Au-catalyzed cascade addition/cyclization/H-transfer reactions of 3-(1-alkynyl)chromones to construct 4H-Furo[3,2-c]pyrans scaffold†
Feng
Hu
a,
Taijie
Chen
b,
Jianwei
Yan
a,
Ming
Cheng
a,
Liping
Huang
a and
Youhong
Hu
*a
aAddress State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, CAS, 555 Zu Chong Zhi Road, Shanghai 201203, P. R. China. E-mail: yhhu@mail.shcnc.ac.cn; Fax: +86-21-50806600-3517; Tel: +86-21-50806600-3517
bDepartment of Chemistry, East China Normal University, 3663 North Zhongshan Road, Shanghai 200062, P. R. China
First published on 15th October 2012
Abstract
Using Au catalyst and ethyl Hantzsch ester as a hydrogen source, 3-(1-alkynyl)chromones were directly transformed to 4H-benzofuro[3,2-c]pyrans in good to excellent yields through a cascade process by addition, cyclization and hydrogen transfer under mild conditions.
Introduction
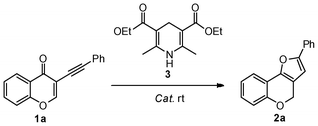
Nicotinamide adenine dinucleotide (NADH) is an important hydride source with the catalysis of glutamate dehydrogenase in nature. Hantzsch ester (HEH) as a synthetic analog of NADH in combination with the different Lewis acids and Brønsted acids have been broadly reported to play as an efficient reagent for hydrogen transfer.1 Au catalysis is a powerful tool in the synthesis of natural products.2 The character of Au complexes as a strong Lewis acid has been utilized in the direct reductive amination of aromatic aldehydes.3 On the other hand, π-acidity is another unique character of Au complex and used to activate the multiple bonds. Continuing our interests to develop various cascade processes to construct diversified natural product-like scaffolds from 3-(1-alkynyl)chromones,4 we envisioned that 1a could be transformed to intermediate M, which was hydrogenated to form 4H-furo[3,2-c]chromene 2a by the Hantzsch ester 3 (Scheme 1).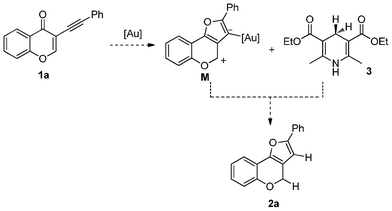 | ||
| Scheme 1 The envisioned cascade reaction. | ||
The 4H-Furo[3,2-c]chromene skeleton can be found in many natural products and exhibits potent biological activity (Scheme 2).5 Most synthetic methods have focused on the construction of pterocarpan system which is the combination of benzofuran and chromene scaffold.6 Currently, the synthesis of substituted 4H-furo[3,2-c]chromene has been rarely investigated.7 As an important bioactive natural product-like template, developing an efficient synthetic method for the library construction to this scaffold could be beneficial for the discovery of lead compounds.
