An example of learning about plastics and their evaluation as a contribution to Education for Sustainable Development in secondary school chemistry teaching†
Mareike
Burmeister
and
Ingo
Eilks
University of Bremen, Germany. E-mail: mburmeister@uni-bremen.de; ingo.eilks@uni-bremen.de
First published on 8th March 2012
Abstract
This paper describes the development and evaluation of a secondary school lesson plan for chemistry education on the topic Education for Sustainable Development (ESD). The lessons focus both on the chemistry of plastics and on learning about the societal evaluation of competing, chemistry-based industrial products. A specific teaching method was developed and applied for the latter purpose: the consumer test method. This method mimics the authentic societal practice of evaluation performed by consumer testing agencies. Applying the consumer test method in the context of this paper is directly tied to the three dimensions most often occurring in prominent sustainability models: ecological, economic and societal sustainability. This paper justifies embedding learning about plastics into the ESD-perspective by using the socio-critical and problem-oriented approach to chemistry teaching. An overview of the lesson plan is given. Experiences and feedback from teachers and students based on the cyclical development by Participatory Action Research are discussed. They reveal the lesson plan's potential to contribute to higher levels of student motivation and ESD understanding.
Scope and scientific background
The heart of the philosophy of sustainable development as defined by the Brundtland-commission about 25 years ago (UN, 1987) remains a lifestyle which can consistently meet “the needs of the present generation without compromising the ability of future generations to meet their own needs.” Today, sustainability thinking is a central focus in every modern society in the fields of politics, economy, ecology, and societal affairs. Nevertheless, there is still discussion about what exactly is meant by the term sustainable development (Burmeister et al., 2012). Four years ago, Johnston et al. (2007) estimated that there are about 300 different definitions of sustainability and sustainable development. There may be even more today. Our current understanding of sustainability is thus not very sharply defined. Different concepts and ideas coexist simultaneously in parallel. Common to all of them, however, is an aim to raise the well-being of society with respect to ecological, economic and societal sustainability (Burmeister et al., 2012).Yet sustainable development is not solely a goal for the economy, ecology or society-at-large. It has also become a regulating idea in the field of education (Rauch, 2004). Beginning with the Agenda 21, educational policy now states that “education is critical for promoting sustainable development and improving the capacity of the people to address environment and development issues” (UNCED, 1992). The current movement towards Education for Sustainable Development (ESD) was the result. This was later expanded into the UN World Decade of Education for Sustainable Development (DESD) for the years 2005–2014 (UNESCO, 2005, 2007; De Haan et al., 2010). The philosophy behind ESD and DESD is an education which focuses on both students' ability to actively participate in society and the development of skills which allow them to actively and sustainably shape their future society (De Haan, 2006, 2010). All educational levels and domains are expected to contribute to ESD, especially also including school chemistry education (Burmeister et al., 2012).
But, why do we view chemistry education as playing a prominent role in ESD? The chemical industry lies at the heart of every industrialized society. Products based on chemical processes are available everywhere in our lives (Bradley, 2005) and chemistry and industry are aiming a greener chemistry (Anastas and Warner, 1998) to achieve sustainable production habits and end-products (Jenck et al., 2004). Yet both industry in particular and chemistry in general are still viewed critically by most societies and suffer from the negative image which is closely associated with such endeavors (Hartings and Fahy, 2011). Seen from this point of view, chemistry teaching should more thoroughly pursue the goal of raising students' ability to better understand the role of chemistry in society (Hofstein and Kesner, 2006). Seen through the lens of socially-relevant science education (Hofstein et al., 2011), chemistry education should increase students' ability to evaluate chemistry related businesses and products in a multidimensional, balanced and carefully reflected fashion. From an ESD standpoint, this should include perspectives such as how chemistry can affect the future, positively contribute to designing sustainable communities, aid in the proper stewardship of natural resources, encourage sustainable economics, and cope with the downside of globalization (Wheeler, 2000). The central role of chemistry for maintaining the current standard of living, while simultaneously bettering the economy towards a more sustainable future, makes chemistry education a prominent domain for fostering ESD. And chemistry curricula are full of potential points of contact. Examples include the debate about the production of different goods and their effects on our personal lives (e.g.Marks and Eilks, 2010), the existence of alternative forms of energy production and use (e.g.Feierabend and Eilks, 2011), or the interaction between local, industrial chemical plants and regional economies and societies (e.g.Hofstein and Kesner, 2006).
The sustainability debate on the use of plastics in modern, industrialized societies can also be placed among these chemistry-related, socio-scientific issues (Wolf et al., 2010). The potential of such products is enormous and the range of possible applications is nearly limitless (Eyerer, 2010a, b). The debate raging over the use of plastics (and potential alternatives to them) ranges into all three dimensions inherent in most sustainability models: ecological, economic and societal. The ecological component concerns itself with the non-degradability of most conventional plastic products. This includes the fact that most recycling mechanisms for end-products remain either incompletely solved or are woefully inadequate (Wolf et al., 2010). For example, the US Environmental Protection Agency (EPA) documented an overall plastic recycling rate of only 7 percent within the USA. Neverthless, even this small rate amounted to 2.1 million tons in 2009, although recycling rates differed widely between different types of plastics (EPA, 2011). The extensive use of plastics in modern society also leads to huge amounts of plastic waste either being land-filled or burned. Currently in the USA, plastics make up about 12 percent of the municipal solid waste stream. In the 1960 this number was only one percent (EPA, 2011). But much of this waste has neither been incorrectly separated from other wastes nor is being combusted under environmentally friendly conditions. Much waste is buried or dumped into the oceans, which increases potential dangers for the animals living in these areas (Barnes and Milner, 2005; Sheavly and Register, 2007). But environmental problems do not solely stem from the polymer materials composing the plastics themselves. Plastic additives during the production process are also frequently found in the environment and, furthermore, have been shown to bioaccumulate in different species. Bioaccumulation in animals–and human beings–can lead to a loss of fertility and is currently suspected of triggering or causing different diseases (Geyer et al., 2000). Another issue concerning both the economy and ecology is that most plastics up to a few years ago have been manufactured from crude oil. A growing supply of polymers made from renewable sources is emerging, currently delivering about 725![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year (European Bioplastics, 2011). However, this amount is only 0.003% when compared to the overall total of 230 million tons a year of fossil fuel-based plastics (PlasticsEurope, 2010). Plastic technologies are also drawn into the discussion of preserving the world's fossil fuel resources and reducing waste streams, since these aspects also raise total carbon dioxide emissions and thus support climate change. On the other hand, the current use of plastics contributes significantly to preserving crude oil reserves and preventing waste. Without plastic packaging, estimates have shown that the tonnage of necessary alternate materials, for example glass or paper, would roughly increase by a factor of four. Worse, emissions of greenhouse gases would rise by approximately 61% (PlasticsEurope, 2010).
