DOI:
10.1039/C2AN36009H
(Paper)
Analyst, 2013,
138, 234-239
Dual-channel cathodic electrochemiluminescence of luminol induced by injection of hot electrons on a niobate semiconductor modified electrode
Received 25th July 2012, Accepted 2nd September 2012
First published on 3rd September 2012
Abstract
In this paper, a new niobate semiconductor photocatalyst Sr0.4H1.2Nb2O6·H2O (HSN) nanoparticle was applied to investigate the cathodic electrochemiluminescent (ECL) behavior of luminol for the first time. The results presented here demonstrated that there were two ECL peaks of luminol at the cathodic potential attributed to immobilization of HSN on the electrode surface. It is implied that HSN can be electrically excited and injected electrons into aqueous electrolytes from this electrode under a quite low potential that only excites luminol. A mechanism for this luminol-ECL system on HSN/GCE has been proposed. Additionally, this HSN/GCE has lots of advantages, such as high stability, good anti-interference ability, simple instrumentation, rapid procedure and ultrasensitive ECL response. It is envisioned that this HSN/GCE has further applications in biosensors.
1. Introduction
Luminol-based electrochemiluminescence (ECL) systems have been developed to construct the ECL sensors since this ECL system offered inexpensive reagent consumption and high emission yields.1 Until now, most studies were carried out at anodic potentials, where luminol is oxidized and reacted with active oxygen anions such as OOH− to emit ECL. Although luminol ECL has been investigated for a long time, it is believed that luminol still has other unknown excitation pathways. Therefore, it needs to be further studied.2 It is reported that cathodic excitation of luminol was induced by injection of hot electrons from the electrode surface into the aqueous solution and the subsequent generation of hydrated electrons as reducing mediators.3–7 Oxide covered aluminium,3,4 magnesium,5 silicon6 and glassy carbon (GC)7 electrodes have been used as cold cathodes during cathodic pulse polarization. However, an extreme alkaline medium should be afforded (pH > 10) in the above-mentioned methods, followed by destruction of the oxide film to yield an ECL signal, which limits its use.Recently, semiconductor nanoparticles (NPs) have attracted a growing interest in ECL studies because of their unique size dependent electrochemical and optical properties.8 Various studies8–11 have used semiconductor NPs as direct luminescent materials for possible luminescent applications. However, most of them have suffered from weak intensity and/or poor stability. On the other hand, some reports have focused on the role of semiconductor NPs in the enhancement of ECL behavior in conventional luminescent systems like luminol12 and Ru(bpy)32+.13 Contributing to the unique property of electron activation and transfer of the semiconductor photocatalyst, the potential applications of NPs materials in luminol cathodic ECL systems have been investigated, such as AuSb,14 C-doped titanium oxide amorphous semi-conductor15 and TiO2.12
Niobate semiconductor, an indirect-bandgap n-type semiconductor which has a unique structure and excellent performance for water splitting, has become a promising photocatalyst. Recently, a novel niobate nanophotocatalyst, Sr0.4H1.2Nb2O6·H2O (HSN) nanopolyhedon, has been developed which exhibits high photocatalytic activities for both pure water splitting and decomposition of benzene.16 However, to the best of our knowledge, the effect of HSN on the behavior of the traditional ECL system has not been investigated.
Herein, we firstly studied the ECL of luminol on the HSN-modified GC electrode (HSN/GCE) in detail. This photocatalyst can effectively generate hot electrons which occur in the region of the semiconductor device featuring high electric fields, enhancing two distinct ECL response peaks at low negative potentials. Some influencing factors were investigated in detail and results showed that on this kind of semiconductor, ECL response to luminol is still very sensitive even without coreaction reagents (such as peroxodisulfate ion, hydrogen peroxide). It was shown that HSN is an excellent electrode material for cathodic ECL of luminol. Hence, this method not only opens a new pathway of the application of HSN in the ECL field, but also provides a potential in other fields such as clinical diagnostics, immunological analysis and environmental monitoring.
