High-school chemistry teaching through environmentally oriented curricula†
Daphna
Mandler
,
Rachel
Mamlok-Naaman
,
Ron
Blonder
,
Malka
Yayon
and
Avi
Hofstein
Department of Science Teaching, Weizmann Institute of Science, Rehovot 76100, Israel. E-mail: rachel.mamlok@weizmann.ac.il
First published on 22nd March 2012
Abstract
Discussions held in the chemical education community have generated a variety of reports and recommendations for reforming the chemistry curriculum. The recommendations refer to teaching chemistry in the context of real-world issues. This has been suggested as a way to enhance students' motivation. It is suggested that real-world problems emphasize the interdisciplinary nature of chemistry and the relevance of chemistry to the students' lives. An attempt was made to incorporate these recommendations into the teaching of chemistry by teaching analytical chemistry together with environmental chemistry. A unit incorporating analytical chemistry in an environmental context was developed, in which the students learn concepts of a specific environmental issue. The unit “I Have Chemistry with the Environment”, consisting of two modules, was developed on the topics of drinking-water quality, and the greenhouse effect. The research questions focus on the change in the attitudes and perceptions of the students toward chemistry and environmental issues, after learning the environmental unit. The results indicate that the students underwent a significant change in their awareness of environmental issues. All the students mentioned that the unit influenced their everyday-life perceptions of environmental issues and that their awareness of environmental issues increased. Another important finding was that more students found that learning the “I Have Chemistry with the Environment” unit encouraged them to learn chemistry. They indicated that they especially appreciated the feeling that they could discover things by themselves. Clearly, the students found that learning the unit was relevant to chemistry learning as well as to their personal lives. Researchers believe that such a program may promote education for sustainable development.
Introduction
The past, near, mid, and long-term future of our society depend on developing the curiosity, imagination, diversity, efficiency, learning, and communication skills of our students. On the one hand, science ultimately connects people and the world in which they live. Thus, as chemical educators numerous intellectual tasks lie before us as we usher in the next decade (Moore, 2006). According to a long-term vision in education, mathematics, science, and engineering, along with an understanding of scientific processes, technology, and global perception, are essential elements that students must develop to further strengthen our society. On the other hand, various reports (National Board for Professional Teaching Standards, 2009) have indicated an increasing need to improve science, mathematics, and technology education at the high-school level.Exploring ways to develop a relevant curricular unit have interested many researchers for more than three decades (Hofstein and Lazarowitz, 1986; Orion and Hofstein, 1994; Kesner et al., 1997; Aldridge et al., 2003; Gilbert, 2006). Discussions held in recent years, within the chemical education community, have generated a variety of reports and recommendations for reforming the chemistry curriculum (Kegley et al., 1996a,1996b; Osborne and Dillon, 2008). Teaching chemistry in the context of real-world issues has been suggested as a way to motivate and interest students. With real-world problems, it is possible to highlight the interdisciplinary nature of chemistry and the relevance of chemistry to the students' lives (Holbrook and Rannikmäe, 2007, 2009; Marks and Eilks, 2009, 2010; Hofstein et al., 2011). Teaching chemistry in the context of real-world issues and implementing it in environmental and societal issues can be a promising way to help students close the gap between school science, applications of science and technology, and their critical evaluation (Albe, 2008; Holbrook, 1998; Ratcliffe, 1998). The selection of such everyday-life contexts of chemistry and technology should be authentic and relevant to students' lives. It was assumed that a context-based curriculum, namely, an environmental context, may improve students' interest in and attitudes toward science lessons in general and in chemistry in particular (e.g., Lee and Erdogan, 2007; Millar, 2006; Osborne et al., 1998). These aspects are of great importance for achieving learning (Osborne and Dillon, 2008).
In this paper we describe an R & D study whose overreaching goal was to develop and implement a chemistry unit that focuses on context, namely, chemistry in an environmental context and to assess its implications regarding chemical education in high schools in Israel. In our opinion, as a first step, school chemistry must show how chemistry is relevant to the students (Marks and Eilks, 2009). It is often argued that school chemistry should start from experiences in students' lives and that it should take place in relevant contexts (Marks and Eilks, 2010). The study that accompanies this developmental unit process examines the question of whether a curriculum that focuses on context that is relevant to students' everyday lives, namely, an environmental context, motivates them to study chemistry in high school.
Use of environmental issues in chemistry education: background and rationale
Environmental issues are prevalent in the news these days—from warnings of impending global catastrophes to local concerns about pollution in previously pure waterways. Although many aspects of these discussions deal more with economics and politics than science, an educated citizen who knows basic chemistry is often appalled by the way the scientific facts and theories are misstated and how the roles of science and technology in both causing and curing these problems are misunderstood. One of the most valuable contributions that chemistry can make is to prepare students to deal rationally with these issues as chemistry-literate citizens in a world in which they are able to confront new problems intelligently. Chemists can help citizens better understand environmental issues by providing the data and theories that define the problem, assess its seriousness, and explain its causes. They can then use this information to develop new processes and materials and help to solve or reduce the environmental problems. This notation is in line with the UN declaration that “Education for Sustainable Development (ESD) is critical for promoting sustainable development and improving the capacity of the people to address environment and development issues… and thus the latter needs to be incorporated as an essential part of learning.” (UNCED, 1992). One of its objectives was to prepare our students to become responsible citizens in the future (Burmeister et al., 2012). This focus of ESD is in line with the focus of the new unit we developed, namely “I have chemistry with the environment”, which aims at educating our students to become environmental responsible citizens in their adult future.Although implementation of environmental issues to chemistry education is widely use in the last decades (American Chemical Society, 2006; Benett and Lubben, 2006; Hofstein and Kesner, 2006), little quantitative research has explored how including relevant examples impacts students' learning of chemistry content or what types of applications of chemistry are most useful in developing understanding. For example, the college curriculum Chemistry Modules (Norton, 2009) uses context to teach chemistry concepts. One experimental study that explored context-based curriculum found that students who study according to this curriculum are more successful in learning certain aspects of content in comparison with their peers in conventional classes (Gutwill-Wise, 2001).
Two high-school curricula that use everyday contexts to make content more appealing to students are supported by some research. Chemistry in the Community (ChemCom), a curriculum designed by the American Chemical Society (American Chemical Society, 2006), conducted one experimental study showing that students using the ChemCom curriculum did better on chemistry posttests than did their peers in a more conventional course (Winther and Volk, 1994). “Salters” Advanced Chemistry in the UK (Bennett et al., 2005), a curriculum used in the United Kingdom, is somewhat similar to ChemCom in the USA. Few studies have documented what students do learn in the Salters curriculum (Barker and Millar, 1999, 2000). One study indicated that there was little difference between what students learned using the Salters approach and what students learned in a more conventional curriculum (Ramsden, 1997). Although little experimental research exists, both curricula continue to be used by high-school teachers who provide ample anecdotal evidence that their students enjoy learning chemistry more when it is put into more familiar contexts (Bennett et al., 2005). Chemistry Modules, ChemCom, and the Salters approach include environmental examples. A five-year evaluation report on ChemCom, published in 1992, stated that students wanted to learn chemistry through environmental contexts (Sutman and Bruce, 1992). To date, no research has investigated which contexts are most effective in teaching specific chemical concepts. According to a study that investigated the majority of the content for a developmental chemistry course in an environmental context (Robelia et al., 2010), environmental topics would spur students toward learning more chemistry and would change their attitudes to become more pro-environment. Other investigators have organized developmental courses around environmental topics, although their studies did not objectively and quantitatively measure student achievement. Swan and Spiro (1995) reported on a course for non-majors. They did not report any quantitative results in their course, nor were any efforts made to methodically compare the outcomes of their course to more conventional courses. Denise Battles and her colleagues at Georgia Southern University also reported successfully integrating environmental topics into four undergraduate science courses for non-majors, including chemistry. Battles et al. (2003) provided student self-reports of how much the course improved their students' understanding of environmental issues, but this study does not provide objective, quantitative information on student achievement.
