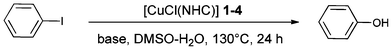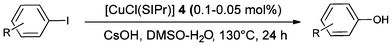Highly active copper-N-heterocyclic carbene catalysts for the synthesis of phenols†
Olivier
Songis
,
Pierre
Boulens
,
Callum G. M.
Benson
and
Catherine S. J.
Cazin
*
EaStCHEM School of Chemistry, University of St Andrews, St Andrews, KY16 9ST, UK. E-mail: cc111@st-andrews.ac.uk
First published on 25th September 2012
Abstract
The hydroxylation of aryl iodides was performed using complexes of the type [CuCl(NHC)]. Excellent conversions were obtained at very low catalyst loadings.
Phenols are widely used building blocks and synthetic intermediates in pharmaceutical and materials production.1 Functionalised phenols are classically obtained by non-oxidative methods using the Sandmeyer reaction or nucleophilic aromatic substitution on phenyl derivatives. However, harsh reaction conditions and the need for functional groups on the substrates are drawbacks limiting the use of these methodologies.2 Milder methods for the preparation of functionalised phenols have been proposed. Maleczka and co-workers have reported a one-pot C–H activation/borylation and oxidation catalysed by iridium.3 Recently, several efficient palladium-catalysed hydroxylation reactions of aryl halides have been developed and successfully applied to the synthesis of substituted phenols.4
The low toxicity and economical attractiveness of copper have led to an extensive use of this metal in catalysis.5 Recently, Taillefer and You reported independently a copper-catalysed hydroxylation of aryl halides under mild conditions.6 Environmental friendly versions of these methodologies were rapidly developed.7 However, these methods present a major drawback: the catalyst loading required, which is on the order of 10 to 20 mol%. Feng recently reported efficient Cu nanoparticle catalysts for the hydroxylation and amination of aryl halides in aqueous solution.8 In this case, good conversions were achieved with a catalyst loading of 1.5 mol%.
During the last decade, Cu systems bearing N-heterocyclic carbenes (NHCs) have been shown to be highly active species enabling various catalytic transformations.9 These have also been successfully used as carbene (NHC) transfer reagents.10 However, such systems have not so far been reported for hydroxylation reactions. Considering the often superior catalytic behaviour of NHC-based systems in a number of transition metal-catalysed reactions, we deemed intriguing the potential of such systems for the synthesis of phenols with the purpose of obtaining efficient systems at loadings lower than state-of-the-art.
Four complexes having proven efficient catalysts in other transformations were selected: [CuCl(NHC)] where NHC is IMes (N,N′-bis-[2,4,6-(trimethyl)phenyl]imidazol-2-ylidene), SIMes (N,N′-bis[2,4,6-(trimethyl)phenyl]imidazolidin-2-ylidene), IPr (N,N′-bis-[2,6-(di-iso-propyl)phenyl]imidazol-2-ylidene) and SIPr (N,N′-bis[2,6-(di-iso-propyl)phenyl]imidazolidin-2-ylidene) (Fig. 1).9,11
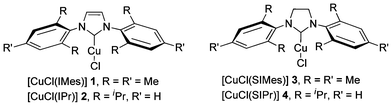 | ||
| Fig. 1 Commercially available Cu–NHC catalysts. | ||
Initial experiments were carried out with iodobenzene as a model substrate, using conditions previously shown as optimal for related systems (CsOH, DMSO–H2O, 130 °C),6b followed by acidic workup.
At a 1 mol% Cu loading, gratifyingly, quantitative conversion to phenol was observed. The catalyst loading was therefore decreased in order to test the limits of our system. Amazingly, at 0.05 mol% Cu (500 ppm), almost complete conversion was observed with all systems tested, corresponding to a turnover number (TON) of nearly 2000 (Table 1, entries 2–5). A further decrease of the catalyst loading to 100 ppm showed the limits of the systems as TONs of ca. 2000 were obtained (Table 1, entries 6–9). Interestingly, all four complexes examined lead to similar results, showing that the NHC, under these reaction conditions, only has a moderate influence on the catalyst activity. Hydroxide bases other than CsOH were next assessed, leading to much poorer results (Table 1, entries 3, 10 and 11). Finally, the use of a H2O–DMSO solvent mixture proved crucial as when reactions were conducted in these solvents alone, only trace amounts of the hydroxylation product were observed (Table 1, entries 12 and 13). This is presumably due to the poor solubility of CsOH in DMSO and to the immiscibility of the aryl iodide in water.
