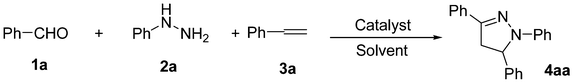
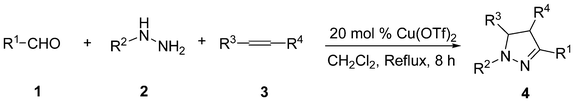
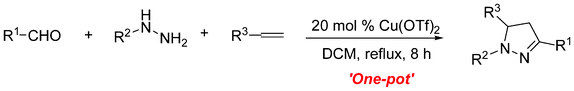Cu(OTf)2-catalyzed three-component annulation reaction: one-pot synthesis of 4,5-dihydropyrazole from aldehydes, hydrazines and alkenes†
Qiang
Wu
,
Pei
Liu
,
Ying-ming
Pan
*,
Yan-li
Xu
and
Heng-shan
Wang
*
Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), School of Chemistry & Chemical Engineering of Guangxi Normal University, Guilin 541004, People's Republic of China. E-mail: panym2004@yahoo.com.cn; wang_hengshan@yahoo.com.cn
First published on 5th September 2012
Abstract
Dihydropyrazole has been recognized as an important framework in pharmaceutical science.1 Many dihydropyrazole-based derivatives have been used as cannabinoid receptor 1 antagonist, monoamine oxidase inhibitors, and cancer cell inhibitors.2 Due to the attractive medicinal properties of the dihydropyrazole skeleton, various efficient approaches have been developed for the preparation of these compounds.3 Nevertheless, the development of the tandem process for the one-pot synthesis of substituted dihydropyrazole directly from simple and readily available substrates is still in great demand. More recently, we reported a convenient one-pot Mannich-type/cyclization/oxidation tandem process for the synthesis of 1,3,5-substituted pyrazoles derivatives from aldehydes, hydrazines and alkynes using p-toluenesulfonic acid monohydrate (PTSA) as a multifunctional catalyst.4 As a result of development on the transition-metal-catalyzed reaction in our group,5 herein, we wish to report a highly efficient three-component annulation reaction for the synthesis of 4,5-dihydropyrazoles directly from aldehydes, hydrazines and alkenes using Cu(OTf)2 as a catalyst. Cu(OTf)2 effectively catalyzes the three-component annulation processes in a single reaction vessel, and a number of functionalities, such as fluoro, chloro, bromo, methyl, methoxy and hydroxyl, will be tolerated.
To identify the suitable conditions for the three-component annulation process, a series of catalysts and solvents were screened (Table 1). Initially, benzaldehyde 1a (0.5 mmol), phenylhydrazine 2a (0.5 mmol), and styrene 3a (0.6 mmol) in the presence of 20 mol% PTSA in CH2Cl2 at room temperature for 8 h gave the substituted dihydropyrazole 4aa in 20% yield, and the yield of 4aa was obviously higher for the tandem reaction performed in CH3COOH (Table 1, entry 2 vs. entry 1). The combination of Cu(OTf)2 and PTSA also produced the highly substituted dihydropyrazole 4aa in 48% yield (Table 1, entry 3). Cu(OTf)2 provided the best results in comparison to other Lewis acids investigated and was effective in catalytic quantities (20 mol%) (Table 1, entry 5). The crystallization of compound 4aa from ethanol gave a single crystal suitable for X-ray analysis. Fig. 1 illustrates the molecular structure of the substituted dihydropyrazole 4aa.
