Tattoo-based potentiometric ion-selective sensors for epidermal pH monitoring
Amay J.
Bandodkar
a,
Vinci W. S.
Hung
ab,
Wenzhao
Jia
a,
Gabriela
Valdés-Ramírez
a,
Joshua R.
Windmiller
a,
Alexandra G.
Martinez
a,
Julian
Ramírez
a,
Garrett
Chan
a,
Kagan
Kerman
b and
Joseph
Wang
*a
aDepartment of Nanoengineering, University of California San Diego, La Jolla, CA 92093-0448, USA. E-mail: josephwang@ucsd.edu
bDepartment of Physical and Environmental Sciences, University of Toronto Scarborough, Toronto, ON M1C 1A4, Canada
First published on 19th October 2012
Abstract
This article presents the fabrication and characterization of novel tattoo-based solid-contact ion-selective electrodes (ISEs) for non-invasive potentiometric monitoring of epidermal pH levels. The new fabrication approach combines commercially available temporary transfer tattoo paper with conventional screen printing and solid-contact polymer ISE methodologies. The resulting tattoo-based potentiometric sensors exhibit rapid and sensitive response to a wide range of pH changes with no carry-over effects. Furthermore, the tattoo ISE sensors endure repetitive mechanical deformation, which is a key requirement of wearable and epidermal sensors. The flexible and conformal nature of the tattoo sensors enable them to be mounted on nearly any exposed skin surface for real-time pH monitoring of the human perspiration, as illustrated from the response during a strenuous physical activity. The resulting tattoo-based ISE sensors offer considerable promise as wearable potentiometric sensors suitable for diverse applications.
1. Introduction
Potentiometric ion selective electrodes (ISEs) have witnessed widespread use in various research, biomedical and industrial domains.1,2 Conventional ion-selective sensors consist of a membrane-based ion-selective electrode and a reference electrode, both of which require an internal solution to ensure a stable and sensitive response.3 Although these sensors have been widely used in a plethora of practical applications, their intrinsic design imposes inherent limitations upon specific in vivo and ex vivo applications, particularly the internal solution complicates the fabrication process and limits their miniaturization. Furthermore, traditional ISEs are often fabricated by employing rigid materials (e.g., glass), which hinder their integration on curvilinear surfaces. The challenges associated with the use of an internal solution were addressed by the development of solid-contact ISEs,4 with the first example demonstrated almost 4 decades ago,5 comprising of an ion-selective membrane casted on a solid metal wire. However, coated-wire sensors suffer from irreproducibility, albeit this issue was later solved by employing conducting polymers as ion-to-electron transducers.6 Similar efforts have been pursued to eliminate the use of the internal solution required for the reference electrode.7 Moreover, researchers have extended the capabilities of ion-selective sensors by fabricating all-plastic flexible ISEs,3,8,9 including complete flexible solid-contact sensing devices using screen printing10,11 and drop-casting techniques.12 Yet, highly flexible and conformal integrated potentiometric sensors, compatible with the non-planarity and irregularities of the human anatomy and enduring prolonged mechanical strain, are highly desired for the realization of epidermal chemical monitoring.This work reports on the development and characterization of ion-selective potentiometric electrodes fabricated on temporary-transfer tattoo paper for direct epidermal pH measurements. An ideal wearable device mandates a conformal geometry that is compliant with skin and can withstand repeated mechanical stress while minimizing intrusion in the wearer's routine. Advances in textile-based sensors have offered considerable advantages in the field of on-body monitoring devices as they conform with the wearer's anatomy while enabling unobtrusive sensing. Recently, wearable devices have been developed for the detection of both physiological13 and environmental14 analytes. An optical device, reported by Curto et al., describes a dermal microfluidic system that is able to respond to pH changes by employing a pH-sensitive dye.15 A necessary requirement for effective real-time physiological monitoring is the continuous contact of the analyte with the sensor surface. We recently addressed this issue by developing electrochemical sensors that can be directly stamped onto the epidermis for on-body sensing.16 We also newly introduced epidermal amperometric sensors based on the fabrication of temporary-transfer tattoo devices.17 This was achieved by combining widely deployed screen printing and temporary transfer tattoo methodologies for the amperometric and voltammetric detection of electroactive molecules such as uric acid, ascorbic acid and TNT. The elasticity of the tattoo substrate, reinforced with addition of dispersed carbon fiber segments within the tattoo ink, allowed the amperometric sensor to attach firmly to the contours of the wearer, while possessing sufficient tensile strength to withstand repetitive mechanical deformation.
