Energy storage characteristics of a new rechargeable solid oxide iron–air battery†
Xuan
Zhao
,
Nansheng
Xu
,
Xue
Li
,
Yunhui
Gong
and
Kevin
Huang
*
Department of Mechanical Engineering, University of South Carolina, SC 29208, USA
First published on 4th September 2012
Abstract
Cost effective and large scale energy storage is critical to renewable energy integration and smart-grid energy infrastructure. Rechargeable batteries have great potential to become a class of cost effective technology suited for large scale energy storage. In this paper, we report the energy storage characteristics of a newly developed rechargeable solid oxide iron–air battery. Investigations of the battery’s performance under various current densities and cycle durations show that iron utilization plays a determining role in storage capacity and round-trip efficiency. Further studies of the battery's cycle life reveal a unique charge-cycle originated degradation mechanism that can be interpreted by a combined vapor-phase transport and electrochemical condensation model. Overall, the energy capacity of the new solid oxide iron–air storage battery should be properly balanced with the round-trip efficiency at optimized iron utilization.
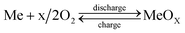
Cost effective and large scale energy storage is essential to the growth of the future's “green energy” infrastructure. Rechargeable batteries and supercapacitors, or a hybrid form of the two, offer a number of advantages over the conventional large scale siting- and geography-constrained pumped-water and compressed-air energy storage systems.1–6 However, rechargeable batteries and supercapacitors will need many breakthroughs in material design and system integration to become commercially viable. In an effort to advance rechargeable batteries towards an efficient, cost competitive energy storage product, we have recently demonstrated proof-of-concept of a new rechargeable solid oxide metal-air battery.7 The new battery combines a regenerative solid oxide electrochemical cell (RSOEC) and a redox cycle unit (RCU) for energy storage. The RSOEC serves as the “electrical functioning unit”, alternating between the fuel cell and electrolyzer modes to realize the discharge and charge cycles, while the RCU acts as the “energy storage unit”, converting electrical-chemical energy in situ via a H2/H2O-mediated metal/metal oxide redox reaction. Fig. 1 shows a schematic of the working principle. The resultant overall chemical reaction occurring inside the battery is essentially a metal–air reaction:
 | (1) |
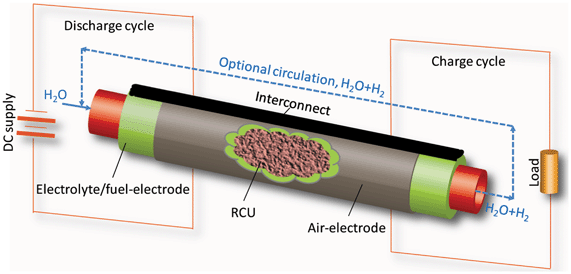 | ||
| Fig. 1 Schematic of working principle of the new solid oxide based metal–air battery. An anode-supported tubular RSOEC is used for illustration purpose. | ||
Among many features of the new battery are three key characteristics: high energy capacity, enabled by multiple-electron charge transfer; fast charging and discharging, resulting from the decoupling of the “energy storage unit” from the “electrical functioning unit”, and the use of earth-abundant and environmentally benign iron-based redox energy storage materials.