000 tons per year (European Bioplastics, 2011). However, this amount is only 0.003% when compared to the overall total of 230 million tons a year of fossil fuel-based plastics (PlasticsEurope, 2010). Plastic technologies are also drawn into the discussion of preserving the world's fossil fuel resources and reducing waste streams, since these aspects also raise total carbon dioxide emissions and thus support climate change. On the other hand, the current use of plastics contributes significantly to preserving crude oil reserves and preventing waste. Without plastic packaging, estimates have shown that the tonnage of necessary alternate materials, for example glass or paper, would roughly increase by a factor of four. Worse, emissions of greenhouse gases would rise by approximately 61% (PlasticsEurope, 2010).
But, there are also many other societal implications of the use of plastics which reach beyond the question of preserving fossil fuel reserves or the environment for future generations. In Western countries, plastic production creates many well-paid jobs and new, innovative products may demand even more employees. The incredibly broad range of current and future applications argues for the economic importance of plastics. The range of use is much broader than just focusing packaging. Innovations in mobility, health care, or even environmental protection techniques are not possible without modern and innovative plastics (Eyerer, 2010a, b). However, even here problems exist. Much of the plastic waste from conventional use produced in industrialized countries is exported to Third World countries (Kitt, 1995) or shipped to China for recycling (Yoshida, 2005). After export, this waste is sometimes handled under questionable working conditions and often quite low environmental regulatory standards. The end result of this kind of “cheap” waste disposal are, more often than not, massive environmental pollution problems, deteriorating public health, and increasing social and/or human rights problems in the country of disposal. These problems boomerang back on the Western world, demanding an answer of our own societies for our moral justification of such political decisions and business practices in the first place. More aspects and potential misconceptions about judging plastics' use in the foreground of sustainability evaluations are discussed in Wolf et al. (2010).
In the end, we recognize many advantages and disadvantages in the use of plastics, which compete with one another in our final deliberations on sustainability. Overall, the plastic debate offers an authentic framework for classroom discourse on the applications of chemistry and technology. It provides insights into how exactly society is handling this debate and which possibilities lie open for an individual to contribute to a collective decision about our future. This includes both the approaches selected for producing, using and handling conventional plastics and any extant alternative technologies, which may lead to new developments for the future.
Even though chemistry plays a key role in sustainable development, widespread networking and dissemination of teaching materials is still a rare phenomenon. It is currently quite difficult for teachers to locate sufficient materials specifically focusing learners' attention on sustainability issues beyond the purely technological perspective covered by most chemistry classes. In many countries, both an organized, widespread team effort and the availability of ESD-driven teaching materials are still lacking. E.g. in Germany, teaching practices which explicitly address and expand upon ESD issues in chemistry education remain the exception rather than the rule (Burmeister et al., 2011). This was the reason why the project presented in this paper addresses the development of effective lesson plans for more firmly embedding ESD into secondary school chemistry education. A group of teachers within a Participatory Action Research project (Eilks and Ralle, 2002) in this project selected developing a new lesson plan based for lower or upper secondary school chemistry classes as its goal, which thoroughly focuses on ESD concerns. Supported by chemical educators, the group decided to focus on plastics and their evaluation. The teachers decided the resulting lesson plan should not only include basic learning about polymers and plastics, but should also bring the societal debate on the sustainable use of plastics to the forefront.
Development of the lesson plan
The lesson plan development followed the Participatory Action Research (PAR) approach in science education as described by Eilks and Ralle (2002). PAR understands evidence-based knowledge from educational research and practical experience from the classroom to compose the two ends of the knowledge spectrum of teaching and learning, both of which are equally important and have their own strengths (McIntyre, 2005). Thus, teachers and researchers in PAR cooperatively develop and investigate science teaching practices. Evidence from educational research and the practical experience of teachers are united through discussion. Within teacher-researcher group processes, knowledge from the different domains is compared and reflected upon with respect to its relevance for innovating teaching practices. A cyclical process is initiated from this point onwards (Fig. 1). Lesson plans are researched, designed, tested, analyzed, and refined. New, innovative teaching concepts and materials are the end-products of this model. The cyclical testing and reflection also enlarges educators' knowledge about teaching and learning on both sides of the project. The participating teachers become better trained and are actively involved in documenting innovative teaching practices. The accompanying research also collects general evidence, which covers both the effects of changed teaching strategies and teachers' and students' personal perceptions of the new teaching approaches and pedagogies (Markic and Eilks, 2011).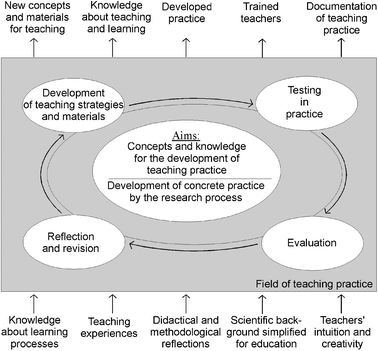 | ||
| Fig. 1 Participatory Action Research in science education (Eilks and Ralle, 2002). | ||
The project described here took place in a teacher group which has been working together for a total of about 12 years now. The group consists of ten teachers from various secondary schools in western Germany. It meets once a month for roughly three hours to discuss developments. The development of this lesson plan took up about one year of the group's time. Proposals were discussed during the meetings, reflected upon, and re-negotiated until a rough draft of the lesson plan was ready. Accompanying the development process, several pre-testings of specific parts of the lesson plan took place. Later reflection was based on testing and feedback results from testing the lesson plan applied in its fully-developed form in different learning groups and taught by different team members.