Thus, the overall objective of curriculum developers should be to create a learning environment that allows students to interact physically and intellectually with instructional materials through relevant hands-on experiences, and through relevant minds-on and inquiry-oriented activities (Tobin et al., 1988). Moreover, according to the literature, learning chemistry in a relevant context enhances students' attitudes toward and perceptions of learning chemistry (Shwartz, 2006).
Development of the unit: “I have chemistry with the environment”
The main goal of the project described here was to develop a chemistry learning unit that focuses on context, namely, chemistry in an environmental context, intended for those students who opted to major in high-school chemistry. In our opinion, as a first step, school chemistry must show how chemistry is relevant to the students (Genseberger, 1997). It is often argued that school chemistry should start from experiences in students' lives and that it should take place in the relevant contexts. This argument raises questions concerning the development process, namely: How can we link everyday life experiences and contexts to the learning of chemistry concepts? How should this be elaborated at the concrete level of a module? How can we create a situation in which the learning of chemistry becomes functional and meaningful from the students' perspective? After choosing an educational context for a curricular module, many questions still remain. How can we create an educational setting in a classroom such that the context becomes and remains relevant for students? What roles will students play in the context (De Vos et al., 2002)? What chemistry concepts operate within the context? And most important of all, how can we construct a sequence of learning activities for students in which a meaningful learning process takes place within the context of developing these chemistry concepts?These questions were the milestones in developing a new curricular unit that focuses on analytical chemistry in an environmental context for high-school students who major in chemistry. Analytical chemistry enables students to learn to ask questions regarding their experiments, such as why a particular method should be used and not another, what results are expected, and what systematic errors are inherent to a particular method (Lunetta et al., 2007).
The development process
The program developed consists of two modules, one focusing on water analysis and the other on the greenhouse effect. The teaching process includes understanding and solving real-world problems, such as global warming or ensuring a clean water supply. Although students were already familiar with the introductory content of both modules from previous chemistry lessons, the modules utilize a single real-world topic as a vehicle for teaching a coherent new set of analytical chemistry concepts (Gutwill-Wise, 2001). The instruction in class uses teaching methods that foster active learning; this requires writing, discussing, and questioning as well as engaging the students in higher-order cognitive activities such as synthesis and evaluation.The approach used in the laboratory was to challenge students to think independently about a real-life problem. Since clean drinking water, for example, is inherently important to everyone (especially in Israel), it was expected that a water experiment sequence would maintain the students' interest. Choosing water as a context to explore also enabled the teachers to incorporate well-understood testing methods.
The first module also focuses on the factors that determine water quality. Water quality is very much influenced by global processes in our industrial world. In this module the students learn how we use different methods to purify water and water is essential to life on earth.
The second module that was developed was “We are the world—The Carbon Cycle” and it mainly focuses on the carbon cycle and the greenhouse effect. This theme was chosen since it focuses on the dynamics and reciprocity between human and nature. Thus, in this module, the students were exposed to a dynamic reciprocity between human and nature in their near and global environment. They were exposed to the influence of human's behavior on global processes and how this influences the delicate balance that exists on earth. The development process involved designing both physical and pedagogical aspects of the learning environment, taking into account the results of chemistry teachers' needs, assessments, and previously reported research. The sequence of the activities and concepts of both modules is described in Fig. 1.
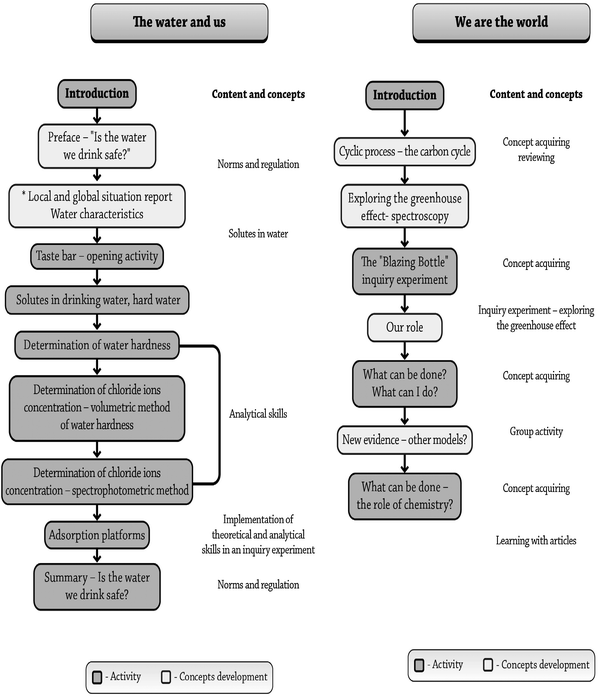 | ||
| Fig. 1 The sequence of the activities and concepts of the two modules: “Water and us” and “We are the world—the carbon cycle”. | ||
“Water and us” module
The module contains chemistry concepts such as concentration, standardized experiments to determine water quality, accuracy, reliability, and the value of the laboratory experiments (Fig. 1), all regarding legal parameters and norms integrated within the unit in such a sequence so that students experience a “need to proceed” to the next activity (Table 1). At the end of the learning process, the students become involved in a “need-to-take-an-action” part, in addition to the academic learning. This process is in line with the approach that students should learn about the role of chemistry in society, and the role of our students as responsible citizens (Zoller and Pushkin, 2007). The students are exposed almost daily via the media to the acute water shortage problem that Israel faces and the critical situation of water reservoirs in Israel and in neighboring countries. We assumed that this kind of exposure should make this subject relevant and interesting to the students.| Context | • The quality of drinking water |
| • The water problem in Israel | |
| Relevance | The students act as those who analyze and judge quality criteria, as the concern about the quality of drinking water continues to rise. |
| Chemistry concepts | • Characteristics of water |
| • Solutes in water and their concentration | |
| • Analytical chemistry | |
| • Purification methods | |
| • Norms and parameters | |
| • Accuracy and reliability | |
| Instructional methods | • The nature of measurement and how to measure |
| • How to select a method of measurement | |
| • Accuracy and reliability of measurement | |
| • Calculating errors (standard deviation, etc.) | |
| • Analytical methods: | |
| • Titration | |
| • Spectroscopy | |
| 1. Calibration curve | |
| 2. Beer–Lambert law | |
| • Absorption, emission, transparency |
The module consists of a driving question such as ‘Is the water clean enough to drink?'. This question is followed by chemical background information regarding the process water undergoes from its origin in natural reservoirs until it reaches our tap at home. The “Water and us” module starts with a question “What is involved in judging water quality?” And then a sequence of concepts, information, and activities guide the students toward the process of exploring the question.