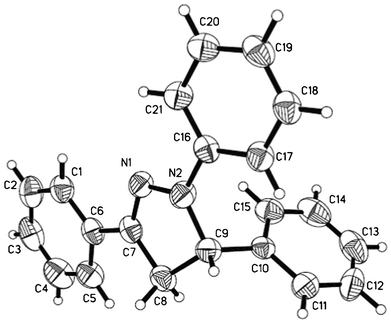 | ||
| Fig. 1 X-ray crystal structure of dihydropyrazole 4aa. The thermal ellipsoids are at the 50% probability level. | ||
| Entry | Catalyst | T/°C | Solvent | Yield (%)b |
|---|---|---|---|---|
| a Reaction conditions: benzaldehyde 1a (0.5 mmol), phenylhydrazine 2a (0.5 mmol), styrene 3a (0.6 mmol), and catalyst (20 mol % to 1a) in solvent (2 mL) for 8 h. b Isolated yield of pure product based on 1a. c The tandem reaction was carried in a 10 mL sealed tube. | ||||
| 1 | PTSA (20 mol %) | rt | CH2Cl2 | 20 |
| 2 | PTSA (20 mol %) | rt | CH3COOH | 31 |
| 3 | PTSA (10 mol %) + Cu(OTf)2 (10 mol %) | rt | CH2Cl2 | 48 |
| 4 | Cu(OTf)2 (20 mol %) | rt | CH2Cl2 | 76 |
| 5 | Cu(OTf)2 (20 mol %) | 40 °C | CH2Cl2 | 89 |
| 6c | Cu(OTf)2 (20 mol %) | 60 °C | CH2Cl2 | 65 |
| 7 | CuCl (20 mol %) | 40 °C | CH2Cl2 | 0 |
| 8 | CuCl2·2H2O (20 mol %) | 40 °C | CH2Cl2 | 0 |
| 9 | Cu(OAc)2 (20 mol %) | 40 °C | CH2Cl2 | 0 |
| 10 | Bi(OTf)3 (20 mol %) | 40 °C | CH2Cl2 | 35 |
| 11 | AgOTf (20 mol %) | 40 °C | CH2Cl2 | 27 |
| 12 | Fe(OTf)3 (20 mol %) | 40 °C | CH2Cl2 | 18 |
| 13 | Cu(OTf)2 (20 mol %) | 40 °C | CH3COOH | 55 |
| 14 | Cu(OTf)2 (20 mol %) | 40 °C | PhCl | 35 |
| 15 | Cu(OTf)2 (20 mol %) | 40 °C | Toluene | 30 |
| 16 | Cu(OTf)2 (20 mol %) | 40 °C | ClCH2CH2Cl | 65 |
Moreover, the temperature has an obvious impact on the yield of 4aa as well. Although the tandem reaction performed in CH2Cl2 at room temperature led to 76% yield of 4aa (Table 1, entry 4), the yield would increase to 89% when the tandem reaction is performed at reflux (Table 1, entry 5). However, when the tandem reaction was performed in CH2Cl2 at 60 °C, the yield of product 4aa decreased to 65% (Table 1, entry 6). Other copper catalysts, such as CuCl, CuCl2·2H2O, and Cu(OAc)2, did not promote the tandem reaction (Table 1, entries 7–9). The tandem reaction was obviously restrained when using Bi(OTf)3, AgOTf or Fe(OTf)3 as a catalyst (Table 1, entries 10–12). The control experiment also confirmed that in the absence of Cu(OTf)2, the reaction led to recovery of starting materials. In addition, it was found that the solvent played a crucial role in the tandem reaction (Table 1, entries 5 and 13–16). The tandem reaction was obviously restrained when it was performed in CH3COOH, chlorobenzene, toluene and 1,2-dichloroethane (DCE) (Table 1, entries 13–16). The above investigations revealed that the best conditions for the three-component annulation reaction involved 20 mol% Cu(OTf)2 in dichloromethane (DCM) at reflux for 8 h.