The current work builds on recent advances charted within the temporary-transfer tattoo electrochemical sensors domain toward the development of epidermal ion-selective potentiometric sensors. To realize such tattoo-based potentiometric devices, we have combined polyaniline-based solid-contact ISEs, commercially available temporary transfer tattoo paper and screen printing techniques using the hybrid fabrication protocol illustrated in Fig. 1(i). The fabrication versatility offered by the screen printing methodology has allowed us to develop ISE tattoos in the form of a ‘smiley face’ with one eye acting as the pH-sensitive ISE while the second one serving as the reference electrode (Fig. 1(ii)), thus concealing the complete sensor contingent in an artistic manner. The ‘smiley face’-shaped tattoo sensors were readily printed on commercially available tattoo base paper using carbon, Ag/AgCl and insulator inks with a distinct stencil pattern employed for each layer. An adhesive sheet was later applied to the printed tattoo paper for subsequent transfer on various substrates. Further details of these steps are illustrated in Fig. 1 and provided in the Experimental section.
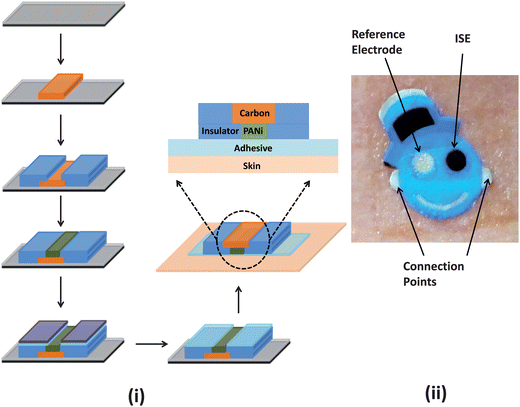 | ||
| Fig. 1 (i) Schematic representation of the fabrication protocol employed in the production of temporary transfer tattoo-based solid-contact ion-selective electrode (ISE) sensors. The sensor was prepared via a thick-film screen printing fabrication technique (only the carbon electrode (orange) and insulator (blue) are shown). The sensor is patterned onto a release agent-coated (grey layer) base paper (black). The carbon electrode is then modified with PANi followed by the application of the adhesive sheet. In order to transfer the tattoo, the protective sheet (purple) is removed from the adhesive layer (light blue) and then placed on the receiving substrate. (ii) Photograph of the tattoo ISE sensor illustrating the two electrodes and the connection points for the voltmeter. | ||
The attractive pH-sensitive conductivity of poly(aniline) (PANi) associated with the reversible emeraldine salt (ES)–emeraldine base (EB) transition (acid–base reaction) has led to its widespread use in the fabrication of solid-state pH sensors.18,19 Furthermore, PANi has minimal cytotoxicity and causes negligible skin irritation and sensitization.20 Highly reproducible thin films of PANi can be easily obtained via electropolymerization, and PANi-based ISEs commonly do not require surface pretreatment. These characteristics along with their attractive performance (further discussed below) make PANi well-suited for developing biocompatible dermal tattoo-based potentiometric sensors. The resulting tattoo ISEs withstand repeated bending and stretching operations, which are of substantial relevance to wearable epidermal sensors. The new fabrication route thus yields body-worn potentiometric sensors that are compliant with the contours of the skin for the realization of non-invasive potentiometric monitoring. In the following sections, the development and characterization of tattoo-based ISE sensor devices for continuous epidermal pH monitoring is described and the performance of such devices under practical scenarios is provided.