“We are the world”—the carbon cycle module
The goal of the “We are the world—The Carbon Cycle” module was to enable the students to better understand global warming and other related environmental issues at the molecular level, and to relate these issues to their everyday lives at the macroscopic level. The students were required to participate in designing experiments through teamwork in order to answer the questions posed by the module (Table 2). Analysis and interpretation of extensive data sets enabled the students to gain experience in thinking critically about their data and in using statistics to enhance the quality of their data sets.| Context—The greenhouse effect | |
| Relevance | The students act as those who can judge the role of humankind regarding global warming. |
| Chemistry concepts | • The cyclic process |
| • The carbon cycles | |
| Spectroscopy | |
| Instructional methods | • An exemplar case of the carbon cycle and its relevance to life |
• An exemplar case of chemical equilibrium CO2(g) ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) CO2(aq) CO2(aq) |
|
| • Electromagnetic radiation: absorbance, emission, and transmission | |
| • Inquiry experiment “Blazing bottles” | |
| The relationship between absorption and temperature | |
| Context—Our role in conserving our planet | |
| Relevance | The students function as experts in order to critically evaluate the following subjects: |
| • Renewable energy | |
| • The use of pesticides—the story of methyl bromide | |
| Chemistry concepts | Scientific models |
| Instructional methods | • Discussing up-to-date news such as Kyoto protocols. |
| • Discussing two papers related to the role of chemists in finding solutions. | |
The greenhouse effect was chosen as the leading theme of the module. The greenhouse effect has become the focus of an international research effort in a scientific community that views the earth and its atmosphere as a unified system affected by the fuel policies of industrial and emerging nations (Andersson and Wallin (2000)). Global climate change couples chemistry with fields such as economics, politics, domestic policy, and foreign affairs. In order to educate students to become literate participants, the “We are the world—The Carbon Cycle” module provides a scientific foundation so that students may become active and knowledgeable participants. Topics include the role of solar radiation and the Earth's atmosphere in creating a habitable climate, past climate change data from scientific sources, and more recent evidence for warming trends. “We are the world—The Carbon Cycle” module addresses complex environmental issues. Students have an opportunity to perform experiments and to interpret their own laboratory data. They then explore debates over whether global climate change is primarily anthropogenic in origin.
The first stage of the module focuses on the following equilibrium CO2(g) ⇆ CO2(aq) ΔH < 0. In this phase the students are motivated to explore the carbon cycle on an exemplar system and to recognize its influence on our everyday lives. Students are familiar with equilibrium systems and factors that influence equilibrium systems from their earlier studies. Now they have an opportunity to reconstruct their knowledge of the equilibrium process and the factors that influence it in a real life scenario. In other words, students apply their previous knowledge and implement known principles in a new system. The students are able to transfer concepts from the molecular level (the micro level), which characterizes the chemical system, to their results at the macroscopic level, which influence their environment. In addition, from the media the students know that the levels of CO2(g) in the atmosphere are elevated, though the information they get is not always reliable and accurate. Later in the learning process, this gives us an opportunity to distinguish between science principles that are used by non-science factors (such as the press) and people with accurate and precise knowledge (such as the students themselves). Before studying this module, the students lacked the scientific knowledge needed in order to be able to judge the information they receive from the media; thus, the students could not evaluate their role in enhancing the greenhouse effect and global warming.
In the next stage, students learn how carbon dioxide can be removed from the atmosphere by natural processes together with those processes that increase the amount of carbon dioxide in the atmosphere. Along with the equilibrium process, students become familiar with the process that alters the sensitive steady-state concentration of carbon dioxide in the atmosphere. The aim is to explore new scenarios with well-known principles. In this exemplary case the students broaden their knowledge at the macro level and apply chemical principles at the molecular level. They become aware that small changes at the molecular level can induce huge effects at the macro level, namely, in our environment.
The focus of the next stage is to help students better understand the greenhouse effect and the role of human actions in global warming. In this phase the students extend their knowledge in order to understand how CO2(g) functions as a greenhouse gas. The phase starts with the question “Is the greenhouse effect good for us or bad for us?” The students intuitively understand that they need more knowledge at the molecular level. This knowledge concerns the relationship between electromagnetic radiation and matter. The next stage is to understand the relationship between electromagnetic radiation and temperature. Students feel the need to extend their knowledge in order to relate between observations and scientific knowledge. The lectures as well as the experiment are designed to help and support students' understanding of the model of the greenhouse effect. In this model, electromagnetic energy, at visible wavelengths coming from the sun, passes through the atmosphere and is absorbed by the surface of the earth. This energy is then reradiated as infrared radiation that does not penetrate the atmosphere as easily. As a result, some of the energy from the sun is trapped in the Earth's atmosphere and facilitates in elevating the temperature at the surface of the earth. Students develop a background for understanding the greenhouse effect by exploring several key topics, including electromagnetic radiation, conversion of visible radiation to infrared radiation, and selective absorption of infrared radiation. We concluded this part of the module with a culminating activity that we hope will enable students to synthesize what they have learned from the previous activities into a coherent understanding of the greenhouse effect. This activity, “the blazing bottles inquiry experiment”, involves directly observing the greenhouse effect. Through this activity the students explore the greenhouse effect. In the first part of the experiment the students test the thermal properties of carbon dioxide with those of air. In the second part of the experiment the students explore this phenomenon by inquiring about the different parameters that influence the extent of the temperature rise upon radiation.
The next stage is the outcome of the previous stages. The students can now critically relate to the observations and data concerning global warming. The students have the scientific knowledge that hopefully will encourage them to become more active in protecting their environment. At the last stage they are exposed to new evidences and the different, most recent models that try to predict what will happen in the coming decades. In addition, the students learn about the Kyoto protocols and other actions that are taken by governments and other organizations in order to reduce CO2(g) emissions. They are encouraged, through articles and related activities, to explore the role of chemistry and chemists in improving our surroundings. They are also requested to suggest how each of us can make a difference by an activity in which the students are required to evaluate what activities they are involved in and what measures they can take in order to reduce the CO2(g) emissions. Now, the students can appreciate their knowledge and can intelligently discuss the actions and immediate measures that need to be taken in order to make a difference. The different stages of the “We are the world—The Carbon Cycle” module are summarized in Fig. 1.
Supporting teachers in implementing context-based environmental modules
Taking into account all the issues previously mentioned, `each module was designed for 45 lessons. In order to engage teachers in the practice of “I Have Chemistry with the Environment”, they were invited to participate in a four-day induction workshop. In the workshop the teachers became familiar with the rationale of the module, i.e., its structure, contents, pedagogy, and ideas regarding how the activities should be integrated into the learning process. This workshop was mainly intended to familiarize the teachers with the various components of the module, when used in the chemistry classroom. In the course of the workshop the teachers underwent various professional development initiatives to enhance their content knowledge (CK) and pedagogical content knowledge (PCK) regarding teaching the topic of chemistry in an environmental context. The teachers were asked to perform four activities that were integrated into the module in order to practice the teaching process suggested by the developers.Throughout the year, close contact was maintained with the teachers. They were provided with continuous support from the developers to ensure that they would successfully implement the website activities, and they were helped according to their needs. In addition, there were four meetings during the school year in which all the teachers met and discussed their problems, shared their experiences, and helped each other. These meetings provided the teachers with an opportunity for reflection and deliberation regarding their work in school. The different components of the support the teachers received during the school year are presented in Fig. 2. Teachers were not involved in the development process. The teachers acted as reference group whom the developers could consult with.
