012, 41, 4799 RSC.
- (a) I. Zaharieva, M. M. Najafpour, M. Wiechen, M. Haumann, P. Kurz and H. Dau, Energy Environ. Sci., 2011, 4, 2400 RSC; (b) M. M. Najafpour, J. Photochem. Photobiol., B, 2011, 104, 111 CrossRef CAS; (c) M. M. Najafpour, M. Amouzadeh Tabrizi, B. Haghighi and Govindjee, Dalton Trans., 2012, 41, 3906 RSC; (d) M. M. Najafpour, Chem. Commun., 2011, 47, 11724 RSC; (e) M. M. Najafpour, F. Rahimi, M. Amini, S. Nayeri and M. Bagherzadeh, Dalton Trans., 2012, 41, 11026 RSC; (f) M. Amini, M. M. Najafpour, S. Nayeri, B. Pashaei and M. Bagherzadeh, RSC Adv., 2012, 2, 3654 RSC; (g) D. Shevela, S. Koroidov, M. M. Najafpour, J. Messinger and P. Kurz, Chem.–Eur. J., 2011, 17(19), 5415 CrossRef CAS.
- (a) H. Popelkova and C. F. Yocum, J. Photochem. Photobiol., B, 2011, 104, 179 CrossRef CAS; (b) T. M. Bricker, J. L. Roose, R. D. Fagerlund, L. K. Frankel and J. J. Eaton-Rye, Biochim. Biophys. Acta, Bioenerg., 2012, 1817, 121 Search PubMed; (c) R. Nagao, T. Suzuki, A. Okumura, A. Niikura, M. Iwai, N. Dohmae, T. Tomo, J.-R. Shen, M. Ikeuchi and I. Enami, Plant Cell Physiol., 2010, 51, 718 Search PubMed; (d) T. M. Bricker and L. K. Frankel, Photosynth. Res., 1998, 56, 157 CrossRef CAS; (e) J. L. Roose, Y. Kashino and H. B. Pakrasi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 2548 Search PubMed; (f) I. Enami, S. Kikuchi, T. Fukuda, H. Ohta and J.-R. Shen, Biochemistry, 1998, 37, 2787 Search PubMed; (g) J. De Las Rivas and J. Barber, Photosynth. Res., 2004, 81, 329 CrossRef CAS; (h) T. Miyaoka Enami, Y. Mochizuki, J. -R. Shen, K. Satoh and S. Katoh, Biochim. Biophys. Acta, Bioenerg., 1989, 973, 35 Search PubMed.
- (a) S. Yu, Top Curr. Chem., 2007, 79, 271 Search PubMed; (b) L. Yang, R. Xing, Q. Shen, K. Jiang, F. Ye, J. Wang and Q. Ren, J. Phys. Chem. B, 2006, 110, 10534 CrossRef CAS; (c) D. Qin, X. Ma, L. Yang, L. Zhang, Z. Ma and J. Zhang, J. Nanopart. Res., 2008, 10, 559 CrossRef CAS; (d) J. Zhang, X. Ma, Y. Guo, L. Yang, Q. Shen, H. Wang and Z. Ma, Mater. Chem. Phys., 2010, 119, 112 CrossRef CAS; (e) P. Huang, Y. Kong, Z. Li, F. Gao and D. Cui, Nanoscale Res. Lett., 2010, 5, 949 CrossRef CAS.
- W. Norde, Colloids Surf., B, 2008, 61(1), 1 CrossRef CAS.
- (a) C. A. Haynes and W. Norde, Colloids Surf., B, 1994, 2(6), 517 CrossRef CAS; (b) T. Arai and W. Norde, Colloids Surf. B Biointerfaces, 1990, 51, 1 CrossRef CAS.
- In a real artificial photosynthetic system, water-oxidation catalysis has to be coupled to other electron acceptor materials than Ce(IV) or [Ru(bipy)3]3+.17,18 For example, Ce(IV) as a very powerful oxidant (E° > +1.7 V) could oxidize and decompose the amino acids of BSA in addition to oxidizing the manganese ions in manganese oxide. In biological water oxidation, the WOC is linked to the reaction center II chlorophyll via a redox-active tyrosine residue on the D1 subunit, labeled as YZ.5 The residue oxidizes the WOC. The YZ/YZ potential is only ∼1.0 V vs. SHE5.
- M. Wiechen, H. Berends and P. Kurz, Dalton Trans., 2012, 41, 21 RSC.
- M. M. Najafpour Govindjee, Dalton Trans., 2011, 40, 9076 RSC.
- (a) M. M. Najafpour, B. Pashaei and S. Nayeri, Dalton Trans., 2012, 41, 7134 RSC; (b) M. M. Najafpour and B. Pashaei, Dalton Trans., 2012, 41, 10156 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21251j |
| This journal is © The Royal Society of Chemistry 2012 |