2. Materials and methods
2.1 Reagents
Potassium phosphate monobasic (KH2PO4), potassium phosphate dibasic (K2HPO4), hydrochloric acid (HCl), Nafion® 117 solution and aniline were obtained from Sigma Aldrich (St. Louis, MO) while citric acid was received from Fisher Chemical (Fair Lawn, NJ). Aniline was further purified by double distillation prior to use. Carbon fibers (8 μm diameter, 6.4 cm length, 93% purity) were procured from Alfa Aesar (Ward Hill, MA) and their length was reduced to ∼0.5 mm (by cutting with a sharp blade) followed by thorough cleaning in acetone. All experiments were conducted at room temperature and all solutions were prepared using ultra-pure deionized water (18.2 MΩ·cm).2.2 Instrumentation
Electrochemical cleaning, deposition, and potentiometric analysis were performed using a CH Instrument (Austin, TX) model 630C electrochemical analyzer. A Mettler Toledo (Columbus, OH) S20 SevenEasy glass-electrode digital pH meter was employed for pH measurements. A miniaturized multimeter (Sinometer MS8216 DMM) was used for on-body measurements.2.3 Fabrication of temporary tattoo sensors
The ISE tattoo was designed to conceal the electrodes in a ‘smiley face’. One eye (green) functioned as the pH-sensitive ISE while the other (silver) acted as the reference electrode (Fig. 1(ii)). As illustrated in Fig. 1(ii), the two ears were employed as connectors for the attachment of the digital multimeter. Design of the sensor pattern was performed in AutoCAD (Autodesk, San Rafael, CA) and fabricated on 75 μm thick stainless steel and mesh stencils (Metal Etch Services, San Marcos, CA). A unique stencil pattern was used for each electrode layer (i.e. carbon, Ag/AgCl, insulator). Conductive Ag/AgCl (E2414), carbon (E3449), and insulator inks (E6165) were procured from Ercon Inc. (Wareham, MA) and a transparent dielectric ink (5036) was obtained from DuPont Inc. (Wilmington, DE). Carbon fiber segments were dispersed within the conductive carbon ink matrix to increase the tensile strength of the electrode. Printing was accomplished via an MPM SPM semi-automatic screen printer (Speedline Technologies, Franklin, MA). Blank temporary transfer tattoo paper and the accompanying adhesive substrate (Papilio, HPS LLC, Rhome, TX) were used without further derivatization.The fabrication process first involved printing the blue insulator ink, followed by the Ag/AgCl and the carbon inks, and finally, by another blue insulator layer. Following each routine, the ink was cured at 90 °C for 15 min. Subsequently, a 30 wt% KCl-doped transparent insulator was screen printed only on the surface of the reference electrode and then cured for 6 min at 90 °C. Finally, a total of 6 μL of the 5% Nafion solution was drop-casted on the Ag/AgCl reference electrode and left to dry overnight. These steps were imperative in order to obtain a stable reference potential; it was observed that, without these additional processing steps, the potential of the reference electrode drifted as a function of the pH of the solution, possibly due to leaching of chloride ions.
Prior to the electropolymerization of aniline, the working electrode was electrochemically cleaned by five cyclic voltammetric scans in 0.5 M HCl over the potential range of −0.3 V to 1.1 V (an external Ag/AgCl reference electrode and an external Pt wire auxiliary electrode were used in this processing step). Surface modification with PANi was performed in a 0.1 M aniline/1 M HCl solution by cyclic voltammetry from −0.2 V to 1.0 V (vs. Ag/AgCl) at 0.1 V s−1. First, electropolymerization was performed for 12 cycles, then a fresh solution was dispensed on the surface, followed by additional 13 cycles. A total of 25 cycles were thus executed for the complete polymerization of the working electrode surface. During the cleaning and polymerization steps, the screen printed Ag/AgCl electrode was protected from the electrolyte solution to avoid its damage by highly acidic solutions and aniline. After air-drying the PANi film, the adhesive sheet was applied to the tattoo. For proper contact between the two electrodes and analyte solution, this adhesive sheet was excised to remove a rectangular-shaped region around the two electrodes (the two eyes). The as-prepared ISE tattoos were then ready for transfer and evaluation.
In order to apply the tattoos, the protective layer from the transparent adhesive sheet was first removed to expose the adhesive. To ensure proper adhesion between the tattoo and substrate, an extra adhesive layer was applied to the substrate. (Note: for applying the adhesive sheet to soft substrates, the adhesive sheet was first applied to a blank tattoo base paper, followed by removal of the protective sheet and attaching it to the substrate. The base paper was subsequently removed by gently applying water and peeling it off.) Later, the sticky adhesive side of the tattoo was placed on the adhesive layer already applied to the substrate. This step was followed by applying water to the base paper and gently peeling it off. The total time for this transfer step was less than 3 min. The entire fabrication and transfer processes are shown in Fig. 1(i).
