Bioinspired phosphorylcholine containing polymer films with silver nanoparticles combining antifouling and antibacterial properties†
Adrian V.
Fuchs‡
ab,
Sandra
Ritz‡
b,
Sabine
Pütz
b,
Volker
Mailänder
b,
Katharina
Landfester
b and
Ulrich
Ziener
*a
aInstitute of Organic Chemistry III, University of Ulm, Albert-Einstein-Allee 11, 89081 Ulm, Germany. E-mail: ulrich.ziener@uni-ulm.de; Fax: +49 731 5022883; Tel: +49 731 5022884
bMax Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany
First published on 14th January 2013
Abstract
The antibacterial (bioactive) and antifouling (biopassive) properties of stable, uniform, high surface coverage films of poly(hydroxyethyl methacrylate-co-2-methacryloyloxyethyl phosphorylcholine) (p(HEMA-co-MPC)) with embedded, non-leaching silver nanoparticles (AgNPs) are reported. Based on the experimental findings, a mechanism of action of AgNPs in antibacterial activity in combination with antifouling characteristics is discussed. Long-term antifouling studies of E. coli determine little to no adhesion on p(HEMA-co-MPC)/Ag films at 2.5 × 106 CFU mL−1 for 7 d, measured using live/dead staining assays. Agar diffusion tests indicate that there is no leaching of Ag from the films and SEM and EDX analyses of the films before and after incubation with E. coli show no attachment of E. coli and no visible change in film morphology or AgNP dispersal. Antibacterial studies are investigated using E. coli K-12 as a model bacterial strain and are tested in static (CFUs) and dynamic contact assays. Antibacterial efficacy of the films containing extremely low AgNP concentration (3.8 ng cm−2) is shown with growth suppression of E. coli in culture medium for 4 h at 1.35 × 105 CFU mL−1 and killing greater than 99% of E. coli in only 1 h of exposure to concentrations up to 1 × 105 CFU mL−1. These hybrid films may propose an exciting direction to long-term antibacterial and antifouling films in clinical applications.
Introduction
Bacterial contaminations triggered by medical implants are still a major issue in hospital acquired infections, so-called nosocomial infections or healthcare-associated infections (HAI). These infections are often caused by the production of biofilms on the surface of medical implants mostly affecting the urinary tract, respiratory tract and bloodstream.1 The investigation of biointerfaces serves as an important step in the development of films and coatings able to prevent fouling by non-specific protein binding and subsequent biofilm formation.2 Combining antifouling and antimicrobial activities in new composite materials could therefore lead to improved antimicrobial effects. Silver nanoparticles (AgNPs) are well known bioactive materials that exhibit a broad antimicrobial effect.3 Studies have shown that the slow oxidative release of Ag+ into solution from AgNPs occurs4 and can further undergo a subsequent reduction to once again form AgNPs.5 It is not yet fully understood if the toxic effects are triggered by direct interaction of AgNPs with bacteria or whether by the contribution from liberated Ag+ ions or probably in a synergy of both.6 Due to their high surface areas and small sizes, AgNPs are able to interact with the sulfur-containing moieties present in many proteins in the bacterial membrane. Subsequent penetration of small AgNPs (e.g. <10 nm) into the cell leads to respiratory enzyme disruption, mitochondrial damage and the generation of reactive oxygen species (ROS) as well as DNA binding and therefore eventual cell death.7,8 Similar mechanisms have been described for silver ions.9E. coli, used here and in previous studies as a suitable model for Gram-negative bacteria,10 has seen an increase in resistance to many antibiotic classes in Europe11 and has a high prevalence in catheter-associated urinary tract infections.12 AgNPs express a cytotoxic effect on E. coli by inhibiting the uptake of phosphate which in turn causes a release