| Entry | Catalyst | Base | Loading (mol%) | Conv.(%)b | TON |
|---|---|---|---|---|---|
| a Reaction conditions: catalyst (0.01–0.05 mol%), C6H5I (1 mmol), DMSO (0.5 mL), H2O (0.5 mL), 24 h, 130 °C. b Determined by GC based on C6H5I, average of 2 runs. c DMSO only (1 mL). d H2O only (1 mL). | |||||
| 1 | — | CsOH | — | <1 | — |
| 2 | [CuCl(IMes)] 1 | CsOH | 0.05 | 98 | 1960 |
| 3 | [CuCl(IPr)] 2 | CsOH | 0.05 | 98 | 1960 |
| 4 | [CuCl(SIMes)] 3 | CsOH | 0.05 | 98 | 1960 |
| 5 | [CuCl(SIPr)] 4 | CsOH | 0.05 | 99 | 1980 |
| 6 | [CuCl(IMes)] 1 | CsOH | 0.01 | 22 | 2200 |
| 7 | [CuCl(IPr)] 2 | CsOH | 0.01 | 23 | 2300 |
| 8 | [CuCl(SIMes)] 3 | CsOH | 0.01 | 21 | 2100 |
| 9 | [CuCl(SIPr)] 4 | CsOH | 0.01 | 25 | 2500 |
| 10 | [CuCl(IPr)] 2 | KOH | 0.05 | 44 | 880 |
| 11 | [CuCl(IPr)] 2 | NaOH | 0.05 | <1 | <1 |
| 12 | [CuCl(IPr)] 2 | CsOH | 0.05 | <1c | <1 |
| 13 | [CuCl(IPr)] 2 | CsOH | 0.05 | <1d | <1 |
The scope of the reaction was next examined under the optimised reaction conditions (Table 2).
| Entry | Ar–I | Ar–OH | Cat. (mol%) | Yield (%)b | TON |
|---|---|---|---|---|---|
a Reaction conditions: catalyst 4 (0.05–0.1 mol%), ArI (1 mmol), CsOH (3 mmol), DMSO/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 mL), 130 °C, 24 h.
b Isolated yield, average of two reactions.
c 110 °C. 1, 1 mL), 130 °C, 24 h.
b Isolated yield, average of two reactions.
c 110 °C.
|
|||||
| 1 |
|
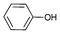
|
0.05 | 88 | 1760 |
| 2 |
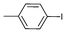
|
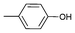
|
0.05 | 82 | 1640 |
| 3 |
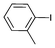
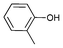
|
|
0.05 | 91 | 1820 |
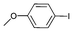
| 4 |
|
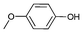
|
0.1 | 92 | 920 |
| 5b |
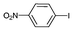
|
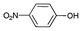
|
0.1 | 80c | 800 |
| 6 |
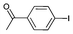
|
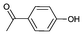
|
0.1 | 63c | 630 |
| 7 |
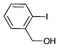
|
|
0.1 | 58 | 580 |
Iodotoluene and iodobenzene were successfully converted to the hydroxylated products in high isolated yields using as low as 500 ppm of catalyst (Table 2, entries 1–3). Using a slightly higher catalyst loading (1000 ppm), aryl iodides bearing methoxy, nitro, acetyl or alcohol groups were successfully converted to the hydroxylation product (Table 2, entries 4–7). In the case of nitrobenzene and acetophenone iodides, the reactions were conducted at 110 °C in order to prevent side-reactions observed at more elevated temperatures.11
Having had experience with the [CuCl(NHC)]/CsOH combination in carboxylation catalysis and having identified the productive role of [Cu(OH)(NHC)] species in this chemistry,12 we suspected the mechanism of hydroxylation might very well involve initial formation of the [Cu(OH)(NHC)] complex. To support our hypothesis, catalytic reactions were tested using the well-defined [Cu(OH)(IPr)]. Lower yields were obtained compared to reactions carried out using the [CuCl(IPr)]/CsOH mixture. This might indicate the necessity of slow dosing of the active species for optimum catalyst activity. Further mechanistic studies are presently being conducted to elucidate the exact catalytic route employed by the copper catalyst in this quite efficient methodology leading to phenols.