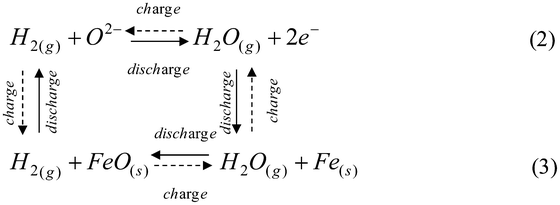
In this paper, we present new results regarding the energy storage characteristics of this new type of “metal–air” battery as a continued effort from our previous proof-of-concept work.7 The studied battery consists of a ZrO2-based RSOEC and an Fe-based RCU. The former utilizes a 150 μm thick ZrO2-based electrolyte disc, as the support for a 50 μm thick Ni–ZrO2-based electrolyte/Ni–GDC fuel electrode, and a 50 μm thick LSM–GDC air-electrode. The latter contains an Fe–FeO redox couple supported on ZrO2. Details about battery assembly can be found in Electronic Supplementary Information.† The first result is related to the phase evolution of iron oxides during the H2-reduction process. The RSOEC's Electromotive Force (EMF) is conveniently used as an indicator of the phase equilibrium. For a ternary system like Fe–O–H under an isothermal and isobaric condition, Gibbs’ phase rule defines only one independent intensive variable for a two-phase system; the independent variable in this case is the partial pressure of oxygen, pO2, or the ratio of partial pressure of H2O and H2, pH2O/pH2. The fixed pO2 (or pH2O/pH2) yields a constant EMF in an oxygen concentration cell like RSOEC. The EMFs in Fig. 2(a) were recorded as a function of time during an H2-reduction process at 800 °C and show several voltage plateaus, each of which represents a two-phase equilibrium that corresponds to a fixed pO2 or pH2O/pH2 according to Gibbs’ phase rule. Thermodynamic assessments indicate that these plateaus correspond to the two-phase equilibria of Fe2O3–Fe3O4 (at ∼0.388 volt), Fe3O4–FeO (at ∼0.938 volt) and FeO–Fe (at ∼0.970 volt). The last plateau at ∼1.30 volts reflects the pO2 in the H2 stream (with a trace amount of H2O in pure H2) equilibrating with a metallic Fe phase. The solid oxide iron–air battery utilizes the FeO–Fe phase equilibrium as a means of storing electrical–chemical energy via the following H2/H2O-mediated reversible electrochemical-chemical looping reactions:
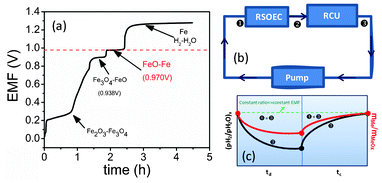 | ||
Fig. 2 (a) EMF recorded during the reduction of Fe2O3 by H2 at 800 °C; (b) key locations inside the battery exposed to reactant gas; (c) variations of pH2/pH2O and mMe/mMeOx at locations  = =  and and  with the state of an electrical cycle. td and tc are times for discharge and charge, respectively. with the state of an electrical cycle. td and tc are times for discharge and charge, respectively. | ||
One pronounced feature of the new battery is its use of a separate RCU, other than the electrode itself, as the energy storage component. This design yields an EMF independent of the cycle state. Fig. 2(b) and (c) schematically illustrate the variations of pH2/pH2O and the mass ratio of the metal and metal oxide, mMe/mMeOx at key locations inside the battery. Since the pH2/pH2O of the reactant gas entering the RSOEC remains constant during the cycle, the energy storage process is accomplished by a corresponding change in the mass ratio of Fe and FeO, mFe/mFeO, which is precisely regulated by the H2/H2O-mediated redox reaction. Such an EMF-constant electrochemical battery cell is advantageous compared to Na–S8 and other liquid metal batteries,9–11 in which the active species is directly incorporated into or extracted from the electrode structure, producing a process-dependent EMF.
The energy storage characteristics of the battery, which were investigated with 50–200 mA cm−2 of current density over 10 min to 6 h of cycle duration, show that the charge storage capacity is strongly dependent on actual iron utilization (UFe), a key parameter reflecting the combined effect of operating current and cycle duration. The variations of battery voltage with charge storage capacities in terms of mAh g−1 Fe and mAh cm−2 at different UFe are shown in Fig. 3. These two capacity terms evaluate the ability of the new battery to store electrical charge on the basis of the weight of energy storage material and the active area of the electrode, respectively. The battery explicitly exhibits a higher charge storage capacity at a higher UFe, but with more pronounced voltage degradation. Given the fact that the kinetic rate (moles s−1 cm−2) of a redox reaction generally decreases with time (a parabolic behavior), the resulting gradual decrease in the production rates of H2 (for discharging) and H2O (for charging) during a deep charge/discharge cycle would essentially increase the actual consumption of H2 and H2O by an RSOEC operating at a constant current, thus lowering voltage as a result of fuel starvation. Other factors, such as the loss of surface area of Fe-particles during high-temperature operation, could also add to the decline of capacity. Finally, the abrupt drop-off in voltage at UFe = 100% signals the shift of the Fe–FeO equilibrium to the adjacent oxygen-lean FeO–Fe3O4 equilibrium. The latter possesses only one third of the Fe–FeO redox couple's capacity, thus rendering the FeO–Fe3O4 equilibrium unfavorable for energy storage.