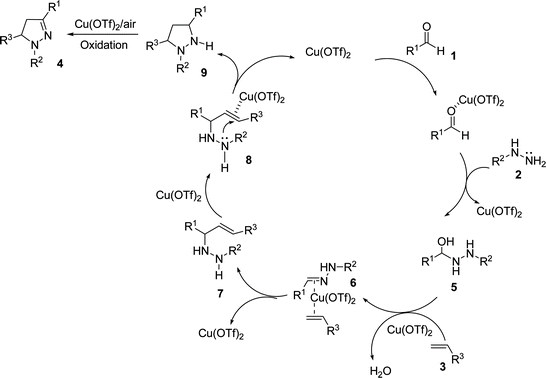
With the identification of the optimal conditions in hand, the scope of the substrates was investigated. Typical results are shown in Table 2. Aldehyde 1, either with an electron-donating or an electron-withdrawing group on the benzene ring, gave the corresponding dihydropyrazoles in good yield. Substrates 1b–1d possessing electron-donating groups at the benzene ring reacted smoothly and afforded the desired products in 83–92% yields (Table 2, entries 2–4, 15 and 20). Other aldehydes 1e–1j possessing electron-withdrawing groups at the benzene ring, such as fluoro, chloro and bromo, also reacted smoothly, providing highly substituted dihydropyrazoles (Table 2, entries 5–10, 16 and 17). In particular, the aromatic aldehydes 1d, 1h and 1j with a substituent on the ortho-position could give the corresponding 4,5-dihydropyrazoles 4da, 4ha and 4ja in high yields (Table 2, entries 4, 8 and 10). Obviously, electron-rich aldehydes provided the desired products in higher yields than electron-poor aldehydes. Additionally, aldehydes bearing a heterocyclic substituent, such as 2-thiophenaldehyde (R1 = 2-thienyl), treated with phenylhydrazine 2a and styrene 3a in the presence of 20 mol% Cu(OTf)2 gave the desired product 4ka in 73% yield (Table 2, entry 11). Also, aldehydes bearing polycyclic aromatic substituents, such as 1-naphthaldehyde and 2-naphthaldehyde, were treated with phenylhydrazine 2a and styrene 3a in the presence of 20 mol% Cu(OTf)2, giving the desired product 4la and 4ma in 76% and 78% yield, respectively (Table 2, entries 12 and 13). Aromatic alkene 3b (R3 = 4–MeOC6H4, R4 = H) with an electron-donating group at the benzene ring gave the corresponding products in higher yields than alkene 3c (R3 = 4–BrC6H4, R4 = H) which possessed an electron-withdrawing group on the benzene ring (Table 2, entries 16–20). Unfortunately, cyclohexene 3d and maleic anhydride 3e, when allowed to react with benzaldehyde 1a and phenylhydrazine 2a, failed to get the desired products (Table 2, entries 21 and 22). Therefore, the results suggested that the three-component annulation reaction should be followed by the proposed pathway shown in Scheme 1, but not the 1,3-dipolar cycloaddition pathway.
 | ||
| Scheme 1 Proposed mechanism. | ||
| Entry | Aldehyde | Hydrazine | Alkene | Yield (%)b |
|---|---|---|---|---|
| a Reaction conditions: aldehyde 1 (0.5 mmol), hydrazine 2 (0.5 mmol), alkene 3 (0.6 mmol), Cu(OTf)2 (20 mol % to 1), CH2Cl2 (2 ml), at reflux for 8 h. b Isolated yield of pure product based on 1. | ||||
| 1 | 1a: R1 = Ph | 2a: R2 = Ph | 3a: R3 = Ph; R4 = H | 89 |
| 2 | 1b: R1 = 4–MeOC6H4 | 2a: R2 = Ph | 3a: R3 = Ph; R4 = H | 92 |
| 3 | 1c: R1 = 4–MeC6H4 | 2b: R2 = 4–ClC6H4 | 3a: R3 = Ph; R4 = H | 83 |
| 4 | 1d: R1 = 2–OHC6H4 | 2a: R2 = Ph | 3a: R3 = Ph; R4 = H | 85 |
| 5 | 1e: R1 = 4–ClC6H4 | 2a: R2 = Ph | 3a: R3 = Ph; R4 = H | 80 |
| 6 | 1f: R1 = 4–BrC6H4 | 2a: R2 = Ph | 3a: R3 = Ph; R4 = H | 81 |
| 7 | 1g: R1 = 3–BrC6H4 | 2a: R2 = Ph | 3a: R3 = Ph; R4 = H | 82 |