of phosphate, mannitol, succinate, proline and glutamine.7,13 Interestingly, it has recently been reported that AgNPs themselves do not inherently have an antimicrobial effect on E. coli, rather it is the released Ag+ ions under aerobic conditions that contribute to bacterial death.14 This was also recently confirmed by Xu et al. who studied AgNPs with a hydrophilic polymer coating that displayed a marked antibacterial effect when not in the presence of a reactive oxygen species scavenger.15Besides the well known antimicrobial effect of silver NPs, composite materials composed of polymers can provide a large variety of antifouling coatings due to their ease of synthesis and compatibility with a range of functionalized materials. A biomimetic approach to antifouling films can be found in derivatives of the phosphorylcholine (PC) moiety,16 a major component in the phospholipids of the cell membrane. One such material is the 2-methacryloyloxyethyl phosphorylcholine (MPC) monomer (Scheme 1) and has been shown through extensive studies by Ishihara and Nakabayashi to be very versatile in the synthesis of biocompatible co-polymers.17
 | ||
| Scheme 1 Highlighting the similarities in the structure of the phospholipid bilayer to 2-methacryloyloxyethyl phosphorylcholine (MPC). | ||
The generally accepted hypothesis of antifouling activity of MPC relies on the ability of the zwitterionic phosphorylcholine surface to strongly bind water and thus create a hydration layer that prevents non-specific protein adhesion. This hypothesis is also supported by the theory of interfacial energy matching. In any case, the strong antifouling properties of MPC-based materials are well known and have the potential to improve the biocompatibility of new materials.18
There are, however, limitations associated with the use of antifouling coatings and films.19 Long-term stability to oxidative degradation and chain cleavage, reliance on defect-free coatings and susceptibility to penetration of low molecular weight proteins and peptides are all issues that need to be addressed. Problems with many antibacterial coatings are that they suffer from fouling effects due to their inability to remove dead bacteria from the interface without a rinse or washing cycle and are typically release-based and therefore undergo consumption of the active agent leading to loss of efficacy over time. Hybrid materials of MPC and AgNPs may provide interesting composites leading to both long-term antifouling and antibacterial effects. We propose that the hydration layer formed through the strong hydrophilicity of MPC may facilitate the gradual dissolution of AgNPs into Ag+. Further, the hypothesized retention of Ag+ within the hydration layer prevents leaching throughout the bulk aqueous phase while non-consumed Ag+ is reduced back to surface-confined AgNPs. Thus, a composite exhibiting a prolonged antibacterial and antifouling lifetime is realized. The combination of bioactive and biopassive materials has recently been reviewed particularly concerning Ag with polymeric nanocomposites.20
Ag has also been investigated with hydrophilic polymers, designed with biomimetic approaches in mind.21 There have been, however, only limited investigations into the use of both AgNPs and MPC (and related derivatives).22 These studies used phosphorylcholine (PC) derivatives to surface modify AgNPs in order to improve cellular uptake, and for increased stability and solubility in aqueous systems. Moreover, to the best of our knowledge, MPC and Ag composite systems have not been studied in relation to their performance in bacterial environments. Recently, we have reported the formation of highly uniform films composed of a p(HEMA-co-MPC) co-polymer containing non-leaching AgNPs.23 The current manuscript aims to investigate the antifouling and antibacterial properties of these films and to determine the mode of action (and hence lifetime) of such films.