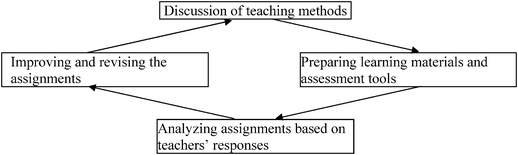 | ||
| Fig. 2 A scheme showing the different components of the teachers' workshop (based on Mamlok-Naamn, Hofstein and Penick, 2007). | ||
Objectives and research questions
The main objectives of this study were: (1) to provide meaningful learning based on an environmental context for learning new chemical concepts and practices, (2) to create a coherent flow of activities in which students learn the chemical concepts by participating in their own activities, and (3) to explore the change in students' attitudes and perception toward chemistry after experiencing learning chemistry through an environmental context.Thus, the research questions used in this study were as follows:
1. Does learning chemistry using a context-based curriculum, namely, an environmental context, influence their attitudes toward chemistry in general and toward learning chemistry in high school in particular?
2. Does learning chemistry in an environmental context influence students' perceptions regarding the relevance of high-school chemistry to their personal life?
3. Is there evidence that learning chemistry when using an environmental context enhances students' awareness of environmental issues?
Research population
The research population consisted of 12th grade students (N ∼ 400) and 18 teachers in 18 classes and 16 schools that chose to major in high-school chemistry. The schools from which the sample was taken could be characterized as urban high schools, which are academic in nature and consist of students from average socioeconomic backgrounds.Research tools
The research study was conducted twice in two successive years, using a combination of both quantitative and qualitative measures (Tobin and Fraser, 1998). By using two different types of tools, we expected to obtain a more comprehensive picture of the change that the students underwent while studying the environmental unit: “I Have Chemistry with the Environment”. The quantitative tool evaluates questions that were asked by the students before and after studying the unit “I Have Chemistry with the Environment”. The qualitative tools were mainly based on semi-structured interviews (Shkedi, 2005) that were conducted with individuals and small groups of students.Questions posed by students
Questions posed by students enabled us to learn about their views on different issues that are of interest in this study (Baram-Tsabari and Yarden, 2005, 2007, 2008; Baram-Tsabari et al., 2006; Hofstein et al., 2005). According to different studies, relying on students' questions is more effective than using their responses to an adult-written questionnaire (Jenkins, 2006). Studying students' questions can provide program developers with an awareness of what students are interested in and what they want to know about a given topic (Chin and Chia, 2004).The students were asked to pose four questions that interest them in relation to chemistry in an environmental context, before studying the unit and at the end of the learning process of the unit. The students' changes in attitudes and perceptions, as a result of studying the unit “I Have Chemistry with the Environment”, were evaluated by analyzing their questions regarding the environment.
The questions were classified into six main categories and subcategories (Blonder, 2008). The main categories were: (1) The subject of the question, (2) the type of required information, (3) the level of the question, (4) the relevance of the question to everyday lives, (5) the connection between chemistry and the environment, and (6) questions about taking action. Two researchers independently classified and categorized 1082 of the questions (pre and post) used in this study. The agreement of classification between the two researchers ranged from 80% to 95% for the different coding schemes. Table 3 presents examples of students' questions collected before and after studying the unit.
| Category | Subcategory | Example |
|---|---|---|
| Subject | Chemistry and the environment | What chemical substances cause environmental pollution? |
| The greenhouse effect | How can I reduce the amount of CO2 that is emitted into the atmosphere? | |
| Water and air quality | Which water is preferable—mineral or tap water? | |
| General environmental questions | What is the connection between the “ozone hole” and cancer? | |
| The information required | Suggestions for solution | In what ways can we minimize water problems? |
| How can we recycle more? | ||
| Explanatory | How air pollution influence the weather? | |
| Factual | Is the water in Israel polluted? | |
| Level of the question | Procedures, methods, and proofs | How can I influence the amount of CO2 that is emitted into the atmosphere? |
| Explanation: what, how | How can the ozone hole influence life on earth? | |
| Facts: what, if | Is water in Israel polluted? | |
| Connection to our everyday lives | Not related | What causes volcanic eruption? |
| What can I do | How can I produce alternative/home energy? | |
| General connection | How can we contribute to our environment? | |
| Connection between chemistry and the environment | No connection | Is there a fear of depletion of natural resources? |
| There is connection | Can we, as chemistry students, perform environmental projects that concentrate on contamination of our environment? | |
| Active/passive | What can I do? | What can we do in order to dramatically decrease pollution? |
| What can be done? | What are the best ways to purify water? | |
| Not relevant | How does air pollution affect the temperature? |
Individual interviews with students
The aim of the interviews with the students was to explore their attitudes toward learning the “I Have Chemistry with the Environment” unit. We focused on the context-based character of the unit and its influence on different aspects: affective, cognitive, the relevance of the subject to students' everyday lives, and the sequence of concepts and activities. The categories of the questions in the students' interviews are presented in Table 4. Altogether, seventeen students were interviewed. Since all the students are high achievers, the students were chosen according to their involvement during the lessons, their teachers' recommendation, and the students' willingness to be involved. Some students were very actively involved during the lessons and some of them were less involved. Table 4 presents the different dimensions of the students' interview questions.| Type of questions asked | Student interview questions (examples) |
|---|---|
| Cognitive | 1. What do you think about learning chemistry in an environmental context in high-school chemistry curricula? |
| 2. Can you compare this unit with other units you have already studied in high school? | |
| 3. Refer to the oral lessons; what would you consider as a strong point, which encourages you to learn this unit, and what are the weak points? | |
| 4. Refer to the laboratory activities; were they similar/different/the same, as other laboratory activities you experienced? | |
| Affective | 1. After watching the movie “The inconvenient truth”, did you want to share it with others? What did you want to share? |
| 2. Do you think that learning the “I Have Chemistry with the Environment” unit will encourage students to learn chemistry in high school? | |
| Relevance | 1. When you see an article concerning the environment, does it interest you? Since you were first exposed to the unit, was there any change in your interest? |
| 2. How would you present this unit in front of 10th grade students who are about to select courses for next year? |
Each student was interviewed for about 30 min. The interview questions were structured to obtain a better understanding of the different dimensions of this study: affective, cognitive, and relevance. The questions were content-validated by 6 scientists from the science education group. Sample of the questions that were asked in the interviews are presented in Table 4. The interview protocols were analyzed in four stages according to Shkedi (2005) qualitative-constructivist method:
Stage 1: Preliminary analysis-categorization
• Dividing the data into small meaningful segments. Each segment is characterized by its heading. The heading reflects the content of the segment.
• Classifying the segments according to their subject, combining the segments according to their subject, and determining categories.
Stage 2: Mapping analysis—identifying connections between categories, combining categories into main categories in such a way that each category should contain several subcategories.
Stage 3: Focus analysis—focusing on the categories which were relevant to the research questions in this study.
Stage 4: Theoretical analysis: identifying the relationship between the categories and subcategories and theoretical concepts and explanations (in this case: context-based learning).
The focus of this research was mainly on the first three stages. Two researchers independently analyzed the 17 interviews according to the four stages. The concordance of classification between researchers ranged from 85% to 95% for the different coding schemes.