3. Results and discussion
3.1 In vitro characterisation
The ISE tattoos were firstly examined in vitro by applying them onto hard plastic substrates prior to on-body epidermal studies. The tattoo sensors were analyzed within the pH range of human sweat (pH 3–7, with a mean around pH 5)21 using standard McIlvaine's buffers. Owing to the continuous fluctuations of the pH of the human perspiration, it is imperative that the ISE tattoos encompass a rapid and near-instantaneous response to pH modulations over this range. Fig. 2(i) displays a characteristic potential-time recording at the tattoo-based potentiometric sensor for decreasing pH levels between 7 and 3 (in one-unit decrements). This real-time recording clearly illustrates that the tattoos exhibit a nearly instantaneous response to varying pH solutions, yielding 80% of their steady-state signal within the first 10 s while a completely stabilized signal was observed within 25 s. The resulting calibration plot (shown in the inset) displays a sub-Nernstian behaviour, with a mean slope (sx) of 50.1 mV/pH and a relative standard deviation (RSD) of 3.72% (n = 4). The pH sensitivity (slope) and conductivity of PANi depend on orientation of the crystalline and amorphous phases of PANi.22 The observed sub-Nernstian response of the PANi tattoo sensors is attributed to inferior orientation of these phases. As will be discussed later, mild mechanical deformations to the tattoos caused reorientation of the conducting and amorphous phases and improved the pH-sensitivity to a near-Nernst response. Batch-to-batch variations between the tattoos also exhibited a low RSD of 4.63% (n = 4), hence indicating that the fabrication of reproducible devices is indeed feasible.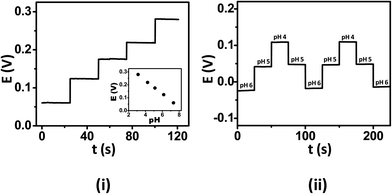 | ||
| Fig. 2 (i) Response of the ISE tattoo sensor, in vitro, upon calibration with unit decrease of pH using standard McIlvaine's buffers. (ii) Potential-time response of the ISE tattoo sensors, in vitro, demonstrates the reproducibility of the sensors in response to large pH fluctuations. | ||
It has been observed that the pH of human perspiration fluctuates according to the respiration rate.23 As such, the tattoo sensors must also exhibit minimal carry-over in order to monitor such dynamically fluctuating pH environments. To investigate this parameter, the ISE tattoos were subjected to operation in varying pH solutions and consecutive measurements recorded without reconditioning or rinsing of the tattoo surface. Fig. 2(ii) demonstrates the dynamic response of the tattoo ISE to alternate and multiple exposures to solutions of different pH. The device responds rapidly and favorably to these dynamic pH changes, rapidly regaining the same potentiometric signal for a given solution pH during this continuous operation. The negligible carry-over of the tattoo-ISE response reflects the fact that the emeraldine salt (ES)–emeraldine base (EB) transition of PANi is fast and reversible. Thus, the tattoo sensors have the capability to perform effectively under continuously varying pH milieu, viz., in situ pH measurement of human perspiration with low carry-over.
A distinctive feature of wearable sensors is their ability to endure prolonged mechanical strain, which is a key requirement of wearable and epidermal sensors. This is especially true in the athletics, fitness and military domains. It is thus essential to examine the influence of relevant mechanical stress upon the sensor performance prior to their integration with the epidermis. For this reason, the influence of mechanical strain permutations, including repeated bending and stretching, upon the potentiometric response were examined. The ISE tattoo sensors were subjected to a total of 50 bending and 40 stretching applications. For these studies, the tattoos were transferred onto GORE-TEX as its viscoelastic behaviour mimics that of skin. In the bending study, the tattoo was bent to 180° and maintained at that position for 5 s prior to release. The response of the tattoo was measured subsequent to 10 bending iterations from pH 7 to 3. In order to analyze the effect of stretching upon the electrochemical performance of the tattoos, the sensors were stretched an additional 10% in lateral extent and maintained at that position for 5 s followed by release and investigation of the response. In case of stretching deformation, the response was measured at an interval of 5 consecutive stretching operations. The deformation created during these studies actually yielded a beneficial effect upon the response of the tattoos. Specifically, in the absence of applied deformation, the tattoos yielded a sub-Nernstian response (52.8 mV/pH), as observed with plastic substrates. The response of the tattoos improved to 59.6 mV/pH within the first 10 bending iterations (Fig. 3(i)). Thereafter, the response stabilized to yield a final slope of 57.5 mV/pH following the 50th bending iteration. The RSD for the entire experiment was 5.71%. A similar trend was observed for the stretching study, where an initial slope of 53.0 mV/pH increased to 58.2 mV/pH following the 10th stretching iteration and finally stabilized at 57.54 mV/pH after the 40th stretch (Fig. 4(i)). A 4.72% RSD was obtained. The sensitivity enhancement observed is hypothesized to originate from the uncoiling and reorientation of the crystal and amorphous phases of PANi and the subsequent improvement in its conductivity owing to mechanical deformation.22 Visual analysis of the tattoo sensors under bending and stretching was performed on human skin. For the bending studies, the tattoo was applied to the cubital fossa and the arm was bent completely until the fingers touched the scapula acromion, thus simulating the extreme deformation expected under heavy epidermal wear (Fig. 3(ii)). In the stretching scenario, the tattoo was applied to the forearm and then stretched repeatedly to the maximum extent (Fig. 4(ii)). The photographs reveal that the potentiometric sensors are quite resilient and that their structural integrity does not easily degrade. Accordingly, the sensors are well-suited for applications involving continuous motion of the substrate, as experienced by the human body. These results thus substantiate that the presented tattoo ISE sensor design is well-positioned for migration towards evaluation on the human epidermis.