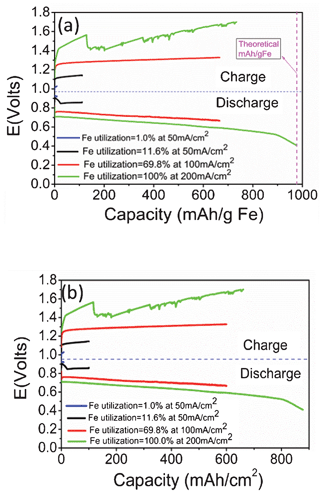 | ||
| Fig. 3 Energy storage characteristics measured at different UFe and 800 °C; (a) weight specific charge storage capacity (over 0.80 g Fe); (b) area-specific charge storage (over 0.88 cm2 active area of electrode). | ||
The relationship between energy capacity, round-trip efficiency and UFe are shown in Fig. 4. Energy capacity follows the theoretical line at low UFe, but quickly deviates to lower values at higher UFe. There are two sources of energy loss for the observed deviation: increased voltage losses from the ohmic and polarization resistances of the RSOEC at higher current density, and raised consumption of H2 and H2O by the RSOEC, due to decreased production rates of H2 and H2O over time in the RCU. The relatively low ionic conductivity and thick yttria stabilized zirconia (YSZ) electrolyte (150 μm) used in this study is a major cause of the higher energy loss. On the other hand, the observed decrease in round-trip efficiency with regard to UFe is attributed to the unbalanced energy inputs and outputs resulting from the RSOEC's polarization and the RCU's parabolic kinetics. Overall, the competing trend exhibited between capacity–UFe and efficiency–UFe suggests that the capacity and efficiency of the new solid oxide iron–air storage battery can be balanced with a proper choice of UFe. One ongoing project in our group is to optimize the performance of functional materials employed in RSOEC and RCU as an effort to achieve high capacity and efficiency at high UFe.
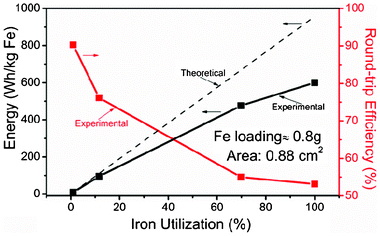 | ||
| Fig. 4 Energy storage capacity and round-trip efficiency as a function of iron utilization. | ||
The cyclic stability of the battery tested over ten continuous cycles is shown in Fig. 5. For each single cycle, the performance appears to be very stable. However, a marked gradual degradation is observed after each charge cycle. The faster degradation of the discharge compared to the charge in every cycle suggests that a performance-detrimental event has occurred during the charge cycle, which is accountable for the degradation found in the subsequent discharge cycle. We hypothesize the following mechanism for the degradation phenomenon. During the discharge cycle, in addition to the dominant reactions described in eqn (2) and (3), a parallel reaction between FeO(s) and H2O(g) can also take place due to its favorable thermodynamics under the battery's operating condition:
| FeO(s) + H2O(g) = Fe(OH)2(g) | (4) |
The calculated equilibrium partial pressure of Fe(OH)2(g), pFe(OH)2 = 2.71 × 10−8 atm (800 °C). During the charge cycle, in addition to the dominant reactions described in eq. (2) and (3), the gaseous Fe(OH)2(g) can also be reduced at the three-phase boundaries (TPBs) in the fuel-electrode via the following electrochemical reaction:
| 2Fe(OH)2(g) + 2e− = 2FeO(s) + O2− + H2(g) + H2O(g) | (5) |
A schematic showing such an electrochemical condensation process is given in Fig. 6. A simple estimation using the equilibrium pFe(OH)2 = 2.71 × 10−8 atm indicates that as high as 0.27 g FeO(s) per cm2 can be deposited onto the TPBs for a 2 h charge cycle shown in Fig. 5(a). With the catalytically inactive FeO(s) accumulating at the TPBs of the Ni-based fuel-electrode over every charge cycle, each following discharge cycle will suffer increased resistances of charge-transfer and mass-transfer as a result of decreased catalytic activity and porosity by the condensed FeO(s). This interpretation is consistent with the degradation trend shown in Fig. 5(a) of the multi-cycle curves. The AC impedance spectra of pre- and post-cycle samples shown in Fig. 5(b) further support the mechanism by demonstrating that the degradation is exclusively linked to the increase in the resistance of the fuel-electrode semicircle. Finally, the proposed electrochemical condensation of FeO(s) at the TPBs has also been experimentally confirmed by energy dispersive X-ray spectroscopy (EDS) analysis of the post-test fuel-electrode: Fig. S3† of Electronic Supplementary Information reveals 0.14 atom% Fe in the fuel electrode. It is also interesting to note from Fig. 5(b) that the use of pure H2 can decrease the battery's resistance to a level even lower than the original one. In line with the proposed model, this improvement can be reasonably understood as a result of freshly reduced fine particles of Fe(s) from the electrochemically condensed FeO(s), and their increased catalytic activity for electrochemical oxidation of H2 when combined with Ni(s).12