Results and data analysis
In the following chapter, we will discuss the data analysis by focusing on several aspects, which follow the research questions:1. The first research question was related to students' ideas regarding a context-based curriculum, namely, an environmental context. Our interest was to explore students' attitudes toward chemistry in general and toward learning chemistry in high school, in particular. Fig. 3 presents the analysis of the questions posed by students before studying the environmental unit and afterwards.
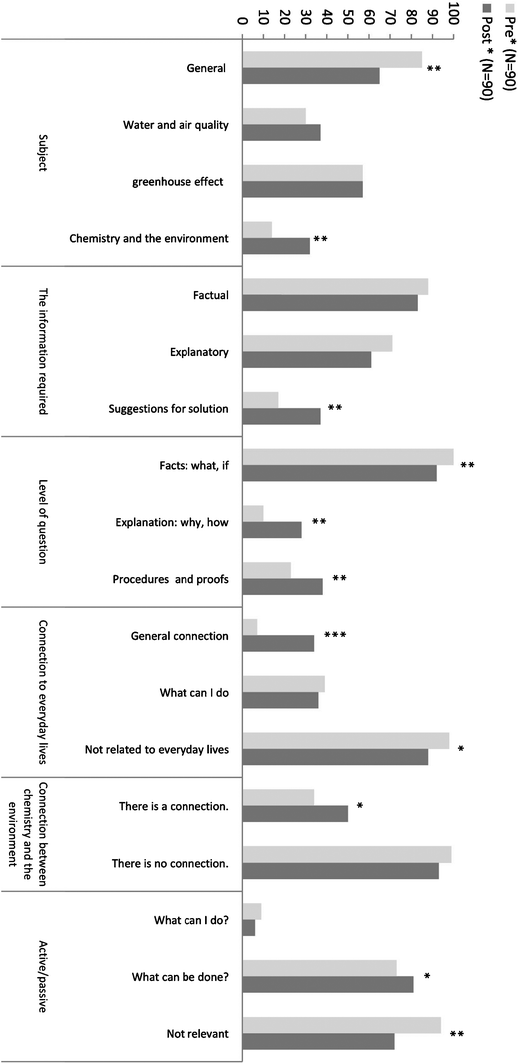 | ||
| Fig. 3 Analysis of students' questions (*0.01 < p < 0.05; **0.001 < p < 0.01; ***p < 0.001). | ||
The results present the percentage of students out of all the students in the sample (N = 90) who had among their questions (either in pre or in post) at least one question that matches a specific subcategory in the main category. The “Subject” category relates to the first research question. Other sub-categories which were found significant within this category were “General environmental questions” and “Chemistry and the environment”. A difference was found regarding the type of questions which the students asked. Before learning the module, most students asked general questions. After learning the module, the questions became more specific and less general. For example, before learning the module, there were questions such as “what is the connection between the “ozone hole and cancer” and “how did life begin”. After learning the module, the students asked more specific questions such as “how can I reduce the amount of CO2 that is emitted into the atmosphere” and “how can I reduce the pollution of groundwater”. Most students asked questions related to environmental issues with chemical concepts, for example, “how can we reduce the concentration of CH4 in the atmosphere” and “how can chemistry help keep our environment clean?” This change may imply that the students find this subject connected to chemistry.
The results from the analysis of the questions posed by students were supported by the results of the interviews which were conducted with them. The main categories, sub-categories, and examples from the interviews with students, are summarized in Table 4. Summarizing the results from the interviews strengthens the answers to the first research question. All students that were interviewed mentioned the importance of learning this unit. One of the students voiced opinions like: “Yesterday I sat with my father and explained to him what the “greenhouse effect” is. At the beginning, he said it was something bad and then I read about it in a book and told him that it is not something bad but when it goes beyond the limit, then it is bad.” And “Chemistry in an environmental context is more important for everyday lives than are acids and bases. I don't always see its connection to everyday lives. I know it exists there but I cannot always make the connection.” The students also expressed their appreciation of the variety of teaching techniques that influenced their opinion toward chemistry in high school: “I would recommend it because it suggests something different” and “It means varying the learning. It means that we will not learn all the time over and over again and then solve problems”. Students reported opinions that show a significant shift toward supporting ideas like “everyone should study environmental issues” and “chemistry and the environment are related”. In addition, after learning “I Have Chemistry with the Environment”, there was a significant increase in the number of students who reported that they talk about chemistry outside of class with families and friends. Furthermore, students think that environmental issues should be common knowledge in school. Moreover, most students reported that they appreciated the unit more than their regular chemistry course. Students wrote that they especially appreciated the feeling that they could discover things themselves: “with all the concentration measurements in the lab, now I know it should concern me. It makes it more interesting.” Also, some students reported that they better understood why they had to perform laboratory activities. They referred to a sense of purpose: “We were doing real experiments, with a purpose.”
The change in students' attitudes toward chemistry in general and toward learning chemistry in high school in particular, after studying chemistry in context, namely, “I Have Chemistry with the Environment” is encouraging. The overall interpretation, based on this study, is that students who are exposed to an environmental context in chemistry find it more related to their everyday lives and thus find it more attractive.
2. The categories which emerged from the analysis of the questions posed by students, which relate to the second research question (“Does learning chemistry in an environmental context influence students' perceptions regarding the relevance of high-school chemistry to their personal life?”) are “The connection to everyday lives”, “The connection between chemistry and the environment” and the “Active/passive” perception. In the category “The connection to everyday lives” two subcategories were found to be significant: “A general connection” and “Not related to everyday lives”. After learning the unit, the percentage of students who understood that environmental issues are connected to their everyday lives increased. Also, after learning the unit, the percentage of students who asked questions that was not related to everyday lives declined. After studying the subject the students asked questions such as “how can we produce alternative/home energy?” or “how can I influence my parents so they will be more aware of environmental issues?” These results imply that there was a change in students' perceptions of the connection between chemistry and everyday lives. Most students' questions linked chemistry and everyday lives. This finding is encouraging since one of the goals of this research is to find a didactical way to bring chemistry closer to the students' daily life.
The analysis of the questions posed by students, was in the category “The connection between chemistry and the environment”, revealed, that the sub-category “There is a connection” was significant. The percentage of students who found a connection between chemistry and the environment increased. This result implies that after learning the module the students connect more between chemical concepts and the environment. They asked questions such as “can we, as chemistry students, do environmental projects that focus on contamination of our environment”, or “How can chemistry help to improve and protect the environment?” This result is aligned with the result in the category “The connection between chemistry and everyday lives”. In both categories the students found that chemistry was connected to subjects in their everyday lives.
Similar results were obtained from the analysis of the questions posed by students in the category “Active/passive”. These results revealed that the sub-category “Not relevant” was significant as well, namely, the percentage of students who asked questions that do not mention any act decreased. The students asked more questions such as “what can we do in order to dramatically decrease the pollution?” Or “how can we minimize the effect of global warming on our lives?” This means that there is evidence that the students are increasingly more interested and willing to take an action in favor of their environment.