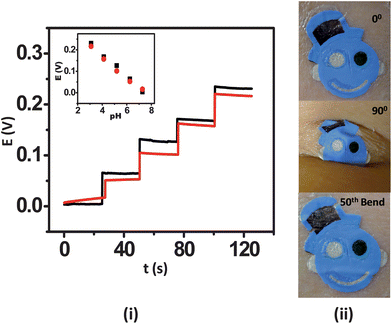 | ||
| Fig. 3 Influence of repeated mechanical strain (bending) upon the response of the tattoo ISE: (i) pH-responsive behavior of the ISE tattoo sensor over the 3–7 pH range prior to stretching (black) and following the 50th (red) bending on GORE-TEX; one unit pH decrement per addition. (ii) Images of the tattoo applied to cubital fossa at 0° bending, 90° bending, and after the 50th bending. | ||
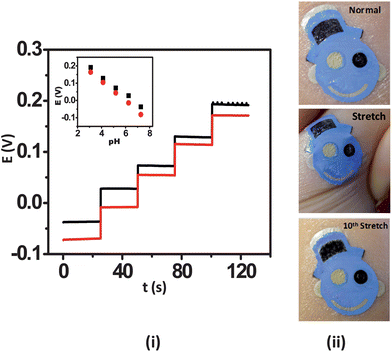 | ||
| Fig. 4 Influence of repeated mechanical strain (stretching) upon the response of the tattoo ISE: (i) pH-responsive behavior of the ISE tattoo sensor prior to stretching (black) and following the 40th (red) stretch on GORE-TEX; one unit pH decrement per addition. (ii) Images of the tattoo applied to the forearm at normal, during stretching, and after the 10th stretch. | ||
3.2 Epidermal studies
There are growing demands for ion-selective sensors in the medical, sports, athletics, and fitness fields where point-of-care devices for the monitoring of physiological conditions are required. Electrolyte (i.e. Na, Cl and K) and pH levels of perspiration can readily yield information regarding the metabolic state of an individual as well as their respiration dynamics during a fitness routine.24 Thus, continuous pH analysis of human perspiration is of great importance in the areas of clinical diagnostics and sports medicine.Following their in vitro characterization, the tattoo ISEs were thus applied to different locations throughout the body (neck, wrist and lower back) of an active, consenting volunteer (sex: male; age: 27; weight: 70 kg; height: 186 cm) while the multimeter readout unit was attached to the body using a commercially available arm-band (Fig. 5(iv)). The multimeter leads were attached firmly to the connection points (ears) of the tattoo ISE sensor using transparent polystyrene tape. The elasticity of the tattoo substrate allows the new potentiometric sensor to attach firmly to these different body locations. These areas were selected in order to vary operational conditions experienced by the sensors, as mechanical stress and local pH are expected to vary among these locations.25 As illustrated in Fig. 5(iv), the complete device can be easily mounted on the epidermis without much hindrance to the wearer. The ISE application process and the subsequent mating of the sensor with the readout instrument required less than 5 min and was readily performed by the volunteer. During the experiment the volunteer used a stationary cycle in a gymnasium for a total of 40 min followed by a 10 min gradual cool down. The volunteer ingested no fluid (dehydrated state) during the entire exercise. Heart rate and cadence were maintained around 165 and 130 RPM, respectively. pH sensing of the subject's perspiration was performed by the ISE tattoos and the data were collected at regular intervals using the miniaturized multimeter. To confirm that the tattoos yielded accurate readings, the regional pH was verified using a conventional pH meter and glass electrode.