Conclusions
In conclusion, the hydroxylation of aryl iodides was achieved using extremely low catalyst loadings of commercially available complexes. While systems reported in the literature require 1.5–20 mol% of copper,6,7 leading to TONs between 10 and 67, our system requires 500–1000 ppm, leading to TONs between 600 and 2000. Hence, the present system displays an improvement in performance of two orders of magnitude over state-of-the art. Furthermore, the systems reported herein do not required the use of additional ligand (10–20 mol%),6,7 potentially simplifying purification steps and minimising waste. As an added synthetic advantage the copper(I) complexes can be readily obtained by the simple reaction of copper(I) oxide with the corresponding imidazolium salt.13 The mechanism and further applications of the well-defined [CuX(NHC)] (X = Cl and OH) systems are presently being explored in our laboratories.Acknowledgements
The authors gratefully acknowledge the Royal Society (University Research Fellowship to CSJC) for funding.References
- (a) J. H. P. Tyman, Synthetic and Natural Phenols, Elsevier, New York, 1996 Search PubMed; (b) Z. Rappoport, The Chemistry of Phenols, Wiley-VCH, Weinheim, 2003 CrossRef.
- (a) T. George, R. Mabon, G. Sweeney, J. B. Sweeney and A. Tavassoli, J. Chem. Soc., Perkin Trans. 1, 2000, 2529 RSC; (b) P. Hanson, J. R. Jones, A. B. Taylor, P. H. Walton and A. W. Timms, J. Chem. Soc., Perkin Trans. 2, 2002, 1135 RSC; (c) C. Hoarau and T. R. R. Pettus, Synlett, 2003, 1, 127 Search PubMed.
- R. E. Maleczka, F. Shi, D. Holmes and M. R. Smith III, J. Am. Chem. Soc., 2003, 125, 7792 CrossRef CAS.
- (a) A. G. Sergeev, T. Schulz, C. Torborg, A. Spannenberg, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 7595 CrossRef CAS; (b) T. Schulz, C. Torborg, B. Schäffner, J. Huang, A. Zapf, R. Kadyrov, A. Börner and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 918 CrossRef CAS; (c) M. C. Willis, Angew. Chem., Int. Ed., 2007, 46, 3402 CrossRef CAS; (d) B. J. Gallon, R. W. Kojima, R. B. Kaner and P. L. Diaconescu, Angew. Chem., Int. Ed., 2007, 46, 7251 CrossRef CAS; (e) G. S. Chen, A. S. C. Chan and F. Y. Kwong, Tetrahedron Lett., 2007, 48, 473 CrossRef CAS; (f) K. W. Anderson, T. Ikawa, R. E. Tundel and S. L. Buchwald, J. Am. Chem. Soc., 2006, 128, 10694 CrossRef CAS.
- N-Heterocyclic Carbenes in Transition Metal Catalysis and Organocatalysis, Ed. C. S. J. Cazin, Springer–Dordrecht Heidelberg London New York, 2011, 32 Search PubMed.
- (a) D. Zhao, N. Wu, S. Zhang, P. Xi, X. Su, J. Lan and J. You, Angew. Chem., Int. Ed., 2009, 48, 8729 CrossRef CAS; (b) A. Tlili, N. Xia, F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 8725 CrossRef CAS.
- (a) L. Jing, J. Wei, L. Zhou, Z. Huang, Z. Lia and X. Zhou, Chem. Commun., 2010, 46, 4767 RSC; (b) D. Yang and H. Fu, Chem.–Eur. J., 2010, 16, 2366 CrossRef CAS.
- H. Xu, Y. Liang, Z. Cai, H. Qi, C. Yang and Y. Feng, J. Org. Chem., 2011, 76, 2296 CrossRef CAS.
- S. Díez-González, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612 CrossRef.
- M. R. L. Furst and C. S. J. Cazin, Chem. Commun., 2010, 46, 6924 RSC.
- The use of aryl bromides and chlorides do not lead to the desired products under the optimised conditions used for the aryl iodides.
- I. I. F. Boogaerts, G. C. Fortman, M. R. L. Furst, C. S. J. Cazin and S. P. Nolan, Angew. Chem., Int. Ed., 2010, 49, 8674 CrossRef CAS.
- C. A. Citadelle, E. Le Nouy, F. Bisaro, A. M. Z. Slawin and C. S. J. Cazin, Dalton Trans., 2010, 39, 4489 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, compound characterisation data and spectra. See DOI: 10.1039/c2ra22193d |
| This journal is © The Royal Society of Chemistry 2012 |