Educational framework
The object of the lesson plan was to teach students the chemistry basics necessary for understanding plastics, while simultaneously increasing the learners' skills in the area of understanding socio-scientific debates. This was undertaken with a clear focus on ESD, which highlighted the aspects of the production, use and disposal of plastics. The teachers made the decision to orient their efforts using the socio-critical and problem-oriented model of chemistry and science teaching as described in Marks and Eilks (2009). In several earlier case studies, this approach had proven itself to be a valuable framework for effectively combining chemistry learning with personal self-reflection upon society's handling of socio-scientific issues (see Eilks, 2002a; Marks et al., 2008; Marks and Eilks, 2010; Feierabend and Eilks, 2011).The socio-critical and problem-oriented approach to chemistry teaching suggests that the lesson plan is best carried out in five steps (Fig. 2). Beginning with an authentic, socio-scientific controversy as an impetus, pupils' motivation to learn essential science background material tends to evidence higher levels of motivation. After contending with basic background knowledge, learners resume the initial debate and must decide whether their newly-acquired, scientific knowledge has helped them to answer the questions which have been posed. As with all socio-scientific controversies, new knowledge can help to better understand the points of contention. It cannot, however, magically resolve the controversy itself. To aid pupils' understand of how social controversy is handled, an authentic topic was selected, which mimics the societal practices employed for carrying out any given debate. Several options exist, for example role playing or business games (e.g.Marks et al., 2008; Feierabend and Eilks, 2011). However, other pedagogies can also be selected, like the journalist method mirroring work in the press or TV (Marks and Eilks, 2010; Marks et al., 2010).
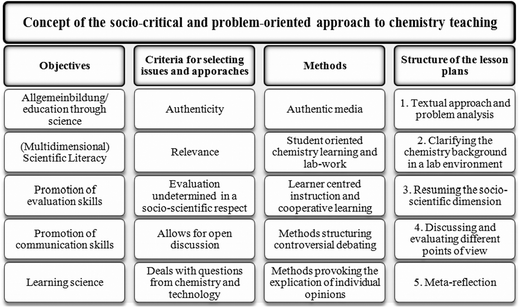 | ||
| Fig. 2 Framework outlining the socio-critical and problem-oriented approach to chemistry and science teaching (Marks and Eilks, 2009). | ||
The criteria necessary for selecting suitable classroom issues are clearly stated by the socio-critical and problem-oriented approach to chemistry teaching. The topics must be current, authentic and relevant. This means that the issue selected must appear in up-to-date media reports that can be used as teaching materials, e.g. television reports, magazine articles, newspaper clippings, advertisements, interest group brochures, political programs, etc. The issues must also be controversial. There must be different stances taken on the topic and these must be expressed in the public forum (Marks and Eilks, 2009). Such different and often contradictory claims challenge students to make up their own minds, think critically, and express their own opinions (Sadler, 2004). It is important that open discussion is possible without opening students to criticism within the group for extreme or less-accepted opinions due to ethical or scientific reasons. Otherwise, no open debate will occur. Nevertheless, all issues require the accumulation of basic chemistry knowledge, which is helpful and necessary for understanding both the debate as a whole and for fulfilling the chemistry syllabus.
All these criteria are fulfilled by the debate on plastics. The main controversy circles around whether we should use plastics in the manner we do or in the current amounts generated. This includes the question of shifts to either alternate uses of plastic products and increased production of bio-plastics. The latter are defined as “bio”-plastics because they are either biodegradable or are produced from renewable bio-sources. However, the label given to “bio” or “green” plastics is not as cut-and-dried an issue as might seem at first glance (Wagner, 2011; European Bioplastics, 2010). This debate is authentic, since television and newspaper reports constantly reveal problems with plastic wastes discovered in the environment, be it on land or in the oceans. Such reports also discuss the political and social dimensions of exporting waste from Western countries to Africa or Southeast Asia (Kitt, 1995; Yoshida, 2005). The entirety of the debate is mentally challenging and ethically laden. Nevertheless, different viewpoints are possible and constantly mentioned in the social debate. And, of course, this topic is related to direct applications of chemistry and technology.
In order to connect the socio-critical and problem-oriented chemistry approach to ESD, the criteria for the issues selected are modified by the fact that the topics must be connected to the sustainability discussion. Since many societal issues are related to this topic, this is not enough in and of itself. The debate chosen must intrinsically and explicitly cover the social, economic and ecological aspects tied to sustainability. It must be clear where both possibilities for sustainable actions and where problems to such approaches exist. The debate on plastics meets also these additional criteria.
Many different methods are available for showing learners how society handles debates on competing scientific and technological developments and evaluates them. A completely new method was developed for the current case study: the consumer test method (Burmeister and Eilks, 2011). This method mimics the authentic societal practices of product testing and product comparison and places these procedures within a jigsaw classroom setting (Aronson et al., 1978). Students are introduced to the consumer test agency with the objective that they personally learn to rate and compare various products. Such consumer testing agencies exist in many countries, for example Warentest in Germany, Which Magazine in the UK, or the Consumer Report in the US. Most students are familiar with such consumer tests from daily newspapers. The public often mistakenly views such publications as an objective measure of quality. Each test looks a bit different, but lesson plans can be developed be using each of them as a pattern.