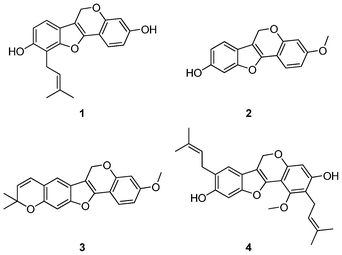 | ||
| Scheme 2 Erypoegin H (1), Lespedezol A1 (2) and their congeners (3 and 4). | ||
Results and discussion
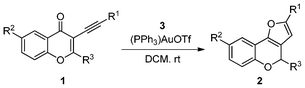
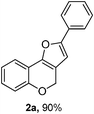
In the initial phase of an effort to explore this proposal, we started our examination of the H-transfer reaction by the following conditions (Table 1). The substrate 1a (0.3 mmol) was reacted with hydrogen source 3 (1.2 equiv.) and (PPh3)AuOTf (0.01 equiv.) in DCM (3 mL) at room temperature under a N2 atmosphere to give the expected product 2a in 90% yield (Table 1, entry 1). When the catalyst was changed from (Ph3P)AuOTf to (PPh3)AuCl (Table 1, entry 2), the yield of the reaction was decreased after completing the reaction in 12 h by checking TLC. Also, AuCl3 could promote the reaction efficiently to afford 2a in 61% yield (Table 1, entry 3). Among other transition metal catalysts, AgOTf (0.015 equiv.) could cause the transformation to generate 3a in 56% yield (Table 1, entry 4). However, Cu(OTf)2 could not proceed the reaction (Table 1, entry 5). After screening commonly used solvents, DCM was superior to MeCN, THF, MeOH and DMF (Table 1, entries 6–9). The reaction was performed in the presence of MeSO3H, a Brønsted acid, the product 2a can not be obtained (Table 1, entry 10).| Entry | Catalyst | Solvent | Time (h) | Yield of 2a (%)b |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), (PPh3)AuCl (0.003 mmol), AgOTf (0.003 mmol) and hydrogen source 3 (0.36 mmol, 1.2 equiv.) in the solvent (3 mL) under a N2 atmosphere at room temperature. b Isolated yield. c AgOTf (0.05 equiv.) was used. | ||||
| 1 | (PPh3)AuOTf | DCM | 3 | 90 |
| 2 | (PPh3)AuCl | DCM | 12 | 40 |
| 3 | AuCl3 | DCM | 3 | 61 |
| 4c | AgOTf | DCM | 24 | 56 |
| 5 | Cu(OTf)2 | DCM | 12 | 0 |
| 6 | (PPh3)AuOTf | MeCN | 3 | 34 |
| 7 | (PPh3)AuOTf | THF | 3 | 52 |
| 8 | (PPh3)AuOTf | MeOH | 12 | <10 |
| 9 | (PPh3)AuOTf | DMF | 3 | trace |
| 10 | MeSO3H | DCM | 12 | 0 |
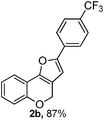
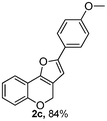
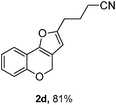
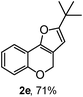
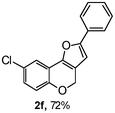
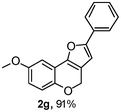
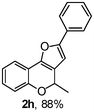
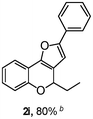
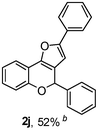
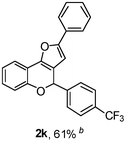
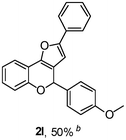
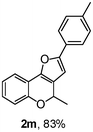
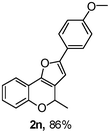
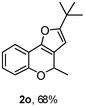
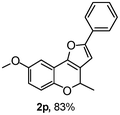
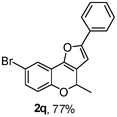
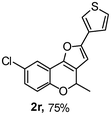
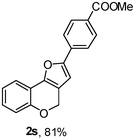
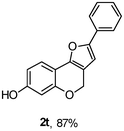
Using the optimal reaction conditions (Table 1, entry 1), we examined the scope of this cascade reaction (Table 2). Obviously, R1 with electronic and steric variation on acetylene moiety afforded the corresponding products in good to moderate yields. Comparing with electronic effects on aromatic substitution of chromone, R2 with electron-donating group (such as OMe) at 6-position of chromone gave the desired product 2g in high yield, which may be stabilized the carbocation of the intermediate. Also, the effect of R3 group was investigated. The results showed the steric effect on the reaction obviously. When R3 was methyl or ethyl group, the reaction was given as reasonable yield (such as 2h, 2i and etc.). The sterically hindered group decreased the yield and prolonged the reaction time (completed after 12 h) without the significant electronic effect (2j and 2k). Due to the slight steric effects of R1 and R3, 2o was obtained in 68% yield. Significantly, the cascade reaction is compatible with various functional groups (such as hydroxy, ester, heteroaryl and halogen) to give good yields.
When compound 1u was used as a expanded substrate in this reaction with 5% of catalyst, the corresponding product 2s was afforded in 61% yield after 24 h (eqn 1). It was interesting that the dehydroxylated product 2v was obtained in 82% yield when 1v was employed (eqn 2, Scheme 3). We speculated that the 1v was easily changed to the allene cation under the conditions of Au catalysis,8 and then followed the H-transfer cyclization to give 2v (Scheme 3).