| 8 | 1h: R1 = 2–BrC6H4 | 2a: R2 = Ph | 3a: R3 = Ph; R4 = H | 78 |
| 9 | 1i: R1 = 4–FC6H4 | 2a: R2 = Ph | 3a: R3 = Ph; R4 = H | 76 |
| 10 | 1j: R1 = 2–FC6H4 | 2a: R2 = Ph | 3a: R3 = Ph; R4 = H | 74 |
| 11 | 1k: R1 = 2-Thienyl | 2a: R2 = Ph | 3a: R3 = Ph; R4 = H | 73 |
| 12 | 1l: R1 = 1-Naphthyl | 2a: R2 = Ph | 3a: R3 = Ph; R4 = H | 76 |
| 13 | 1m: R1 = 2-Naphthyl | 2a: R2 = Ph | 3a: R3 = Ph; R4 = H | 78 |
| 14 | 1a: R1 = Ph | 2b: R2 = 4–ClC6H4 | 3a: R3 = Ph; R4 = H | 86 |
| 15 | 1b: R1 = 4–MeOC6H4 | 2b: R2 = 4–ClC6H4 | 3a: R3 = Ph; R4 = H | 90 |
| 16 | 1f: R1 = 4–BrC6H4 | 2a: R2 = Ph | 3b: R3 = 4–MeOC6H4; R4 = H | 85 |
| 17 | 1h: R1 = 2–BrC6H4 | 2a: R2 = Ph | 3b: R3 = 4–MeOC6H4; R4 = H | 82 |
| 18 | 1a: R1 = Ph | 2a: R2 = Ph | 3b: R3 = 4–MeOC6H4; R4 = H | 87 |
| 19 | 1a: R1 = Ph | 2a: R2 = Ph | 3c: R3 = 4–BrC6H4; R4 = H | 81 |
| 20 | 1b: R1 = 4–MeOC6H4 | 2a: R2 = Ph | 3c:R3 = 4–BrC6H4; R4 = H | 83 |
| 21 | 1a: R1 = Ph | 2a: R2 = Ph | 3d: R3 = R4 = –(CH2)4– | 0 |
| 22 | 1a: R1 = Ph | 2a: R2 = Ph | 3e: R3 = R4 = –COOCO– | 0 |
We propose the process detailed in Scheme 1 as the most likely mechanism for this Mannich-type/cyclization/oxidation transformation.6 Firstly, the reaction proceeds via an initial nucleophilic attack of hydrazine 1 on aldehyde 2 resulting in an adduct 5 which is converted into hydrazone 6 through loss of water. Secondly, the distance has been shortened by the complexation between copper(II) triflate, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, leading to the formation of a Mannich-type intermediate 7. This is followed by the intramolecular cyclization to afford tetrahydropyrazole 9 due to the attack of γ-NH on C
C, leading to the formation of a Mannich-type intermediate 7. This is followed by the intramolecular cyclization to afford tetrahydropyrazole 9 due to the attack of γ-NH on C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C which is activated by chelating to Cu(II), which then undergoes oxidation in the air atmosphere to afford 4,5-dihydropyrazole 4.
C which is activated by chelating to Cu(II), which then undergoes oxidation in the air atmosphere to afford 4,5-dihydropyrazole 4.
In summary, we have developed an effective three-component annulation reaction for the synthesis of substituted 4,5-dihydropyrazoles directly from aldehydes, hydrazines and alkenes using Cu(OTf)2 as a catalyst. This tandem reaction proceeded smoothly without exclusion of moisture or air from the reaction mixture, which gave rapid access to a variety of substituted dihydropyrazoles.
Acknowledgements
We thank the project 973 (2011CB512005) and Guangxi Natural Science Foundation of China (2012GXNSFAA053027, 2011GXNSFD018010 and 2010GXNSFF013001) for financial support.References
- For selected examples, see: (a) H. H. Seltzman, Drug Dev. Res., 2009, 70, 601 CrossRef CAS; (b) W. G. Bensen, Pain, 2003, 515 Search PubMed; (c) C. Wasylyk, H. Zheng, C. Castell, L. Debussche, M.-C. Multon and B. Wasylyk, Cancer Res., 2008, 68, 1275 CrossRef CAS.