Consumer tests choose different testing dimensions. These competing factors are weighted against one another, e.g. by using percentages. But, it often remains unclear to the reader that such weighting is not an objective measure. It is a decision made by an individual or group, and the justifications for the weighting are not always transparent. This aspect can actually influence the final product rating more heavily than the various, individual categories selected for testing do. In order to merge the consumer testing method with ESD teaching, we took the three dimensions of sustainability discourse (ecological, economic and societal aspects) and transformed them into the categories tested by the learners. They were connected with a fourth dimension about the potential and properties with respect to make a good and valuable range of applications. The influence of individually-made decisions also plays an enormous role when assigning marks in each of the testing categories selected. Such decisions play a large role, especially because objective criteria are not always available to the testers. Pupils must learn about the importance of individual decisions when they are driven by personal values and ethical concerns.
In our case, the consumer testing method analyzes three different kinds of plastics: TPS, PVC and PET. Each of these plastics has specific (dis)advantages, which all contribute to the final evaluation (Wolf et al., 2010). Table 1 summarizes the typical (dis)advantages of the plastics selected for this lesson plan. Students quickly realize that there are nearly always conflicting or contradictory tendencies within each test dimension, which work against each other. This is also reflected in the public debate about new products and technologies whenever they take all the sustainability dimensions into account.
| Thermoplastic starches (TPS) | |
| • Largely composed of renewable resources. | • Unstable, therefore only useful in products requiring biodegradability, e.g. mulch foils, sutures or food packaging. |
| • Biologically decomposable, which makes them especially suited for uses in agriculture, medicine and product packaging. | • Biodegradable does not mean compostable, causing problems for regional disposal firms. |
| • No dangers in their production, which occurs under relatively mild conditions and under minimal energy requirements in optimized production facilities. | • Intensive agricultural practices necessary to win raw materials, usually through gene-manipulated crops. |
| • Neither reactants nor products are dangerous for animals or humans. | • Up until now no recycling possible. |
| • The demand for bio plastics is constantly on the rise, causing a booming market for them. | • Composting leads to product loss without energy gain. |
| • Production is currently non-competitive with respect to production facilities, production capacities, and market pricing. | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Polyethene terephthalate (PET) | |
| • Good physical properties: stabile, transparent, and flexible, especially good for packaging materials. | • Good for many, but not all, uses. For example, it cannot be made into a rubberlike, elastic state. |
| • Can be spun into fibers and is especially indispensable to the textile industry (Polyester). | • Permeable to gases, so that carbon dioxide-containing drinks in PET bottles can lose their fizz and possibly pick up aromas from their storage environment. |
| • Can be easily recycled and the products of recycling evidence hardly any loss of quality. | • Is manufactured from fossil fuels. |
| • Is safe for humans and the environment, combusting to CO2 and water, so that it can be burned in waste plants without problem. | • Is primarily recycled in China, where social and environmental conditions normally do not meet Western standards. Imported PET waste is typically recycled into fleece cloth, which is exported—frequently containing additives which are forbidden in Western lands. |
| • Manufacturing is well-established and has been optimized, so that cheap economic production is possible. | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Polyvinyl chloride (PVC) | |
| • Well-researched plastic with many uses. | • Creates carcinogenic substances in the intermediate production steps, thus increasing the risk for humans and environment in the case of industrial accidents. |
| • There are hard, soft and elastic forms of PVC, depending on the required use. | • Combustion of PVC frees poisonous gases (e.g. HCl), which causes additional problems in the case of unintended fires. |
| • Durable and especially fitted for outdoors use, e.g. windows, or locations under heavy traffic like flooring. | • Can only be burned in modern waste disposal systems with advanced filtering systems. Otherwise, mixed waste must be stored (illegal in the EU), so that waste is often exported to foreign lands. |
| • Cheap in almost every area. | • Is produced from fossil fuels. |
| • Chlorine, stemming from the synthesis of sodium hydroxide, is itself a reactant, which reduces disposal and extra production costs. | |
| • Pure PVC can be recycled. | |
Structure of the lesson plan
The lesson plan developed by the PAR group follows the five-step model described by Marks and Eilks (2009). It begins with authentic, yet contradictory excerpts taken from 1) an industry brochure on alternative (bio-)polymers and 2) a TV documentary film dealing with the plastic waste problem in our oceans. Pupils are asked to create headlines for small newspaper articles. The headlines are displayed on the blackboard to provoke a broad range of potential questions. Some of these deal with the properties of plastics, others address the public debate about dealing with plastic waste, and yet others ask a shift to alternative bio plastics might be beneficial.A lab phase was structured around the learning-at-stations method (Eilks, 2002b) in order to clarify the necessary chemistry background knowledge. Nine different stations offer different tasks to perform. Some stations are experimental in nature, others are theoretical (Fig. 3). They variously cover the physical properties of different plastics, the structures and production of polymers, comparative properties, polymer synthesis, and model-based explanations of the different types of plastics (elastomers, duroplasts and thermoplasts). The students are given 2–3 periods (45 min each) to learn the properties of the different plastics and the chemistry of different polymers. Additional content can be added depending on grade level, for example in advanced courses on upper secondary level we added stations on the reaction mechanisms for forming PVC, PET and TPS.