Results and discussion
Description of p(HEMA-co-MPC)/Ag films
The polymeric, AgNP doped films used in this study are composed of biocompatible 2-hydroxyethyl methacrylate (HEMA) and zwitterionic, biomimetic MPC (p(HEMA-co-MPC)) colloids containing either 10 or 40% MPC and between 5–15% AgNPs (all w.r.t. HEMA). Uniform films on silicon substrates were formed through an inverse miniemulsion polymerization, gaseous hydrazine reduction of the Ag+ to Ag0, followed by colloid deposition and subsequent solvent annealing. Films are typically 100–500 nm thick containing 10–40 nm AgNPs (identified by the white particles, see S1†) dispersed throughout (Fig. 1). The films were identified as non-leaching Ag surfaces by ICP-OES up to 28 days. For a detailed description of the film formation and characterization we refer to Fuchs et al.23 The composition and nomenclature of these may be found in Table 1. | ||
| Fig. 1 The visual characteristics of the films used in the study as seen in (a) the top view SEM image scratched along the left hand side for reference and (b) the side view backscattered SEM image taken at a 15° angle. | ||
Antifouling effect of p(HEMA-co-MPC)/Ag films
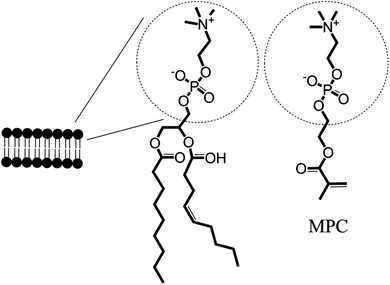
The films used here are dual functional. They contain both an antifouling and an antibacterial functionality. Thus, investigations aim to characterize each of these systematically. First, an investigation of biofilm formation to test the non-specific binding of E. coli was performed on films without Ag and containing MPC concentrations in the co-polymer of 10 or 40% w.r.t. HEMA. Next surfaces were incubated with 5 × 106 colony forming units (CFUs) of E. coli, washed and stained with dyes for living (SYTO9, green) and dead (propidium iodide, red) cells. The results of the biofilm formation, visualized in Fig. 2, show that significant adhesion occurs on the silicon control substrates over a period of 1 or 24 h (Fig. 2a, d). Importantly, minimal adhesion was seen on surfaces containing either 10 or 40% MPC over the same time spans (Fig. 2b, c or e, f). This indifference to MPC concentration was seen previously in films containing 5, 10 or 30% MPC in investigations concerning HL-60 cell attachment24 or whole blood.25 Concerning bacterial adhesion, Fujii et al.24,26 witnessed minimal or no adhesion to a co-polymer of MPC and n-butyl methacrylate after 48 h exposure to both Gram-positive and -negative bacteria. Here, the MPC films significantly reduce the adhesion of E. coli to these surfaces so much so that during fluorescence microscopy studies it was noticed that, as the aqueous solution evaporated from the films, the withdrawing solvent front was seen to ‘carry’ the E. coli off the sample and concentrate them along the (aqueous) solvent front (S2†).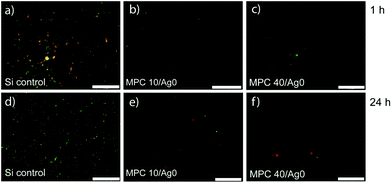 | ||
| Fig. 2 Biofilm formation of E. coli on p(HEMA-co-MPC) films without AgNPs containing 10 or 40% MPC (w.r.t. HEMA). Silicon control (a, d), MPC10/Ag0 (b, e), and MPC40/Ag0 (c, d) were submersed in an E. coli suspension (2.5 × 106 CFU mL−1) in minimal LB-medium for 1 h or 24 h and washed four times with saline thereafter. Scale bar is 20 μm. | ||
When investigating the biofilm formation of films with 10% included Ag (Fig. 3), it was established that the same antifouling properties were present as that of the films without Ag (Fig. 2). Long-term antifouling effectiveness is proven after 7 d of exposure since the surfaces still contain greatly reduced numbers of E. coli compared to the silicon control (Fig. 3e).
 | ||
| Fig. 3 Biofilm formation of E. coli on MPC10/Ag10 films. Silicon controls (a, d) and MPC10/Ag10 films (b, e) were submersed in an E. coli suspension (2.5 × 106 CFU mL−1) in minimal LB-medium for 1 h or 7 d. The surfaces were transferred in fresh containers and washed four times with saline. Backscattered SEM images of the same films (c, f). The scale bar for a, b, d, and e is 20 μm while for c and f it is 250 nm. | ||
Also, the films used in the biofilm formation experiments do not show an obvious change in AgNP distribution as a function of E. coli exposure time, suggesting a form of non-leaching Ag conservation (Fig. 3e, f and S3†). There is, however, a decrease in the number of apparent nanoparticles when compared to the appearance of the film before analysis (Fig. 1b) and this may be explained using the partial dissolution and subsequent reduction of Ag (and Cu) NPs as seen by Glover et al.5 Because of the lack of adhering E. coli, due to the high antifouling properties of the films, it is not evident by this method if the AgNPs contribute significantly to any antimicrobial properties.