2. Experimental section
2.1 Chemicals
3-Aminophthalhydrazide (luminol) was purchased from Aldrich Chem. Co. (USA). A stock solution of 1.0 × 10−4 mol L−1 luminol was prepared and stored at 4 °C. All other reagents were of analytical reagent grade and used without further purification. Argon and oxygen of 99.99% purity were used. All solutions were prepared with using Milli-Q reagent water (Milli-Q, Millipore, 18.2 MΩ resistivity.2.2 Apparatus
The ECL detection system consists of a BPCL Ultra Weak Luminescence Analyzer (Institute of Biophysics, Chinese Academy of Science, Beijing, China) and a CHI 660a electrochemical system (CH Instruments, USA). A three-electrode electrochemical cell with an optically flat bottom was used, including a glassy carbon electrode as the working electrode, a platinum wire as the counter electrode and an Ag/AgCl (sat. KCl) electrode as the reference electrode. The electrochemical cell was placed directly in front of a photomultiplier (PMT, operated at −780 V) and the PMT window was only opened to the working electrode to reduce the interference of ECL from the counter electrode.Scanning electron microscopy (SEM) images was performed with a Nova NanoSEM 230 microscopy (FEI Corp.). Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images were recorded using a JEOL model JEM 2010 EX microscope at an accelerating voltage of 200 kV.
The fluorescence measurements were performed at room temperature on a Varian Cary Eclipse Fluorescence Spectrophotometer. The emission spectra were collected from 370 to 470 nm with the excitation wavelength of 312 nm. Both the excitation and emission slit widths were set to 10.0 nm.
2.3 Preparation of Sr0.4H1.2Nb2O6·H2O nanopolyhedra
The Sr0.4H1.2Nb2O6·H2O (HSN) samples were prepared according to a previous report16 with a minor modification. Briefly, a mixture of these-obtained Nb2O5·nH2O and Sr(NO3)2 in a molar ratio of Nb5+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sr2+ = 2
Sr2+ = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was dispersed into 70 mL deionized water. Then, 1 mL NH3·H2O (25%) was added into the suspensions under vigorous stirring. The resultant mixture was transferred to a sealed Teflon-lined stainless steel autoclave and heated in an oven at 160 °C for 48 h. After cooling, the products were centrifuged, washed with deionized water and dried at 60 °C in an oven.
1 was dispersed into 70 mL deionized water. Then, 1 mL NH3·H2O (25%) was added into the suspensions under vigorous stirring. The resultant mixture was transferred to a sealed Teflon-lined stainless steel autoclave and heated in an oven at 160 °C for 48 h. After cooling, the products were centrifuged, washed with deionized water and dried at 60 °C in an oven.2.4 Fabrication of Sr0.4H1.2Nb2O6·H2O (HSN) nanopolyhedra modified electrode
The glassy carbon electrode (GCE, 3 mm in diameter) was polished to a mirror-like finish with 1.0, 0.3, and 0.05 μm alumina slurries, respectively. The electrode was successively sonicated in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 nitric acid, acetone and doubly distilled water, and then dried in nitrogen. HSN/GCE was prepared by spreading 5 μL of HSN suspension (5 mg mL−1 in ethanol solution) on the electrode surface, and dried under room temperature.
1 nitric acid, acetone and doubly distilled water, and then dried in nitrogen. HSN/GCE was prepared by spreading 5 μL of HSN suspension (5 mg mL−1 in ethanol solution) on the electrode surface, and dried under room temperature.2.5 ECL and ECL spectral measurements
The above treated GCE, as the working electrode, was immersed into an ECL cell containing 2 mL of PBS buffer solution (0.1 mol L−1, pH 9) and 2 μL, 1.0 × 10−4 mol L−1 luminol. The cell was laid in the dark chamber without stirring. In this work, linear sweep voltammetry (LSV) was chosen as the scan mode. The scan rate was 50 mV s−1. Under an argon or oxygen atmosphere, argon or oxygen were bubbled through the solutions for 15 min. All experiments were carried out at ambient room temperature. The height of the ECL signal was used for calibration.ECL spectra of both ECL peaks at different potentials were measured by inserting the filters (band-pass filter) at wavelengths of 350, 400, 425, 440, 460, 490, and 570 nm between the PMT and the ECL cell. The curves of ECL intensity versus λ are consistent with ECL spectra.