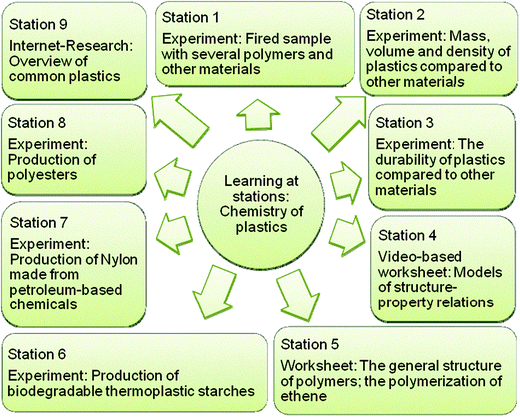 | ||
| Fig. 3 Learning at stations for plastics and polymer chemistry. | ||
The socio-scientific debate is resumed after the lab-phase. The students must discover which of the initial questions have already been answered—this will include most of the questions concerning the basic chemistry behind plastics. Yet many questions remain open, including those dealing with judgment and decision-making when it comes to the waste problem and promoting alternative technologies.
A simple concept of sustainability is introduced to the pupils to provide them with a basis for evaluation. This makes sure that the learners understand that sustainability discourse always covers the ecological, economic and societal domains. Consumer interests and the material properties of plastics are also shown to be important factors. After this, the consumer testing phase begins. Students mimic product testing procedures as they are performed by consumer testing agencies. They must evaluate three different types of plastics:
• PVC, which is often viewed critically by society due to disposal problems in the past, despite its cheap production and its broad range of applications
• PET, which is neutrally viewed due to its ease of recycling, but is often exported to Third World countries with questionable social and environmental standards, and
• TPS, which is positively seen because of its biodegradability and manufacture from renewable sources, but is prohibitively expensive and limited in application.
The consumer testing method is structured around the jigsaw classroom method (Aronson et al., 1978). Students are introduced to the consumer testing agency and shown how to evaluate and rate PVC, PET and TPS for themselves. They are asked to cover four dimensions: three from sustainability discourse and one analyzing the properties in use (see Fig. 5). However, the students were not given the specific weighting of the four dimensions. The weighting is left up to the participants. They must negotiate among themselves, e.g., whether the ecological component (raw material sources and degradability) or economic aspects (availability and costs for production) should be weighted more heavily. The students are asked to weight these dimensions three times: first individually, then in a group, and finally as a whole class. The weighting factors decided upon are then applied to the consumer test occurring later. It will also be discovered that they strongly affect the final rating for every plastic. After this, the learners are given an evaluation sheet, which mirrors similar reports published in daily newspapers. We used one structured and layouted parallel to Warentest, the best known consumer test agency in Germany (Fig. 4).
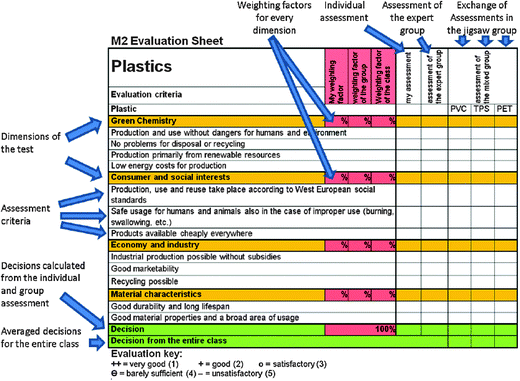 | ||
| Fig. 4 Evaluation sheet for the consumer test method with commentary. | ||
The class is divided into three groups, each working on one of the three types of plastic. Each group receives a text for their specific sort of plastic, which explains its production, use, advantages and disadvantages. The students must rank the special (dis)advantages of their particular plastic and give them marks ranging from very good (++) to unsatisfactory (−) for each criterion on the evaluation sheet. Once again, the pupils first work individually, then discuss and find commonalities for their rankings within the whole group. The next phase mixes the jigsaw-classroom groups afresh, so that each grouping contains at least one specialist for each kind of plastic. The newly-constituted groups exchange their knowledge and discuss their evaluations. A second round of product discussion, negotiation and calculation of grades for each of the plastics takes place. From these calculations, the students quickly see that the (dis)advantages for each plastic quickly counterbalance each other. In almost every case, each type of plastic receives quite similar final marks, normally ranging between good and satisfactory. This quite often shocks the pupils, since the final marks usually differ by only a few percent, despite the obvious disparities between the products. It also becomes quickly clear that different learning groups reach different ranking schemes with respect to the relative weighting percentages and the final marks.
At the end of the lesson, the whole unit is reflected upon. This is especially true for the processes of weighting and negotiating during the consumer test phase. The final marks are examined and compared to the individual ratings. It quickly becomes clear that the initial weighting of the dimensions far outweighs the final, cumulative result of the individual decisions. The value-driven balancing of different sustainability dimensions proves itself to be much more influential on the final results than decisions reached for the individual areas. The learners quickly recognize that the central aim of the consumer test method is not to grade or rank different types of plastic from the viewpoint of economic, ecological, or societal aspects. They see that such methods try to examine weighting and discussion processes when evaluating competing dimensions like the different dimensions inherent in questions of sustainability. The students recognize that each step in the process is an individual decision which is influenced by values and ethical concerns. Because of this, some students weight the ecology dimension at 60%, others only at 20%. The same is true for the other dimensions. These decisions not only influence the weighting of different dimensions, but also any grade assigned to a specific item. For example, some testers grade PVC use with “good”, others with only “satisfactory”. Much debate occurs during the process and the students recognize that this discussion is influenced not only by scientific facts, but also by personal attitudes and values. This is always the case for any reflections on technology when its use and impact on sustainable development are considered.