Antibacterial activity of p(HEMA-co-MPC)/Ag films
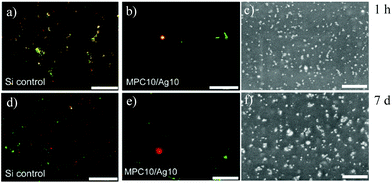
The antimicrobial activity of the p(HEMA-co-MPC)/Ag films was investigated through the use of a number of different assays.First, an agar diffusion test was performed to investigate the leaching behavior of Ag from the p(HEMA-co-MPC)/Ag films in combination with the effect of increasing Ag concentrations. Therefore films MPC10/Ag0, MPC10/Ag5, MPC10/Ag10 and MPC10/Ag15 (see Table 1) were placed face-down on spread E. coli films (Fig. 4). No obvious zone of E. coli growth inhibition was seen around films with 5, 10 and 15% Ag, suggesting no diffusion of Ag (Fig. 4a). Previous ICP-OES investigations of these films also support this conclusion.23 As controls, a silicon substrate (Si), MPC10/Ag0, and a film of MPC10/Ag0 having been exposed to hydrazine (used in the reduction of Ag+ during the production of the films) were also investigated. Again, no zone of inhibition was seen (Fig. 4b), and E. coli growth was unaffected underneath all control films. This result is not trivial as it also confirms that MPC alone, i.e. without AgNPs, displays no significant antibacterial effect. Also, MPC10/Ag5 did not appear to affect the growth of E. coli under the film, i.e. in direct contact with the film, and suggests that the concentration of 5% AgNPs is insufficient to cause an antibacterial effect. Apart from showing no zone of inhibition, MPC10/Ag10 and MPC10/Ag15 also displayed a definite antibacterial effect by killing E. coli in direct contact with the underside of the films. This growth inhibition was maintained for more than 2 weeks. It can be concluded therefore that films with at least 10% Ag are capable of long-term antibacterial effects via a direct contact mechanism while no contribution was measured from any possible Ag leachate.
 | ||
| Fig. 4 Agar diffusion test of p(HEMA-co-MPC) films with and without Ag: (a) MPC10/Ag5, MPC10/Ag10 and MPC10/Ag15 as well as a control of pure silicon (Si), and (b) control samples: Si, MPC10/Ag0, and MPC10/Ag0 after treatment with hydrazine for the reduction of Ag ions into nanoparticles. | ||
The antimicrobial activity of non-leaching, antimicrobial treated specimens can be evaluated by using the Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents Under Dynamic Contact Conditions (E2149-10; ASTM International). Therefore MPC10/Ag10 films were subjected to orbital rotation (200 rpm) for 1 h submerged in buffer solutions containing E. coli concentrations of ∼1 × 105 CFU mL−1 as adapted from the standard test method. Using a buffer solution instead of a growth medium provides metabolic stasis of the population and prevents the overlay with the growth phase of the bacteria (and therefore reduces the variability). Shown in Fig. 5 are the resultant solutions spread on agar plates and the reduction in % CFUs.