2.6 Terephthalic acid fluorescence measurements
The terephthalic acid fluorescent probing technique (TA-FL) has been developed for the detection of hydroxyl radicals.17 TA molecule can readily react with OH˙ radicals to produce 2-hydroxyterephthalic acid (TAOH) with an obvious fluorescence at around 426 nm. Hence, the hydroxyl radical can be indirectly monitored by fluorescence intensity changes of the TA solution. Before the fluorescence measurements, the above HSN/GCE was immersed into a cell containing 2 mL of PBS buffer solution (0.1 mol L−1, pH 9) and 8 mM terephthalic acid (TA), and scanned under constant potential mode at −0.8 V for 30 min by the CHI 660a electrochemical system. Then, 300 μL of the treated solution was immediately transferred to the cuvette and the fluorescence measurements were performed .3. Results and discussion
3.1 Morphologies and structures of HSN
The morphologies and structures of HSN were characterized using SEM and TEM. As shown in the SEM image (Fig. 1A), the sample consisted of nanopolyhedra ranging from 30 to 70 nm and was well-dispersed. It was also confirmed by the TEM image (Fig. 1B). Clear lattice fringes could also be observed in Fig. 1C. The interplanar spacing was consistent with the d-spacing of the corresponding lattice plane. The fringes of d ¼ 0.61 nm matched that of the (111) crystal graphic plane of cubic HSN. A typical selected area electron diffraction (SAED) pattern (Fig. 1D) revealed that the sample had single-crystalline character. |
| | Fig. 1 SEM (a), TEM (b), HRTEM images (c), and typical SAED pattern (d) of the HSN sample. | |
3.2 Electrochemistry and ECL behaviors of luminol on different electrodes
Electrochemistry and ECL of luminol on different electrodes were investigated. In Fig. 2A, under the linear sweep voltammograms (LSV) mode, a remarkable peak appeared at the potential around −0.5 V on bare GCE in air-saturated PBS buffer, which was defined as the reduction of oxygen in the solution. Interestingly, on the HSN/GCE two cathodic peaks (C1, C2 in curve b and c, respectively) were observed at about −0.35 and −0.72 V, respectively, regardless of the presence (curve c) or absence (curve b) of luminol. Moreover, these two curves seem very similar, indicating that luminol has no electrochemical response under this potential range. In IECL/E curves, two distinct ECL peaks (E1 and E2) corresponding to these two cathodic peaks were observed on the HSN/GCE in luminol solution (curve c in Fig. 2B). While on bare GCE (curve a in Fig. 2B), there was not even a clear ECL peak. Also, no ECL response appeared on the HSN/GCE in the absence of luminol (curve b in Fig. 2B). The results indicate that ECL responses come from luminol, and more importantly, reveal that the HSN semiconductor nanoparticles play a predominant role on the cathodic ECL of luminol.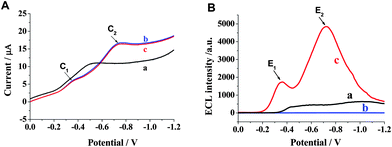 |
| | Fig. 2 LSV (A) and IECL/E (B) curves on (a) bare GCE in 0.1 mol L−1 PBS (pH 9.0) containing 1.0 × 10−7 mol L−1 luminol, (b) HSN/GCE in 0.1 mol L−1 PBS (pH 9.0) without luminol and (c) HSN/GCE in 0.1 mol L−1 PBS (pH 9.0) containing 1.0 × 10−7 mol L−1 luminol. Scan rate, 50 mV s−1. | |
3.3 Effect of various factors
Firstly, the effect of concentration of HSN was investigated (the spread volume was fixed at 5 μL). It was found that the ECL intensity of luminol increased with the addition of HSN. The strongest ECL responses at two cathodic potentials were obtained when the concentration of HSN was 5 mg mL−1 (see Fig. 3A). Thus, 5 mg mL−1 of the HSN solution was selected for subsequent studies. |