Evaluation of the lesson plan
Method
The fully developed lesson plan was carried out in five classes spanning a spectrum from grade 9 (age range 14–15) to grade 13 (age range 18–19). Altogether, 95 students participated in the case study. Data was collected by protocolling the teachers' group discussions during the monthly meetings of the action research group. Student feedback was collected using a combination of two written questionnaires. Both questionnaires were filled out directly after completion of the lesson plan. One open-ended questionnaire covered students' reflections on: (1) what they considered to be the most important things that they had learned, (2) their personal opinion about the lesson plan, and (3) what they now believed about the different types of plastics. Open data was analyzed by qualitative content analysis (Mayring, 2000). A 4-step Likert questionnaire with 12 items asked the students for their opinion about the lesson plan. Participants' answers included whether they liked the topic, how they viewed the methods which had been employed, and if they had experienced a change in their personal attitudes towards the issues of sustainability, consumer testing and plastics (Fig. 5). All three sources of data were inter-related for control of reliability in the means of triangulation.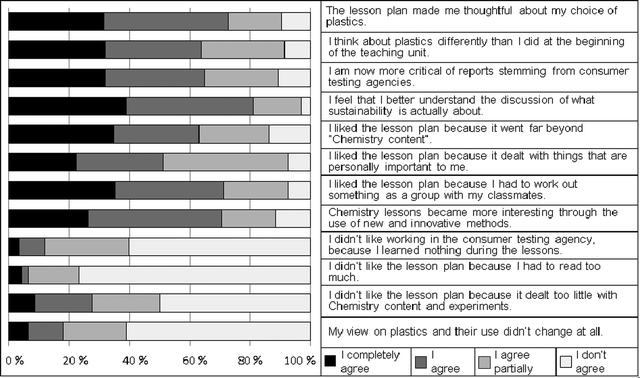 | ||
| Fig. 5 Student feedback in the Likert-questionnaire. | ||
Findings from the teachers' group discussions
Within PAR sessions, participating teachers stated that the lesson plan was proving to be both highly feasible and intensely motivating for their students. The teachers described the learners involving themselves intimately with both inquiry activities and cooperative learning processes. The more controversial the questions selected were, the more challenging the discussion became. For example, the teachers described that many students had great difficulty in assigning their own weighting factors and in jointly reaching agreements on a given factor. This phase incited intense discussions about the different sorts of plastics. More importantly, the questions incited endless debate about the specific role that the evaluation dimensions should play when categorizing plastic products. The durability and affordable prices coupled with PVC ran headlong into this product's environmentally persistent nature and poisonous by-products if burned under uncontrolled conditions. The teachers reported that the students contrasted this with the degradability of TPS with its high production costs and limited applicability. The contradictions inherent between the different dimensions led to productive confusion and an intense reflection upon the evaluation process itself. It was an interesting observation that, regardless of the different weightings and marks proposed by all of the learning groups, the results always flattened into an average value between “good” and “satisfactory”, with no extreme evaluations emerging for the different plastics. Each plastic was evaluated as neither very good, nor very bad. From the teachers' reports, this was not what the pupils had expected to see, as the students also bluntly stated in the open questionnaire. Some of the teachers also said that they had never thought about consumer testing activities in such an intense manner and that their view of such activities had shifted drastically.Findings from the students' questionnaires
Many students stated in the open questionnaire that the lesson plan had been interesting and that it is important to learn more than just “chemistry”. Many of the learners saw chemistry learning as represented by this lesson plan to be relevant for their lives and futures:“It was informative. I saw the topic of plastics from a new angle. I even am interested in this topic outside of class.” Or: “I have found a new viewpoint concerning plastics and my personal use of them.”
Some students indicated that they had gained knowledge about sustainability issues and the manner in which consumer tests are devised and implemented.
“I especially liked the look into the everyday work of product testing employees.”
Some pupils explicitly remarked that they now were more critical towards the use of plastic products:
“Chemistry class has shown me in the past few weeks that we need to be mindful of which products we purchase.” Or: “This was useful for the future, since you now pay attention to what you buy.”
Only a few of the pupils offered criticism about the lesson plan. Students from higher grades said that the lessons did not include enough focus on theory, e.g. chemical reactions and formulae, and that they had had to discuss too many things:
“The chemistry teaching of the past weeks took on a new and interesting approach to old contents. However, the lessons seemed to put too much value on the determination of societal content, so that a theoretical link to chemistry as a scientific discipline was missing.” Or. “The topic was interesting with respect to societal issues, but superficial with respect to chemistry content, formulae, reactions…”
These students expressed a feeling that this type of lesson is not really “chemistry”, but would better fit into the social sciences:
“It was something different and other aspects were touched upon in comparison with normal chemistry books. But in the long run boring, especially the analysis and weighting of a particular plastic, since this actually belongs to the Social Sciences and not to chemistry.” Or: “In my opinion, this has less to do with chemistry than it does with Economics and product testing.”
This observation reveals an interesting insight into the perception of chemistry held by these pupils. Discussing controversial issues and evaluating chemical content does not seem to belong to their definition of chemistry class (Marks and Eilks, 2010). In the opinion of these students, debate is not considered to be an integral part of chemistry teaching and learning. It appears that conventional teaching approaches have led these pupils to a point which yields a very abbreviated, distorted picture of what chemistry really is to them. These students do not believe that modern chemistry is part of a societal and economical endeavor more far-reaching than a simple construct consisting only of formulae and theories. Despite this, the open questionnaire showed that this point of view was not shared by the majority of the students and delivered quite positive overall feedback on the lesson plan.
The positive results from the teachers' reports and the open questionnaire were supported by the Likert items (Fig. 5). Most students agreed that the lesson plan had been motivating and was useful for their future lives. A vast majority of the students agreed that the lesson plan had made them thoughtful about their choice of plastics and that they now saw plastics in a different light than they had before the lesson plan. They also stated that they had become more critical towards reports coming from consumer testing agencies. A majority of the learners liked the lesson plan, because offered more than only pure chemistry content. An overwhelming majority stated that they now had the feeling that they better understood the sustainability discussion. The student-centered methodology was particularly appreciated and the pupils expressed the feeling that the consumer test method had contributed their learning success. Only a minority said that the lesson plan had too little focus on chemical theory. Most students rejected this statement emphatically.