The analysis of the students' answers to the interviews suggested that their increased motivation and awareness of environmental phenomena have grown. They indicated the relevance and importance of this subject to their everyday lives. Some of them emphasized the change in their habits, such as reducing the use of plastic bags. It is worth mention that there were also some comments which the students expressed their lack of motivation to change their habits. One student said: “I like to use the car and I like big cars. I don't think I will change my habits since it makes me happy”. These comments were considerably fewer comparable to the comments which favored the environmental issues. Most students reported that they found the unit more interesting than their regular chemistry course. Students expressed their appreciation of the self-discovery process: “with all the concentration measurements in the lab, now I know it should concern me. It makes it more interesting.” The students expressed a preference for a chemistry approach that makes connections to the real world: “This is a subject that is highly connected to our everyday lives.” This provides some evidence to Linn's (2000) reasoning that “if students connect ideas from science class to personally-relevant contexts then they will be poised to revisit these ideas outside of class” (p. 783). In contrast, “when students cannot connect school and home understanding, they follow a path of isolating, fragmenting, and ignoring the science instruction they encounter in school” (Linn 2000, p. 783). The students commented that chemistry in an environmental context helped them make real-life connections to chemistry content and that this increased the relevance of chemistry to their lives: “Chemistry in an environmental context is more important for everyday lives than are acids and bases. I don't always see its connection to everyday lives. I know it exists there but I cannot always make the connection.” The students indicated that the unit was “meaningful to them, that they were doing activities with a sense of purpose, and that this “why and how” was missing in their regular chemistry lessons.”
3. The analysis of the data regarding the third research question (“Is there evidence that learning chemistry when using an environmental context enhances students' awareness of environmental issues?”), revealed interesting results. The categories which emerged from the questions posed by students, showed, that in the category “The information required” the sub-category that was found significant was “Suggestions for solutions”. At the post level the percentage of students who asked questions related to searching for a solution increased. Most students ask questions such as “in what ways can we minimize water problems?” and “how can we recycle more?” This means that there were an increased number of questions reflecting an interest in looking for solutions. After learning the topic, more students looked for ways to improve their environment.
In the category “Level of the question” all the subcategories were found to be significant. The results show that after learning the unit, fewer students asked questions about facts. Most of them, compared with those in the pre phase, looked for explanations. Moreover, the students looked for procedures and ways to improve and searched less for facts. For example, they asked more questions such as “how can we significantly reduce pollution? Or “how can we synthesize bio diesel? Instead of “is the water in Israel polluted?” These results, together with the results in the category “the information required”, imply that learning the unit influences the level of questions that were asked by the students. After the learning process, the students' requests to acquire additional knowledge increased.
The results from the interviews indicate that the students' awareness of environmental issues grew significantly. All students that were interviewed noted the change in their attitude toward environmental issues. Some of them expressed it strongly and made comments like “It is also important to be involved and to understand what is going on” and “I think that chemistry in an environmental context prepares us better for life”. They also remarked on their future role as citizens and said “Now, we are growing up and we want to be the ones who will be responsible for our future” and “we can also be more involved and will understand things better”. These findings strengthened the conclusion that learning the unit influences the students' awareness of environmental issues.
Summary and conclusions
Motivating the students, while providing a good understanding of chemical concepts, was ranked among the most important concerns in chemistry education (Zoller and Pushkin, 2007; Marks and Eilks, 2010; Parchmann et al., 2006). In order to study whether inclusion of an environmental context influences high-school students' attitudes, perception and thus motivates them toward learning chemistry, a curricular unit was developed, namely, “I Have Chemistry with the Environment” unit”. The unit concentrates on two context-based topics or modules. In each of these contexts students were given opportunities to make connections between chemistry concepts and real-life applications such as the water quality of their surroundings (in the “Water and us” module), and the relationship between everyday actions and the greenhouse effect. Since chemistry concepts from the traditional program were taught in previous chemistry lessons, it could be argued that learning the unit was an application of the previously learned concepts, for example, the topic of oxidation-reduction and chemical equilibrium. However, there were concepts that emerged through the context that were different from the students' previous experience. This included, in particular, topics such as spectroscopy with regard to the greenhouse effect. In addition, solubility and precipitation reactions required new conceptual links with water treatment and the greenhouse effect. In the “Water and us” module, students learned how the concentration of calcium ions affects the hardness of water. The concept of ‘hard water’ was introduced, enabling students to explore how the ‘hardness’ of water could be removed. These real-life connections were not merely applications of previously learned concepts; rather, they were concepts that emerged through real-life contexts that were different from students' previous experience. For example, “With all the concentration measurements in the lab, now I know it should concern me. It makes it more interesting.” Learning concepts such as solubility, through this context, now enabled the students to make real-life connections to water treatment.Many studies have shown that if students are able to see how learning activities relate to their “real life” and have a greater sense of ownership of the investigation, they are likely to be more motivated and successful in the area of science that they are investigating (Hofstein and Kesner, 2006; Hofstein and Lunetta, 2004). Making real-life connections between chemistry concepts and the contexts in which they were realized has been shown to have positive affective outcomes for many students who have studied science through a context-based approach (Ramsden, 1997).
Burmeister et al. (2012) suggest thinking in four models of implementation of sustainable development into formal chemistry education. The second and the third models are relevant to this study. The second model is defined as “Adding sustainability strategies as content in chemistry education”. In this model basic chemical principals are taught in the context of environmental and sustainable (ESD) examples. The third model suggested is “Using controversial sustainability issues for socio-scientific issues which drive chemistry education”. This model include chemical basis of knowledge and reflection on environmental debate. The development of the new unit discussed here lies mostly in the second model and has some characteristics from the third model. The strength of this approach is that it is skill-oriented in focusing at environmental issues. This approach is in line with the claims of Holbrook (2005), who asks for more education through science instead of science through education.
The research findings revealed from this study imply that teaching chemistry, using environmental questions in a context-based approach, is perceived by most of the students in this research as relevant to their lives. Learning in environmental context may suggest an effective method of motivating the students to learn chemistry. The inclusion of an environmental context in the chemistry experiments had a significant impact on students' awareness of how chemistry is connected to the real world.
The inclusion of an environmental context in the chemistry curriculum seemed to have had an impact on students' awareness of how chemistry is connected to the real world, with students remarking about their perceptions of chemistry as useful and applicable to their daily lives. Students also became more aware of the relationships between chemistry and the society in which they live.
A summary of the results from students' questioning behavior and the interviews shows that the students connected more between concepts in chemistry and the environmental phenomena and that in their questions they related more specifically to their everyday lives. These findings may imply that the students find chemistry relevant to their personal lives as well as to the society in which they live. Moreover, the students increased their awareness of environmental issues. This finding implies that using more environmental examples in teaching chemistry, in particular, may increase students' awareness of environmental issues, especially issues that are connected to their everyday lives. However, increasing student awareness of environmental issues is not an easy task since students are at the age at which they mostly concentrate on their personal and social issues. Educating them and increasing their awareness in high school can influence their attitudes toward environmental issues in their future.
It is important to note the aspects of the laboratory experience that were altered in the environmental laboratory compared with the regular laboratory course. First, students learned chemical concepts by experiencing real-world environmental problems using state-of-the-art instrumentation. In addition, a variety of approaches to teaching (group activities, collaborative learning, role taking, and independent projects) were incorporated into the curriculum of “I Have Chemistry with the Environment. Students' comments indicated that they had gained a wider perspective about the nature of science processes and that they were more critical of data obtained using scientific methods especially in the laboratory activities (Hofstein et al., 2011).