| | Fig. 3 (A) Effect of different concentrations of HSN-modified electrode to the ECL intensities; (B) effect of different pH values to the ECL intensities on the HSN/GCE in pH 9.0 PBS containing 1.0 × 10−7 mol L−1 luminol. | |
The effect of pH on the ECL intensity of luminol was studied, and the results are shown in Fig. 3B. It was found that in the pH range of 7.4–9.0 (PBS buffer), two ECL peaks were observed at the cathodic potential. At the potential of −0.72 V (E2), the ECL response was weak under neutral conditions, whereas it could be increased either in acidic or alkaline solution, especially, the intensity was enhanced significantly in alkaline solution. At the potential of −0.35 V (E1), the ECL intensity enhanced slightly with the increase in pH from 6.5 to 9.0. When the pH was higher than 9.3, E1 disappeared due to quick degradation of OOH− in alkaline solution. Finally we chose pH 9.0 for the subsequent experiments.
The effect of luminol concentration has been examined (data not shown). It was found that there was no ECL response on the HSN/GCE in the absence of luminol. While the intensities of two ECL peaks increased with the increase in luminol concentration, indicating that ECL responses come from luminol. Moreover, the intensities of both E1 and E2 were measured, and found to be linear with the concentration of luminol in the range of 5.0 × 10−9 to 2.0 × 10−7 mol L−1. The detection limit could reach 8.0 × 10−10 mol L−1 without any co-reaction reagents (E2 was used for the calculation).
3.4 ECL spectra of the two ECL peaks
The ECL spectra of the two peaks in the IECL/E curves were analyzed under air-saturated conditions. ECL spectrum measurements (see Fig. 4) showed that the maximum emission of both E1 and E2 were at ca. 430 nm, corresponding to the light emission of 3-aminophthalate.18 It can be concluded that both of the ECL peaks are initiated by luminol reactions.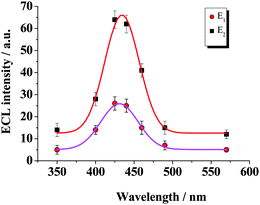 |
| | Fig. 4 ECL spectra of ECL peaks at different potentials in air-saturated PBS buffer (0.1 mol L−1, pH 9.0) containing 1.0 × 10−7 mol L−1 luminol. E1 at −0.35 V and E2 at −0.72 V. | |
3.5 Electrochemistry and ECL behavior of HSN/GCE under different atmospheres
Generally, the ECL of luminol involves the important intermediates; superoxide radical OOH−, O2˙− or hydroxyl radical OH˙.19,20 These free radicals have been confirmed to be tightly correlative with dissolved oxygen.21 Hence, the electrochemistry and ECL behavior of HSN/GCE under different atmospheres were studied in detail. As shown in Fig. 5, air-saturated luminol solutions exhibited a strong cathodic ECL on the HSN/GCE. Two cathodic peaks and the ECL intensities increased under an oxygen atmosphere, especially a remarkable enhancement of E1 (curve c). However, all the peaks decreased under an Ar atmosphere, even C1 and corresponding E1 almost disappeared (curve b). It is confirmed that E1 depends on the dissolved oxygen in the solution and E2 was also oxygen relevant.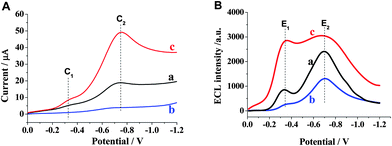 |
| | Fig. 5 LSV (A) and IECL/E (B) curves of luminol ECL on HSN/GCE in air-saturated (a), deoxygenated (b), and oxygen-saturated solutions (c). Solution: 0.1 mol L−1 PBS (pH 9.0) containing 5.0 × 10−8 mol L−1 luminol. | |
3.6 Possible mechanism