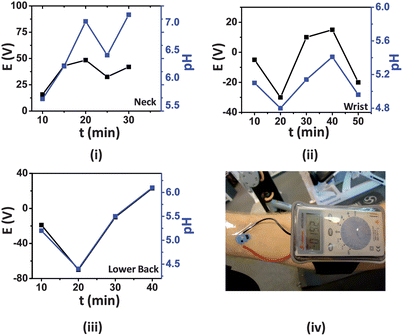 | ||
| Fig. 5 Real-time response (black) of the ISE tattoos applied to (i) neck, (ii) wrist and (iii) lower back. The plot in blue represents the pH measured by the conventional pH meter. (iv) Photograph showing the entire device (tattoo and digital multimeter) attached to the wrist for such epidermal measurements of pH in human perspiration. | ||
Although an athlete initially perspires at a low rate, this is soon followed by heavier perspiration as physical activity continues. Thus, an important requirement for the tattoos is their ability to yield precise readings during a wide range of sweat flow rates. It was observed that during the first 10 min of exercise, the tattoos provided no response as the amount of perspiration generated was not sufficient to record a consistent open circuit potential. This was also true for the pH glass electrode. However, at the 10 min mark, sweat excretion became sufficient for the tattoo sensors to yield a stable reading. Initially, the pH measured at the three positions by the tattoo ISE sensors were nearly equivalent (∼pH 5.3) (Fig. 5). The real-time sweat pH data obtained from the ISE sensors can be explained based on varying sweat rate at the respective body parts. The volunteer perspired most profusely in the vicinity of the neck, followed by the lower back and the wrists. As the sweat excretion rate increases, the relative concentration of lactate and pyruvate decreases due to dilution, and the pH concomitantly increases.21Fig. 5(i–iii) clearly illustrate that the tattoo ISE sensors performed favorably with a mean slope of ∼54 mV/pH and their potentiometric response at the different body locations followed closely the pH values recorded with the glass electrode.
During the entire course of the experiment, it was observed that the tattoo ISEs performed well during both moderate and profuse perspiration. However, owing to the combination of excessive sweating and the highly curvilinear morphology of the skin on the neck, the neck-based tattoo ISE sensor functioned reliably for about 30 min. It is also important to note that the tattoos functioned satisfactorily even when minor cracks were observed (as long as connection to the multimeter was maintained). This is attributed to the fact that the potentiometric response is independent of electrode area, in contrast to area-dependent voltammetric and amperometric measurements. This represents an inherent merit of potentiometric measurements over alternative electrochemical techniques toward potential epidermal monitoring. Thus, the ISE tattoo sensors are attractive for measuring the pH of human perspiration under practical scenarios, including complex body motions typically experienced during fitness and athletic routines.
4. Conclusions
We have successfully demonstrated the fabrication of tattoo-based solid-contact ISEs for epidermal pH monitoring by combining commercially available temporary transfer tattoo paper with conventional screen printing and solid-contact polymer ISE methodologies. The new fabrication route yields highly flexible body-worn potentiometric sensors that are compliant with the skin and concealed in an artistic tattoo pattern. Repeated bending and stretching of the tattoos exhibited minimal effect on their mechanical integrity and potentiometric behavior. In addition, the sensors exhibited no apparent carry-over effects, as is desired for successful monitoring of dynamic events. The devices were later applied to the lower back, neck and wrist of the human body and evaluated for real-time measurement of the pH levels of human perspiration during exercise. Advantageously, the tattoos performed in a near-Nernstian manner under such practical scenarios and provided stable signals even when operating under profuse perspiration. Furthermore, the tattoo ISE sensors were able to tolerate the complex mechanical deformations experienced by the human skin during exercise. The tattoo ISE sensors thus exhibit substantial potential as practical, body-worn devices for continuous physiological monitoring. The new potentiometric sensing concept can be readily expanded towards epidermal monitoring of other clinically relevant sweat electrolytes such as sodium, potassium, calcium, or magnesium.Acknowledgements
This work was supported by the National Science Foundation (Awards CBET-1066531 and CHE-1057562) and NSERC Canada. A.G.M., J.R. and V.W.S.H acknowledge support from the CALIT 2 Summer Undergraduate Scholarship Program, NIH IMSD program, and NSERC for the Michael Smith Foreign Study supplement, respectively. J.R.W. acknowledges support from the UCSD William J. von Liebig Center under the Southern California Clean Energy Technology Acceleration Program sponsored by the U.S. DOE.References
- R. A. Durst, Electroanalysis, 2012, 24, 15–22 CrossRef CAS
.