Educational implications
The positive outcomes of implementing a context-based approach in this study may suggest that context-based approaches help students make connections between chemistry concepts and real-world phenomena (Marks and Eilks, 2010); moreover, the links between the concepts are clearer in a context-based program. In fact, a context-based program appears more likely to engage students in meaningful tasks with real-world connections.Linking the environmental issue immediately to the chemical principle suggests an effective way to engage the students. It may imply that when the students' “need to know” includes chemistry, students maintain their focus and concentration throughout lengthy, complex, and quantitative analyses because they perceive the goals of learning as corresponding to their needs.
Societal awareness of global warming, hazardous waste dumps, groundwater contamination, and overdevelopment has increased in the last decades. Chemistry is involved in both studying and solving environmental problems that are immediately relevant, and it may provide the student with significant motivation to better understand the underlying chemical principles and the interdisciplinary aspects of the issues (Bulte et al., 2005). An added benefit of studying chemistry in an environmental context may be the fact that the instructors do not know the answer to questions such as “How hard is the water we are drinking?” This is a setting in which the students and instructors work together to solve a scientific problem, a situation in which mutual scientific curiosity and support may encourage mutual exploration.
In this study, a subtle treatment led to modest results. It is suggested that if more environmental examples were used in class and more references were made to those examples during chemistry lessons, this treatment may lead to significant learning of general chemistry because students may see chemistry as a way of better understanding and as a better way of solving environmental problems. In addition to more environmental contexts for chemistry, more information about how students could improve environmental problems may have a great impact on environmental attitudes. This conclusion is in line with the results of the NEETF (Coyle, 2005) survey (Robelia et al., 2010). The NEETF environmental literacy report recommended that people receive concrete suggestions on how to change their behavior, with an emphasis on how they can join others who are doing the same thing (Coyle, 2005). The report noted the positive correlation between environmental knowledge and most environmental behaviors. The more you know, the more likely you are to act to take positive steps to improve the environment (Coyle, 2005). The NEETF report also emphasized that environmental knowledge develops over a lifetime as people are exposed to information in different contexts. Adding information about the environment to the chemistry curriculum can be a way to significantly increase learning in content chemistry as well as serve as a source of environmental knowledge. Adding environmental contexts can also increase students' perceptions of how relevant chemistry is to real-life problems (Robelia et al., 2010).
Perhaps the changes in the curriculum in this study were not enough to induce large differences in the attitudes of our students toward learning chemistry. Larger changes in the curriculum need to be made in order to induce greater effects on students' attitudes and to create an environmental mindset. This is in agreement with Zoller's recommendations to embed extensive environmental case studies as a context for chemistry content in general chemistry courses in order to increase environmental awareness (Zoller and Pushkin, 2007).
Acknowledgements
We thank the Davidson Institute of Science Education at the Weizmann Institute of Science for their continuing support. We also thank Mrs Yetty Varon and Mrs Tirtsa Kauders from the Department of Science Teaching, the Weizmann Institute of Science, for their professional help.References
- Albe V., (2008), When scientific knowledge, daily life experience, epistemological and social considerations intersect: Students' argumentation in group discussions on a socio-scientific issue, Res. Sci. Educ., 38, 67–90.
- Aldridge J., Fraser B., Fisher D., Trinidad S. and Wood D., (2003, April), Monitoring the success of an outcomes-based, technology-rich learning environment, Paper presented at the meeting of the American Educational Research Association, Chicago, IL.
- American Chemical Society, (2006), Chemistry in the community: ChemCom (6th ed.), New York, NY: Freeman.
- Andersson B. and Wallin A., (2000), Students' understanding of the greenhouse effect, the societal consequences of reducing CO2 emissions and the problem of ozone layer depletion, J. Res. Sci. Teach., 37, 1096–1111.
- Baram-Tsabari A. and Yarden A., (2005), Characterizing children's spontaneous interests in science and technology, Int. J. Sci. Educ., 27, 803–826.
- Baram-Tsabari A. and Yarden A., (2007), Interest in biology: A developmental shift characterized using self-generated questions, Am. Biol. Teach., 69, 546–554.
- Baram-Tsabari A. and Yarden A., (2008), Girls' biology, boys' physics: Evidence from free-choice science learning settings, Res. Sci. Tech. Educ., 26, 75–92.
- Baram-Tsabari A., Sethi R. J., Bry L. and Yarden A., (2006), Using questions sent to an Ask-a-Scientist site to identify children's interests in science, Sci. Educ., 90, 1050–1072.
- Barker V. and Millar R., (1999), Students' reasoning about chemical reactions: What changes occur during a context-based post-16 chemistry course? Int. J. Sci. Educ., 21, 645–665.
- Barker V. and Millar R., (2000), Students' reasoning about basic chemical thermodynamics and chemical bonding: What changes occur during a context-based post-16 chemistry course? Int. J. Sci. Educ., 22, 1171–1200.
- Battles D. A., Franks M. E., Morrison-Shetlar A. I., Orvis J. N., Rich F. J. and Deal T. J., (2003), Environmental literacy for all students: Evaluation of environmental science courses developed for a new core curriculum, J. Coll. Sci. Teach., 32, 458–465.
- Bennett J., Graesel C. and Parchmann I., (2005), Context-based and conventional approaches to teaching chemistry: Comparing teachers' views, Int. J. Sci. . Educ., 27, 1521–1547.
- Benett J. and Lubben F., (2006), Context-based chemistry: The Salters approach, Int. J. Sci. Educ., 28, 999–1015.
- Blonder R., (2008), Scientific-chemical viewpoints regarding smoking: A science laboratory for all, J. Chem. Educ., 85, 248–250.
- Bulte A., Klaassen K., Westbroek H., Stolk M., Prins G., Genseberger R. and Pilot A., (2005), Modules for a new chemistry curriculum: Research on a meaningful relation between contexts and concepts, In P. Nentwig and D. Waddington (ed.), Making it relevant: Context based learning of science (pp. 273–299), Münster: Waxmann.
- Burmeister M., Rauch F. and Eilks I., (2012), Education for Sustainable Development (ESD) and chemistry education, Chem. Educ. Res. Pract., 13(2) 10.1039/c1rp90060a.
- Chin C. and Chia L. G., (2004), Problem-based learning: Using students' questions to drive knowledge construction, Sci. Educ., 88, 707–727.
- Coyle K., (2005), Environmental literacy in America: What ten years of NEETF/Roper research and related studies say about environmental literacy in the U.S., Washington, DC: The National Environmental Education and Training Foundation. Retrieved from http://www.neefusa.org/pdf/ELR2005.pdf.
- De Vos W., Bulte A. and Pilot A., (2002), Chemistry curricula for general education: Analysis and elements of a design, In J. K. Gilbert, O. De Jong, R. Justi, D. F. Treagust and J. H. Van Driel, (ed.), Chemical education: Towards research-based practice (pp. 101–124), Dordrecht: Kluwer.
- Genseberger R. J., (1997), Physics and chemistry education aimed at students' interests: A study about curriculum development at the Open School (Unpublished doctoral dissertation), Utrecht University, Utrecht, The Netherlands.
- Gilbert J. K., (2006), On the nature of “context” in chemical education, Int. J. Sci. Educ., 28, 957–976.