Next, we elucidated the possible mechanism behind above ECL behavior. It is said that O2 is electrochemically reduced to the intermediate O2˙−,22 which can further generate to other reactive oxygen species. The capability of HSN to effectively generate O2˙− and OH˙ radicals has been proven by electron spin resonance assay (ESR) in previous work.16 Furthermore, by the terephthalic acid fluorescent spectrum experiment (TA-FL) the existence of OH˙ on the electrically excited HSN/GCE (Fig. 6) has been confirmed. Therefore, the n-type (which is rich in free electrons) HSN should be responsible for the hot electron injection. It can be assumed that, the electrons of HSN on the valence band can be transferred by direct tunnel emission with sufficient energy by electrical excitation. Then the excited hot electrons enter the conduction band (CB) of water near the HSN/GCE and turn into hydrated electrons after thermalization and salvation.3 Hence, oxygen in the solution could be reduced by the hot electrons at the surface of the electrode. The possible mechanisms for E1 are as follows:3| | | ehot− (electrode) → equasifree− (in the CB of water) | (1) |
 |
| | Fig. 6 FL responses of TA solution under (a) untreated, (b) treated by bare GCE and (c) treated by HSN/GCE. Solution: pH 9.0 PBS containing 8 mM TA. | |
Additionally, here HSN may play the role to help form and stabilize the reductive oxygen species.
E2 at −0.72 V vs. Ag/AgCl involves the reaction between luminol and the action group (OH˙), where OH˙ is formed by further reaction of O2˙− with hot electrons after hermalization and salvation (eaq−). The possible mechanisms are described as follow:3,23,24
| | | O2˙− + 2eaq− + 2H2O → OH˙ + 3OH− | (5) |
The scheme of the whole process is described in Fig. 7. Briefly, once the dissolved oxygen in the solution is withdrawn, eqn (3)–(7) are prohibited, leading to the disappearance of E1 and the decrease of E2. On the contrary, in oxygen-rich solution the generation of O2˙− is facilitated, and more hot electrons combine with oxygen and indirectly help the formation of OH˙. Thus, it can well explain why E1 almost disappears and E2 significantly decreases in an Ar atmosphere as well as the reason for the increase of those peaks under an oxygen atmosphere. Further studies focusing on revealing the exact mechanism of these two ECL reactions on HSN/GCE are ongoing in our laboratory.
 |
| | Fig. 7 Scheme of possible mechanisms for ECL of luminol. | |
3.7 Stability and reproducibility of ECL on HSN modified electrode
The stability of this HSN-modified electrode was further examined, and the results showed that the relative standard deviation (RSD) of the ECL response (n = 12) was found to be 2.0% for this modified electrode in air-saturated luminol solutions, indicating that the HSN-modified electrode has excellent stability (see Fig. 8). The validity of a HSN nanopolyhedra modified electrode could reach at least 40 days if it is stored in dark. Excellent reproducibility of ECL can be achieved without any pretreatment. The stable ECL response might be attributed to following three factors: firstly, HSN nanoparticles could be electrically excited and showed high catalytic activities for generating reactive oxygen species; secondly, the electrogenerated reactive oxygen species is more stable at the HSN layer; thirdly, the modification of HSN can prevent the GCE surface from being fouled by electrochemical polymerization of luminol. |
| | Fig. 8 Stability of ECL on the HSN-modified GCE in pH 9.0 PBS containing 1.0 × 10−7 mol L−1 luminol. | |