- (a) A. B. Need, R. J. Davis, J. T. Alexander-Chacko, B. Eastwood, E. Chernet, L. A. Phebus, D. K. Sindelar and G. G. Nomikos, Psychopharmacology, 2006, 184, 26 CrossRef CAS; (b) H. Dmytro, Z. Borys, V. Olexandr, Z. Lucjusz, G. Andrzej and L. Roman, Eur. J. Med. Chem., 2009, 44, 1396 CrossRef; (c) F. Manna, F. Chimenti, R. Fioravanti, A. Bolasco, D. Secci, P. Chimenti, C. Ferlinib and G. Scambia, Bioorg. Med. Chem. Lett., 2005, 15, 4632 CrossRef CAS; (d) D. Havrylyuk, B. Zimenkovsky, O. Vasylenko, L. Zaprutko, A. Gzella and R. Lesyk, Eur. J. Med. Chem., 2009, 44, 1396 CrossRef CAS; (e) B. Insuasty, A. Tigreros, F. Orozco, J. Quiroga, R. Abonía, M. Nogueras, A. Sanchez and J. Cobo, Bioorg. Med. Chem., 2010, 18, 4965 CrossRef CAS; (f) X. H. Liu, J. Zhu, A. N. Zhou, B. A. Song, H. L. Zhu, L. S. bai, P. S. Bhadury and C. X. Pan, Bioorg. Med. Chem., 2009, 17, 1207 CrossRef CAS; (g) M. Shaharyar, M. M. Abdullah, M. A. Bakht and J. Majeed, Eur. J. Med. Chem., 2010, 45, 114 CrossRef CAS.
- For selected recent dihydropyrazole syntheses, see: (a) S. Fustero, M. Sánchez-Roselló, P. Barrio and A. Simón-Fuentes, Chem. Rev., 2011, 111, 6984 CrossRef CAS and references cited therein; (b) X.-H. Liu, B.-F. Ruan, J. Li, F.-H. Chen, B.-A. Song, H.-L. Zhu, P.S. Bhadury and J. Zhao, Mini-Rev. Med. Chem., 2011, 11, 771 CrossRef CAS and references cited therein; (c) X.-H. Liu, B.-F. Ruan, J.-X. Liu, B.-A. Song, L.-H. Jing, J. Li, Y. Yang, H.-L. Zhu and X.-B. Qi, Bioorg. Med. Chem. Lett., 2011, 21, 2916 CrossRef CAS; (d) T. Okitsu, K. Sato and A. Wada, Org. Lett., 2010, 12, 3506 CrossRef CAS; (e) Y. Suzuki, S. Naoe, S. Oishi, N. Fujii and H. Ohno, Org. Lett., 2012, 14, 326 CrossRef CAS.
- P. Liu, Y. M. Pan, Y. L. Xu and H. S. Wang, Org. Biomol. Chem., 2012, 10, 4696 CAS.
- (a) Y. L. Xu, Y. M. Pan, Q. Wu, H. S. Wang and P. Z. Liu, J. Org. Chem., 2011, 76, 8472 CrossRef CAS; (b) Y. L. Xu, Y. M. Pan, P. Liu, H. S. Wang, X. Y. Tian and G. F. Su, J. Org. Chem., 2012, 77, 3557 CrossRef CAS.
- (a) S. Ogoshi, T. Haba and M. Ohashi, J. Am. Chem. Soc., 2009, 131, 10350 CrossRef CAS; (b) L. A. Adrio, L. S. Quek, J. G. Taylor and K. K. Hii, Tetrahedron, 2009, 65, 10334 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and NMR data of the prepared compounds. See DOI: 10.1039/c2ra21106h |
| This journal is © The Royal Society of Chemistry 2012 |