Discussion
Both the students as well as the teachers described a highly motivating lesson plan. The topic was considered being unconventional, but interesting and challenging. The broader focus beyond chemistry content learning was considered as an enrichment of chemistry teaching both from the teachers as well as the students view. But, also both—teachers and students—described potential of the lesson plan to make chemistry learning more relevant and provoking debate with potential to promote higher order cognitive skills like competencies in communication and evaluation. All these considerations got support from all three sources of data and thus evidence for this claims got support in the means of triangulated data. From the students' feedback in the Likert questionnaire there are also indications that students learned about sustainability issues and started becoming more critical concerning the use of chemistry related products in their life and future. This is also supported by single claims in the open questionnaire and in the teachers' impression. Anyhow, data cannot say whether this consideration will lead to any change in later acting in the students' future life, nor whether the positive perception of relevance in this lesson plan will have impact on the students' general consideration of chemistry teaching's relevance. But, this might be an even too high expectation for the performance of only one lesson plan of this type.Conclusions
Based on Participatory Action Research (Eilks and Ralle, 2002), this project was able to cyclically refine the teaching materials developed due to feedback stemming from the teachers and students involved. Although initial problems existed, caused by the complexity of the consumer test method, a solid lesson plan was achieved by later cycles of testing. In the final analysis, the lesson plan presented for plastics and their evaluation in the foreground of ESD proved to be a very highly motivating addition to the German chemistry curriculum. This was especially true because of the inclusion of the consumer test method, which added an authentic, socially-relevant, and controversial facet to the learning process. This was supported by the responses of both students and teachers within the case study described here.Like previous examples of such teaching units have demonstrated (Marks and Eilks, 2009; Marks and Eilks, 2010), the socio-critical and problem-oriented approach to teaching has proven itself to be a very feasible platform for undertaking such lesson planning. The case study presented here reveals that this approach can provide fertile ground for the fruitful integration of societally-oriented chemistry education, based on important aspects of environmental or ESD-driven chemistry education, as there were also indications in similar lesson plans connected to socio-scientific issues from environmentally related topics (Eilks, 2002a; Marks and Eilks, 2010; Feierabend and Eilks, 2011). There are initial indications that students became increasingly contemplative with respect to both the environment and their personal decisions made concerning sustainability issues and available resources. Therefore, respective practices should be applied more often in chemistry education.
References
- Anastas P. T. and Warner J. C., (1998), Green Chemistry Theory and Practice, New York: Oxford University.
- Aronson E., Blaney N., Stephin C., Sikes J. and Snapp M., (1978), The jigsaw classroom, Beverly Hills: Sage.
- Barnes D. K. A. and Milner P., (2005), Drifting plastic and its consequences for sessile organism dispersal in the Atlantic Ocean, Marine Biology, 146, 815–825.
- Bradley J. D., (2005), Chemistry education for development, Chem. Educ. Int., 7, retrieved from the World Wide Web, July 01, 2011, at http://old.iupac.org/publications/cei/vol6/index.html.
- Burmeister M. and Eilks I., (2011), Teaching about plastics and education for sustainable development in chemistry teaching, Paper presented at the 9th ESERA conference, France: Lyon.
- Burmeister M., Rauch F. and Eilks I., (2012), Education for Sustainable Development (ESD) and secondary school chemistry education, Chem. Educ. Res. Pract., 13(2), DOI: 10.1039/c1rp90060a.
- Burmeister M., Jokmin S. and Eilks I., (2011), Bildung für nachhaltige Entwicklung und Green Chemistry im Chemieunterricht, Chemie konkret, 18, 123–128.
- De Haan G., (2006), The BLK ‘21’ programme in Germany: a ‘Gestaltungskompetenz’-based model for education for sustainable development, Environ. Educ. Res., 12, 19–32.
- De Haan G., (2010), The development of ESD-related competencies in supportive institutional frameworks, In International Review of Education, Vol. 56, Numbers 2–3/June 2010, pp. 315–328.
- De Haan G., Borman I. and Leicht A., (2010), Special issue: The midway point of the UN Decade of Education for Sustainable Development: Where do we stand? Int. Rev. Educ., 56, 199–372.
- Eilks I., (2002a), “Learning at Stations” in secondary level chemistry lessons. Sci. Educ. Int., 13(1), 11–18.
- Eilks I., (2002b), Teaching ‘Biodiesel’: A sociocritical and problem-oriented approach to chemistry teaching, and students' first views on it, Chem. Educ. Res. Pract., 3, 67–75.
- Eilks I. and Ralle B., (2002), Participatory Action Research within Chemical Education. In B. Ralle and I. Eilks (ed.), Research in chemical education–What does this mean? (pp. 87–98). Aachen: Shaker.
- EPA, (2011), Wastes–Resource Conservation–Common Wastes & Materials: Plastics. US Environmental Protection Agency, Retrieved from the world wide web, October 2, 2011 at http://www.epa.gov/osw/conserve/materials/plastics.htm.
- European Bioplastics, (2010), Fact Sheet March 2010—Home composting, Retrieved from the world wide web, August 2, 2011 at http://en.european-bioplastics.org/wp-content/uploads/2011/04/fs/FactSheet_Home_Composting.pdf.
- European Bioplastics, (2011), Driving the evolution of plastics, Retrieved from the world wide web, October 2, 2011 at http://en.european-bioplastics.org/wp-content/uploads/2011/04/EuBP_image_brochure_2011.pdf.
- Eyerer P., (2010a), Plastics: Classification, characterization, and economic data. In P. Eyerer (ed.), Polymers—Opportunities and risks (pp. 1–19). Dordrecht: Springer.