3.8 Potential analytical application for detection of ascorbic acid
Ascorbic acid is known as an antioxidant in vivo, which can effectively suppress the O2˙− and hydroxyl radical OH˙−.25 The dissolved oxygen was consumed when ascorbic acid was added in the solution, leading to the decrease of ECL signals (see Fig. 9). It was found that E2 was more sensitively inhibited by ascorbic acid than E1, and the inhibited ECL intensity of E2 has a linear relationship with the denary logarithm of ascorbic acid concentration in the range of 3.0 × 10−8 to 1.2 × 10−6 mol L−1 (inset of Fig. 9) with a detection limit of 3.0 × 10−9 mol L−1 (S/N = 3), which is lower than that from a graphite/poly(methylmethacrylate) composite electrode26 and an electrically heated ionic-liquid/multiwall carbon nanotube composite electrode.27 Additionally, the effect of some compounds such as K+, Na+, SO42−, NO3−, amylum, lactose, glucose, citric acid, sucrose that may coexist in biosamples was studied. The results indicated that 1000 fold for K+, Na+, SO42− and NO3−; 500 fold for glucose, lactose, sucrose and amylum to 2.0 × 10−7 mol L−1 ascorbic acid did not interfere with the detection signal. |
| | Fig. 9 ECL responses to different concentrations of ascorbic acid (from a to g: 0, 0.03, 0.06, 0.15, 0.30, 0.60, 1.20 mol L−1). Scan rate: 50 mV s−1; luminol: 2.0 × 10−7 mol L−1. Inset: calibration curve for ascorbic acid. | |
4. Conclusion
In summary, the electrochemical and ECL properties of luminol on a novel semiconductor photocatalyst (HSN)-modified electrode were studied. The results presented here demonstrated that there were two ECL peaks of luminol at a cathodic potential which were attributed to immobilization of HSN on the electrode surface. This implies that HSN can be electrically excited and inject electrons into aqueous electrolytes from this electrode under a quite low potential. This HSN/GCE has many advantages, such as high stability, good anti-interference ability, simple instrumentation, rapid procedure and ultrasensitive ECL response, and it is envisioned that this HSN/GCE will have further applications in biosensors.Acknowledgements
This project was financially supported by the NSFC (41076059, 81202912), the Key Foundation of Education Department of Fujian Province (JK2009016), the Natural Sciences Foundation of Fujian Province (2011J01035), and the Program for Changjiang Scholars and Innovative Research Team in University (no. IRT1116).References
- M. M. Richter, Chem. Rev., 2004, 104, 3003–3036 CrossRef CAS.
- S. Kulmala and J. Suomi, Anal. Chim. Acta, 2003, 500, 21–69 CrossRef CAS.
- S. Kulmala, T. A. Kleme, A. Kulmala, D. Papkovsky and K. Loikas, Anal. Chem., 1998, 70, 1112–1118 CrossRef CAS.
- M. Hảkansson, Q. H. Jiang, J. Suomi, K. Loikas, M. Nauma, T. A. Kleme, J. Kankare, P. Juhala, J. U. Eskola and S. Kulmala, Anal. Chim. Acta, 2006, 556, 450–454 CrossRef.
- Q. Jiang, A. Spehar, M. Håkansson, J. Suomi, T. Ala-Kleme and S. Kulmala, Electrochim. Acta, 2006, 51, 2706–2714 CrossRef CAS.
- Q. Jiang, H. Ketamo, A. J. Niskanen, J. Suomi, M. Håkansson and S. Kulmala, Electrochim. Acta, 2006, 51, 3332–3337 CrossRef CAS.
- A. H. Wu, J. J. Sun, R. J. Zheng, H. H. Yang and G. N. Chen, Talanta, 2010, 81, 934–940 CrossRef CAS.
- Y. Bae, N. Myung and A. J. Bard, Nano Lett., 2004, 4, 1153–1161 CrossRef CAS.
- G. H. Zou and H. X. Ju, Anal. Chem., 2004, 76, 6871–6876 CrossRef CAS.
- H. Y. Wang, F. Q. Zhang, Z. Tan, Q. F. Chen and L. Wang, Electrochem. Commun., 2010, 12, 650–652 CrossRef CAS.