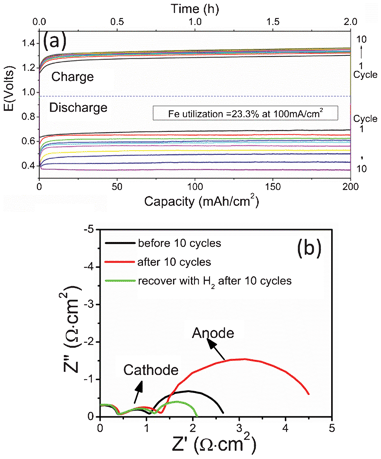 | ||
| Fig. 5 (a) Cyclic stability recorded at UFe = 23.3% and 800 °C; (b) AC impedance spectra showing close association of the degradation with the fuel-electrode. | ||
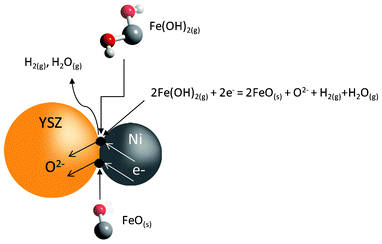 | ||
| Fig. 6 Schematic of electrochemical condensation of FeO(s) at the triple-phase boundaries (TPBs) of the fuel-electrode. | ||
In conclusion, the new solid oxide iron–air rechargeable battery has demonstrated the potential for high charge and energy storage capacities, as well as high efficiency. The capacity and efficiency characteristics of this battery are strongly dependent on the degree of iron utilization: higher charge and energy storage capacity, but lower round-trip efficiency, can be produced at higher iron utilization, and lower charge and energy storage capacity, but higher round-trip efficiency, can be produced at lower iron utilization. Improving electrochemical performance of the RSOEC components and catalytic activity of the RCU materials are attractive approaches to boost efficiency while maintaining high capacity. On the other hand, studies of the battery's cycle life reveal a unique charge-cycle originated degradation mechanism. A combined vapor-phase transport and electrochemical condensation mechanism is proposed to interpret the degradation behavior, which is favorably confirmed by EDS analysis of the post-test sample. Overall, the presented fundamental findings are essential to the successful development and deployment of an efficient, cost effective, environmentally friendly, and sustainable solid oxide metal–air rechargeable battery for grid energy storage. Recent efforts in our laboratory have been devoted to lowering the battery's operating temperature to the 600–650 °C range as a means of improving the cyclic stability, as well as designing and eventually testing a hundred-watt multi-battery bundle/stack system operating at a practically meaningful voltage and current density.
Acknowledgements
The authors acknowledge the Solid Oxide Fuel Cell Center of Excellence and Department of Mechanical Engineering in the University of South Carolina for providing seed funding to this work.References
- P. Poizot and F. Dolhem, Energy Environ. Sci., 2011, 4, 2003–2019 CAS.
- T. R. Cook, D. K. Dogutan, S. Y. Reece, Y. Surendranath, T. S. Teets and D. G. Nocera, Chem. Rev., 2010, 110, 6474–6502 CrossRef CAS.
- C. J. Yang and R. B. Jackson, Renewable Sustainable Energy Rev., 2011, 15, 839–844 CrossRef.
- Z. Yang, J. Zhang, M. C. W. Kintner-Meyer, X. Lu, D. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 3577–3613 CrossRef CAS.
- J. P. Barton and D. G. Infield, IEEE Trans. Energy Convers., 2004, 19, 441–448 CrossRef.
- R. Saidur, N. A. Rahim and M. Hasanuzzaman, Renewable Sustainable Energy Rev., 2010, 14, 1135–1153 CrossRef.
- N. Xu, X. Li, X. Zhao, J. B. Goodenough and K. Huang, Energy Environ. Sci., 2011, 4, 4942–4946 CAS.
- T. Oshima, M. Kajita and A. Okuno, Int. J. Appl. Ceram. Technol., 2004, 1, 269–276 CrossRef CAS.
- D. J. Bradwell, H. Kim, A. H. C. Sirk and D. R. Sadoway, J. Am. Chem. Soc., 2012, 134, 1895–1897 CrossRef CAS.
- H. Shimotake, G. Rogers and E. J. Cairns, Ind. Eng. Chem. Process Des. Dev., 1969, 8, 51–56 CAS.
- E. J. Cairns and H. Shimotake, Science, 1969, 164, 1347–1355 CAS.
- H. Kan and H. Lee, Catal. Commun., 2010, 12, 36–39 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21992a |
| This journal is © The Royal Society of Chemistry 2012 |