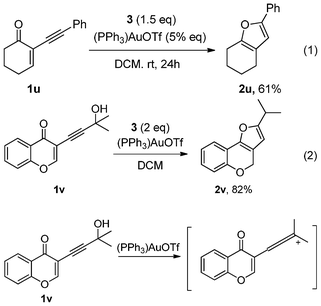 | ||
| Scheme 3 Proposed generation of allene. | ||
In addition, pyridine 1-oxide could be employed as an oxygen source for oxygen transfer with substrate 1a to generate the product 2w in 72% yield after 6 h (Scheme 4).
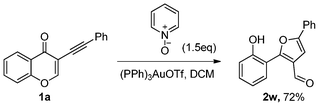 | ||
| Scheme 4 Oxygen-transfer reaction. | ||
Conclusion
In summary, HEH as an analog of biological hydride source, in combination with Au catalysis, exhibits an efficient cyclization/hydrogen transfer access to directly assemble a natural product-like 4H-furo[3,2-c]pyrans scaffold from readily accessible starting material 3-(1-alkynyl)chromones. It is noteworthy that this reaction can be extended with broad substrates tolerating the various substitution under the mild conditions to give excellent yields.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (21172232) and Ministry of Science and Technology of China (2009CB940903).References
- (a) M. Rueping, E. Sugiono and F. R. Schoepke, Synlett, 2010, 852 CrossRef CAS; (b) J. B. Tuttle, S. G. Ouellet and D. W. C. MacMillan, J. Am. Chem. Soc., 2006, 128, 12662 CrossRef CAS; (c) N. J. A. Martin, L. Ozores and B. List, J. Am. Chem. Soc., 2007, 129, 8976 CrossRef CAS; (d) N. J. A. Martin and B. List, J. Am. Chem. Soc., 2006, 128, 13368 CrossRef CAS.
- For some recent reviews see: (a) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994 CrossRef CAS; (b) H. Huang, Y. Zhou and H. Liu, Beilstein J. Org. Chem., 2011, 7, 897 CrossRef CAS; (c) A. Corma, A. Leyva-Pérez and M. J. Sabater, Chem. Rev., 2011, 111, 1657 CrossRef CAS; (d) M. Bandini, Chem. Soc. Rev., 2011, 40, 1358 RSC; (e) C. Aubert, L. Fensterbank, P. Garcia, M. Malacria and A. Simonneau, Chem. Rev., 2011, 111, 1954 CrossRef CAS; (f) H. C. Shen, Tetrahedron, 2008, 64, 7847 CrossRef CAS; (g) H. C. Shen, Tetrahedron, 2008, 64, 3885 CrossRef CAS; (h) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef CAS; (i) Z. Li, C. Brouwer and C. He, Chem. Rev., 2008, 108, 3239 CrossRef CAS; (j) A. Furstner, Chem. Soc. Rev., 2009, 38, 3208 RSC; (k) V. Michelet, P. Y. Toullec and J. P. Genêt, Angew. Chem. Int. Ed., 2008, 47, 4268 CrossRef CAS; (l) A. S. K. Hashmi and M. Rudolph, Chem. Soc. Rev., 2008, 37, 1766 RSC; (m) E. S. Jiménez-Núñez and A. M. Echavarren, Chem. Rev., 2008, 108, 3326 CrossRef; (n) A. Arcadi, Chem. Rev., 2008, 108, 3266 CrossRef CAS; (o) R. Skouta and C. J. Li, Tetrahedron, 2008, 64, 4917 CrossRef CAS; (p) T. C. Boorman and I. Larrosa, Chem. Soc. Rev., 2011, 40, 1910 RSC.
- M. Zhang, H. Yang, Y. Zhang, C. Zhu, W. Li, Y. Cheng and H. Hu, Chem. Commun., 2011, 6605 RSC.