- Eyerer P., (2010b), Synthesis (manufacture, production) of plastics. In P. Eyerer (ed.), Polymers—Opportunities and risks (pp. 19–47). Dordrecht: Springer.
- Feierabend T. and Eilks I., (2011), Teaching the societal dimension of chemistry using a socio-critical and problem-oriented lesson plan on bioethanol usage, J. Chem. Educ., published online first July 10, 2011.
- Geyer H. J., Rimkus G. G., Scheunert I., Kaune A., Schramm K.-W., Kettrup A., Zeeman M., Muir D. C. G., Hansen L. G. and Mackay D., (2000), Bioaccumulation and Occurrence of Endocrine-Disrupting Chemicals (EDCs), Persistent Organic Pollutants (POPs), and Other Organic Compounds in Fish and Other Organisms Including Humans. In: B. Beek (ed.), The Handbook of Environmental Chemistry. Heidelberg: Springer-Verlag.
- Hartings M. R. and Fahy D., (2011), Communicating chemistry for public engagement, Nat. Chem., 3, 674–677.
- Hofstein A., Eilks I., and Bybee R., (2011), Societal issues and their importance for contemporary science education: a pedagogical justification and the state of the art in Israel, Germany and the USA, Int. J. Sci. Math. Educ., 9, 1459–1483.
- Hofstein A. and Kesner M., (2006), Industrial chemistry and school chemistry: making chemistry studies more relevant, Int. J. Sci. Educ., 28, 1017–1039.
- Jenck J. F., Agterberg F. and Droescher M. J., (2004), Products and processes for a sustainable chemical industry: a review of achievements and prospects, Green Chem., 6, 544–556.
- Johnston P., Everard M., Santillo D. and Robèrt K.-H., (2007), Reclaiming the Definition of Sustainability, Env. Sci. Pollut. Res., 14(1), 60–66.
- Kitt J., (1995), Waste exports to the developing world: A global response, Georgetown Int. Environ. Law Rev., 7, 485–512.
- Markic S. and Eilks I., (2011), Effects of a long-term Participatory Action Research project on science teachers' professional development, Eurasia J. Math. Sci. Tech. Educ., 7, 149–160.
- Marks R., Bertram S. and Eilks I., (2008), Learning chemistry and beyond with a lesson plan on “potato crisps”, which follows a socio-critical and problem-oriented approach to Chemistry lessons—A case study, Chem. Educ. Res. Pract., 9, 267–276.
- Marks R. and Eilks I., (2009), Promoting Scientific Literacy using a socio-critical and problem-oriented approach in chemistry education: Concept, examples, experiences, Int. J. Environ. Sci. Educ., 4, 131–145.
- Marks R. and Eilks I., (2010), Research-based development of a lesson plan on shower gels and musk fragrances following a socio-critical and problem-oriented approach to chemistry teaching, Chem. Educ. Res. Pract., 11, 129–141.
- Marks R., Otten J. and Eilks I., (2010), Writing news spots about chemistry—A way to promote students' competencies in communication and evaluation, Sch. Sci. Rev., 92(339), 99–108.
- Mayring P., (2000), Qualitative content analysis, Forum: Qualitative Social Research, 1(2), www.qualitative-research.net/fqs (assessed 01/02/2007).
- McIntyre D., (2005), Bridging the gap between research and practice, Cambridge J. Educ., 35, 357–382.
- PlasticsEurope, (2010), Plastics—the Facts 2010. An analysis of European plastics production, demand and recovery for 2009, Retrieved from the World Wide Web, October 2, 2011 at http://www.plasticseurope.de/cust/documentrequest.aspx?DocID=46843.
- Rauch F., (2004), Education for sustainability: A regulative idea and trigger for innovation, In W. Scott and S. Gough (ed.), Key issues in sustainable development and learning: A critical review (pp. 149–151). London: Roudlege Falmer.
- Sadler T. D., (2004), Informal reasoning regarding socioscientific issues: A critical review of research, J. Res. Sci. Teach., 41(5), 513–536.
- Sheavly S. B. and Register K. M., (2007), Marine Debris & Plastics: Environmental Concerns, Sources, Impacts and Solutions, J. Polym. Environ., 15, 301–305.
- UN, (1987), Report of the World Commission on Environment and Development, Retrieved from the World Wide Web, July 10, 2011 at http://www.un-documents.net/wced-ocf.htm.
- UNCED, (1992), Agenda 21, Retrieved from the World Wide Web, July 10, 2011 at http://www.un.org/esa/dsd/agenda21/.
- UNESCO, (2005), World decade of education for sustainable development, Retrieved from the World Wide Web, July 10, 2011 at http://www.unesco.org/new/en/education/themes/leading-the-international-agenda/education-for-sustainable-development/.
- UNESCO, (2007), The UN Decade of Education for Sustainable Development (DESD 2005–2014). The first two years. Paris: UNESCO.
- Wagner F., (2011), The truth about bioplastics, Retrieved from the World Wide Web, November 28, 2011 at http://www.greenlivingonline.com/article/truth-about-bioplastics.
- Wheeler K., (2000), Sustainability from five perspectives, In K. A. Wheeler and A. P. Bijur (ed.), Education for a sustainable future (pp. 2–6). New York: Kluwer.
- Wolf M.-A., Baitz M. and Kreissig J., (2010), Assessing the sustainability of polymer products. In P. Eyerer (ed.), Polymers—Opportunities and risks II: Sustainability, product design and processing (pp. 1–55). Dordrecht: Springer.
- Yoshida A., (2005), China: the World's Largest Recyclable Waste Importer. In M. Kojima (ed.), International trade of recyclable materials in Asia (pp. 33–52). Mihamaku: IDE-Jetro.
Footnote |
| † This article is part of a themed issue on sustainable development and green chemistry in chemistry education. |
| This journal is © The Royal Society of Chemistry 2012 |