- Gutwill-Wise J. P., (2001), The impact of active and context-based learning in introductory chemistry courses: An early evaluation of the modular approach, J. Chem. Educ., 78, 684–90.
- Hofstein A. and Kesner M., (2006), Industrial chemistry and school chemistry: Making chemistry studies more relevant, Int. J. Sci. Educ., 28, 1017–1039.
- Hofstein A. and Lazarowitz R., (1986), A comparison of the actual and preferred classroom learning environment in biology and chemistry as perceived by high school students, J. Res. Sci. Teach., 23, 189–199.
- Hofstein A. and Lunetta V. N., (2004), The laboratory in science education: Foundation for the 21st century, Sci. Educ., 88, 28–54.
- Hofstein A., Eilks I. and Bybee R., (2011), Societal issues and their importance for contemporary science education: A pedagogical justification and the state-of-the-art in Israel, Germany and the USA, Int. J. Sci. Math. Educ., 9, 1459–1483.
- Hofstein A., Navon O., Kipnis M. and Mamlok-Naaman R., (2005), Developing students' ability to ask more and better questions resulting from inquiry-type chemistry laboratories, J. Res. Sci. Teach., 42, 791–806.
- Holbrook J., (1998), Operationalising scientific and technological literacy: A new approach to science teaching, Sci. Educ. Int., 9, 13–18.
- Holbrook J., (2005), Making chemistry teaching relevant, Chemical Education International, 7, Retrieved from the World Wide Web, July 01, 2011, at http://old.iupac.org/publications/cei/vol6/index.html.
- Holbrook J. and Rannikmäe M., (2007), The nature of science education for enhancing scientific literacy, Int. J. Sci. Educ., 29, 1347–1362.
- Holbrook J. and Rannikmäe M., (2009), The meaning of scientific literacy, Int. J. Environ. Sci. Educ., 4, 275–288.
- Jenkins E. W., (2006), The student voice and school science education, Stud. Sci. Educ., 42, 49–88.
- Kegley S., Stacy A. M. and Carroll M. C., (1996), Environmental chemistry in the general chemistry laboratory, part I: A context-based approach to teaching chemistry, Chem. Educator, 1. Retrieved from http://chemeducator.org/bibs/0001004/00010052.htm.
- Kegley S., Stacy A. M. and Carroll M. C., (1996), Environmental chemistry in the general chemistry laboratory, part II: Evaluation of an alternative curriculum, Chem. Educator, 1. Retrieved from http://chemeducator.org/bibs/0001004/00010053.htm.
- Kesner M., Hofstein A. and Ben-Zvi R., (1997), Student and teacher perceptions of industrial chemistry case studies, Int. J. Sci. Educ., 19, 725–738.
- Lee M. K. and Erdogan I., (2007), The effect of science–technology–society teaching on students' attitudes toward science and certain aspects of creativity, Int. J. Sci. Educ., 29, 1315–1328.
- Linn M. C., (2000), Designing the knowledge integration environment, Int. J. Sci. Educ., 2, 781–796.
- Lunetta V. N., Hofstein A. and Clough M. P., (2007), Learning and teaching in the school science laboratory: An analysis of research, theory, and practice, In S. K. Abell and N. G. Lederman (ed.), Handbook of research in science education (pp. 393–342), Mahwah, NJ: Erlbaum.
- Mamlok-Naamn R., Hofstein A. and Penick J., (2007), Involving Science Teachers in the Development and Implementation of Assessment Tools for "Science for All" Type Curricula, J. Sci. Teach. Educ., 18, 497–524.
- Marks R. and Eilks I., (2009), Promoting scientific literacy using a sociocritical and problem-oriented approach to chemistry teaching: Concept, examples, and experiences, Int. J. Environ. Sci. Educ., 4, 231–245.
- Marks R. and Eilks I., (2010), Research-based development of a lesson plan on shower gels and musk fragrances following a socio-critical and problem-oriented approach to chemistry teaching, Chem. Educ. Res. Pract., 11, 129–141.
- Millar R., (2006), Twenty-first century science: Insights from the design and implementation of a scientific literacy approach in school science, Int. J. Sci. Educ., 28, 1499–1521.
- Moore J. W., (2006), Keeping our cool, J. Chem. Educ., 83, 1255.
- National Board for Professional Teaching Standards., (2009), Voices of influence: Advancing high-quality teaching through national board certification, Arlington, VA. Retrieved from http://www.nbpts.org/userfiles/File/voices_FINAL.pdf.
- Norton W. W., (2009), About the Chem Connections project, Retrieved from: http://www.wwnorton.com/college/chemistry/chemconnections/modules.html.
- Orion N. and Hofstein A., (1994), Factors that influence learning during a scientific field trip in a natural environment, J. Res. Sci. Teach., 31, 1097–1119.
- Osborne J. and Dillon J., (2008), Science education in Europe: Critical reflections, London: Nuffield Foundation.
- Osborne J., Driver R. and Simon S., (1998), Attitudes to science: Issues and concerns, Sch. Sci. Rev., 79, 27–33.
- Parchmann I., Gräsel C., Baer A., Nentwig P., Demuth R. and Ralle B., (2006), “Chemie im Kontext”: A symbiotic implementation of a context-based teaching and learning approach, Int. J. Sci. Educ., 28, 1041–1062.
- Ramsden J., (1997), How does a context-based approach influence understanding of key chemical ideas at 16? Int. J. Sci. Educ., 19, 697–710.
- Ratcliffe M., (1998), Discussing socioscientific issues in science lessons: Pupils' action and the teacher's role, Sch. Sci. Rev., 79, 55–59.
- Robelia B., McNeill K., Wammer K. and Lawrenz F., (2010), Investigating the impact of adding an environmental focus to a developmental chemistry course, J. Chem. Educ., 87, 216–221.
- Shkedi A., (2005), Multiple case narrative: A qualitative approach to studying of multiple populations. Amsterdam: Benjamins.
- Sutman F. and Bruce M., (1992), Chemistry in the community – ChemCom: A five-year evaluation, J. Chem. Educ., 69, 564–567.
- Swan J. A. and Spiro T. G., (1995), Context in chemistry: Integrating environmental chemistry with the chemistry curriculum, J. Chem. Educ., 72, 967–970.
- Tobin K. and Fraser B. J., (1998), Qualitative and quantitative landscapes of classroom learning environment, In B. J. Fraser and K. G. Tobin (ed.), International handbook of science education (pp. 623–640), Dordrecht: Kluwer.
- Tobin K., Capie W. and Bettencourt E., (1988), Active teaching for higher cognitive learning in science, Int. J. Sci. Educ., 10, 17–27.
- UNCED, (1992), Agenda 21, Retrieved from the World Wide Web, July 10, 2011 at http://www.un.org/esa/dsd/agenda21/.
- Winther A. A. and Volk T. L. J., (1994), Comparing achievement of inner-city high school students in traditional versus STS-based chemistry courses, J. Chem. Educ., 71, 501–505.
- Zoller U. and Pushkin D., (2007), Matching Higher-Order Cognitive Skills (HOCS) promotion goals with problem-based laboratory practice in a freshman organic chemistry course, Chem. Educ. Res. Pract., 8, 153–171.
Footnote |
| † This article is part of a themed issue on sustainable development and green chemistry in chemistry education. |
| This journal is © The Royal Society of Chemistry 2012 |