- (a) G. Cheng and Y. Hu, J. Org. Chem., 2008, 73, 4732 CrossRef CAS; (b) L. Zhao, G. Cheng and Y. Hu, Tetrahedron Lett., 2008, 49, 7364 CrossRef CAS; (c) L. Zhao, F. Xie, G. Cheng and Y. Hu, Angew. Chem., Int. Ed., 2009, 48, 6520 CrossRef CAS; (d) F. Xie, X. Pan, S. Lin and Y. Hu, Org. Biomol. Chem., 2010, 8, 1378 RSC; (e) Y. Liu, L. Huang, F. Xie and Y. Hu, J. Org. Chem., 2010, 75, 6304 CrossRef CAS; (f) J. Gong, F. Xie, H. Chen and Y. Hu, Org. Lett., 2010, 12, 3848 CrossRef CAS; (g) F. Xie, H. Chen and Y. Hu, Org. Lett., 2010, 12, 3086 CrossRef CAS; (h) Y. Liu, L. Huang, F. Xie, X. Chen and Y. Hu, Org. Biomol. Chem., 2011, 9, 2680 RSC.
- (a) R. S. Khupse and P. W. Erhardt, J. Nat. Prod., 2008, 71, 275 CrossRef CAS; (b) A. Fürstner, E. K. Heilmann and P. W. Davies, Angew. Chem. In. Ed, 2007, 46, 4760 CrossRef.
- (a) B. A. Chauder, A. V. Kalinin, N. J. Taylor and V. Snieckus, Angew. Chem. Int. Ed., 1999, 38, 1435 CrossRef CAS; (b) H. Leutbecher, J. Conrad, I. Klaiber and U. Beifuss, Synlett, 2005, 16, 3126 Search PubMed; (c) T. Tricotet, P. Fleming, J. Cotter, A. M. L. Hogan, C. Strohmann, V. H. Gessner and D. F. O'Shea, J. Am. Chem. Soc., 2009, 131, 3142 CrossRef CAS; (d) R. C. Larock and L. W. Harrison, J. Am. Chem. Soc., 1984, 106, 4218 CrossRef CAS; (e) C. C. Li, Z. X. Xie, Y. D. Zhang, J. H. Chen and Z. Yang, J. Org. Chem., 2003, 68, 8500 CrossRef CAS; (f) H. Leutbecher, S. Hajdok, C. Braunberger, M. Neumann, S. Mika, J. Conrad and U. Beifuss, Green Chem., 2009, 11, 676 RSC; (g) T. Yao, D. Yue and R. C. Larock, J. Org. Chem., 2005, 70, 9985 CrossRef CAS; (h) P. Pahari and J. Rohr, J. Org. Chem., 2009, 74, 2750 CrossRef CAS; (i) N. Al-Maharik and N. P. Botting, Tetrahedron, 2004, 60, 1637 CrossRef CAS.
- (a) D. P. Sant'Ana, V. D. Pinho, M. C. L. S. Maior and P. R. R. Costa, Tetrahedron Lett., 2009, 50, 3753 CrossRef CAS; (b) G. Cheng and Y. Hu, Chem. Commun., 2007, 3285 RSC; (c) N. Takeda, O. Miyata and T. Naito, Eur. J. Org. Chem., 2007, 2007, 1491 CrossRef; (d) A. Fürstner and P. W. Davies, J. Am. Chem. Soc., 2005, 127, 15024 CrossRef; (e) T. A. Engler, J. P. Reddy, K. D. Combrink and D. Vander Velde, J. Org. Chem., 1990, 55, 1248 CrossRef CAS.
- (a) C.-F. Xu, M. Xu, L.-Q. Yang and C.-Y. Li, J. Org. Chem., 2012, 77, 3010 CrossRef CAS; (b) B. M. Trost and M. T. Rudd, J. Am. Chem. Soc., 2005, 127, 4763 CrossRef CAS; (c) R. Sanz, D. Miguel, A. Martínez, J. M. Álvarez-Gutiérrez and F. Rodríguez, Org. Lett., 2007, 9, 727 CrossRef CAS; (d) Y. Shigemasa, H. Oikawa, S. Ohrai, H. Sashiwa and H. Saimoto, Bull. Chem. Soc. Jpn, 1992, 65, 2594 CrossRef CAS; (e) M. Niggemann and M. J. Meel, Angew. Chem. Int. Ed., 2010, 49, 3684 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data for new compounds. See DOI: 10.1039/c2ra22268j |
| This journal is © The Royal Society of Chemistry 2012 |