- R. P. Buck and E. Lindner, Anal. Chem., 2001, 73, 88A–97A CrossRef CAS
.
- A. Michalska and K. Maksymiuk, Anal. Chim. Acta, 2004, 523, 97–105 CrossRef CAS
.
- D. H. Cho, K. C. Chung and M. Y. Park, Talanta, 1998, 47, 815–821 CrossRef CAS
.
- R. W. Cattrall and H. Freiser, Anal. Chem., 1971, 43, 1905–1906 CrossRef CAS
.
- J. Bobacka, Electroanalysis, 2006, 18, 7–18 CrossRef CAS
.
- Ł. Tymecki, E. Zwierkowska and R. Koncki, Anal. Chim. Acta, 2004, 526, 3–11 CrossRef
.
- Ł. Tymecki, E. Zwierkowska, S. Głąb and R. Koncki, Sens. Actuators, B, 2003, 96, 482–488 CrossRef
.
- J. L. Hill, L. S. Gettes, M. R. Lynch and N. C. Hebert, Am. J. Physiol., 1978, 235, H455–H459 CAS
.
- Y. Wang, H. Xu, X. Yang, Z. Luo, J. Zhang and G. Li, Sens. Actuators, B, 2012, 173, 630–635 CrossRef CAS
.
- F. X. Rius-Ruiz, G. A. Crespo, D. Bejarano-Nosas, P. Blondeau, J. Riu and F. X. Rius, Anal. Chem., 2011, 83, 8810–8815 CrossRef CAS
.
- M. Novell, M. Parrilla, G. A. Crespo, F. X. Rius and F. J. Andrade, Anal. Chem., 2012, 84, 4695–4702 CrossRef CAS
.
- B. Schazmann, D. Morris, C. Slater, S. Beirne, C. Fay, R. Reuveny, N. Moyna and D. Diamond, Anal. Methods, 2010, 2, 342–348 RSC
.
- J. R. Windmiller and J. Wang, Electroanalysis, 2012 DOI:10.1002/elan.201200349
.
- V. F. Curto, S. Coyle, R. Byrne, N. Angelov, D. Diamond and F. Benito-Lopez, Sens. Actuators, B, 2012, 171–172, 1327–1334 CrossRef CAS
.
- J. R. Windmiller, A. J. Bandodkar, S. Parkhomovsky and J. Wang, Analyst, 2012, 137, 1570–1575 RSC
.
- J. R. Windmiller, A. J. Bandodkar, G. Valdés-Ramírez, S. Parkhomovsky, A. G. Martinez and J. Wang, Chem. Commun., 2012, 48, 6794–6796 RSC
.
- X. Zhang, B. Ogorevc and J. Wang, Anal. Chim. Acta, 2002, 452, 1–10 CrossRef CAS
.
- A. A. Karyakin, M. Vuki, L. V. Lukachova, E. E. Karyakina, A. V. Orlov, G. P. Karpachova and J. Wang, Anal. Chem., 1999, 71, 2534–2540 CrossRef CAS
.
- P. Humpolicek, V. Kasparkova, P. Saha and J. Stejskal, Synth. Met., 2012, 162, 722–727 CrossRef CAS
.
- J. L. Matousek and K. L. Campbell, Adv. Vet. Dermatol., 2002, 13, 293–300 CrossRef
.
- L. Abell, P. N. Adams and A. P. Monkman, Polymer, 1996, 37, 5927–5931 CrossRef CAS
.
- D. Kaiser, R. Songo-Williams and E. Drak, Pflügers Arch., 1974, 349, 63–72 CrossRef CAS
.
- W. A. Latzka and S. J. Montain, Clin. Sports Med., 1999, 18, 513–524 CrossRef CAS
.
- M. J. Patterson, S. D. R. Galloway and M. A. Nimmo, Exp. Physiol., 2000, 85, 869–875 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2013 |