- H. P. Huang, G. F. Jie, R. J. Cui and J. J. Zhu, Electrochem. Commun., 2009, 11, 816–818 CrossRef CAS.
- R. H. Ye, L. Z. Huang, B. Qiu, Z. W. Song, Z. Y. Lin and G. N. Chen, Luminescence, 2011, 26, 531–535 CrossRef CAS.
- H. N. Choi, S. H. Cho and W. Y. Lee, Anal. Chem., 2003, 75, 4250–4256 CrossRef CAS.
- A. H. Wu, J. J. Sun, Y. M. Fang, X. L. Su and G. N. Chen, Talanta, 2010, 82, 1455–1461 CrossRef CAS.
- J. Wang, R. R. Zhao, M. Z. Xu and G. N. Chen, Electrochim. Acta, 2010, 56, 74–79 CrossRef CAS.
- S. J. Liang, X. W. Wang, Y. Chen, J. Zhu, Y. Y. Zhang, X. X. Wang, Z. H. Li and L. Wu, Nanoscale, 2010, 2, 2262–2268 RSC.
- J. C. Barreto, G. S. Smith, N. H. P. Strobel, P. A. McQuillin and T. A. Miller, Life Sci., 1994, 56(4), 89–96 CrossRef.
- K. A. Fähnrich, M. Pravda and G. G. Guibault, Talanta, 2001, 54, 531–559 CrossRef.
- I. Parejo, C. Petrakis and P. Kefalas, J. Pharmacol. Toxicol. Methods, 2000, 43, 183–196 CrossRef CAS.
- M. Fujihira and C. Ganzorig, Mater. Sci. Eng., B, 2001, 85, 203–208 CrossRef.
- L. Luo and Z. Zhang, Anal. Chim. Acta, 2006, 580, 14–17 CrossRef CAS.
- L. Y. Zheng, Y. W. Chi, Q. Q. Shu, Y. Q. Dong, L. Zhang and G. N. Chen, J. Phys. Chem. C, 2009, 113, 20316–20321 CAS.
- T. Hirakawa and Y. Nosaka, Langmuir, 2002, 18, 3247–3254 CrossRef CAS.
- M. R. Hoffmann, S. T. Martin, W. D. Choi and W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- M. Huang, W. Liu, Q. Li and C. F. Wu, Brain Res., 2002, 944, 90–96 CrossRef CAS.
- H. Dai, X. P. Wu, Y. M. Wang, W. C. Zhou and G. N. Chen, Electrochim. Acta, 2008, 53, 5113–5117 CrossRef CAS.
- Y. T. Chen, X. P. Chen, Z. Y. Lin, H. Dai, B. Qiu, J. J. Sun, L. Zhang and G. N. Chen, Electrochem. Commun., 2009, 11, 1142–1145 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2013 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sr2+ = 2
Sr2+ = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was dispersed into 70 mL deionized water. Then, 1 mL NH3·H2O (25%) was added into the suspensions under vigorous stirring. The resultant mixture was transferred to a sealed Teflon-lined stainless steel autoclave and heated in an oven at 160 °C for 48 h. After cooling, the products were centrifuged, washed with deionized water and dried at 60 °C in an oven.
1 was dispersed into 70 mL deionized water. Then, 1 mL NH3·H2O (25%) was added into the suspensions under vigorous stirring. The resultant mixture was transferred to a sealed Teflon-lined stainless steel autoclave and heated in an oven at 160 °C for 48 h. After cooling, the products were centrifuged, washed with deionized water and dried at 60 °C in an oven.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 nitric acid, acetone and doubly distilled water, and then dried in nitrogen. HSN/GCE was prepared by spreading 5 μL of HSN suspension (5 mg mL−1 in ethanol solution) on the electrode surface, and dried under room temperature.
1 nitric acid, acetone and doubly distilled water, and then dried in nitrogen. HSN/GCE was prepared by spreading 5 μL of HSN suspension (5 mg mL−1 in ethanol solution) on the electrode surface, and dried under room temperature.