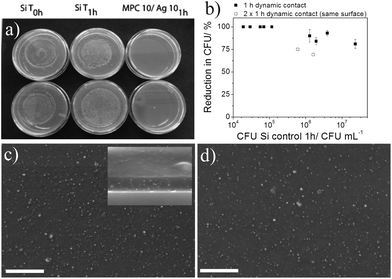 | ||
| Fig. 5 Antibacterial activity of MPC10/Ag10 films tested in a 1 h dynamic contact assay under non-growing conditions. (a) Agar plates of E. coli spreads after 1 h dynamic contact to Si substrates (left, middle) and MPC10/Ag10 films (right) (duplicate samples). (b) Reduction of E. coli (% CFUs) relative to Si wafer control after 1 h dynamic contact with MPC10/Ag10 films with respect to increasing bacteria cell numbers. (c) Backscattered SEM images of the MPC10/Ag10 film after 1 h contact to an E. coli suspension (5 × 105 CFU mL−1) with the inset showing a side-view and (d) the same film after a second hour of contact to an E. coli suspension (1 × 106 CFU mL−1). Bright spots indicate AgNPs and the scale bar is 1 μm. | ||
From this, it is seen that p(HEMA-co-MPC) films with 10% Ag are capable of killing all (>99%) CFUs of E. coli up to concentrations of 1 × 105 CFU mL−1 within 1 h under dynamic contact conditions. Further dynamic contact assays with increasing bacterial numbers revealed that even higher numbers of E. coli (up to ∼1 × 107 CFU mL−1) can be effectively reduced (∼75%) in a 1 h contact time (Fig. 3b). To assess the stability of the films we repeated the dynamic contact assay with the same film (2 × 1 h dynamic contact assay). The film was washed after the first dynamic contact assay, dried and reused 1 week later for a second test. The results still showed a 75% and 68% reduction of bacterial growth (open squares in Fig. 3b). Films at elevated E. coli concentration (>1 × 105 CFU mL−1) showed a reduction in CFUs after exposure, though did not appear to follow obvious trends. This may be a consequence of saturation of E. coli cells effectively shielding the surface and has been witnessed before with E. coli concentrations greater than 1 × 108 CFU mL−1.27
It was calculated that the MPC10/Ag10 films contained approximately 20 ng of nanoparticulate Ag (3.8 ng cm−2). The exact amount on the surface of the films cannot, however, be determined. High local concentrations of Ag and Ag+ can develop unwanted side effects on the host tissue like delaying wound healing or cytotoxicity.28 Ag concentrations have been previously studied and showed that typical loadings of Ag in ointments and dressings of approximately 100 μg cm−2 lead to cytotoxic effects in vitro.29 Other studies showed that the antimicrobial activity of polymeric thin films composed of poly(allylamine hydrochloride) (PAH) and poly(acrylic acid) (PAA) with a loading of 400 ng cm−2 AgNPs caused a reduction of 1 × 106 CFU mL−1 (99.9999%) against Staphylococcus epidermidis. The loading with this concentration of Ag was not toxic for the mammalian fibroblast cell line (NIH-3T3).30 Another study by Baker et al.31 showed that loadings of 8 μg cm−2 of AgNPs on surfaces were completely cytotoxic to E. coli. Visually, the films remain stable and uniform after either the first hour (Fig. 5c) or the second hour (Fig. 5d) dynamic contact analyses. The AgNP size and distribution frequency are comparable as evidenced by comparing the ‘bright’ spots on the backscattered SEM images (see S1† for further clarification and analysis). After the same film was subjected to a second hour of dynamic contact, the films, while providing reduced antibacterial efficacy, do not visually alter. Interestingly, upon comparison of the side view of MPC10/Ag10 before (Fig. 1b) and after (Fig. 5c inset) antibacterial analysis, the overall frequency of AgNPs appears to reduce. This may be due to the migratory effect described by Glover et al.5 where they highlight the partial dissolution of Ag or Cu NPs and subsequent reduction into smaller NPs. After analysis, film washing could effectively remove these smaller AgNPs from the film, and would explain the consistent AgNP concentration seen irrespective of bacterial exposure time. This may also support why the second hour of dynamic contact experiments shows reduced antibacterial activity as some of the surface exposed AgNPs may have been washed from the surface between the 1st and 2nd hour analyses. The findings by Xiu et al. also support this observation since AgNPs under anaerobic conditions do not exhibit antibacterial properties on E. coli.14 Our studies are performed under the likelihood that oxygen is present and therefore so too are Ag+ ions. Since no detectable Ag leaching was measured, it suggests that AgNPs exert an antimicrobial effect through a contact active mechanism whereby Ag+ ions are transferred to the E. coli which leads to subsequent death. To study if the AgNPs are consumed by E. coli used in these experiments we analyzed for Ag by elemental analysis using EDX (Fig. 6).
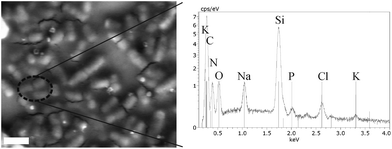 | ||
| Fig. 6 Backscattered SEM image and the accompanying EDX spectrum of E. coli after a dynamic contact assay. The scale bar is 2 μm. | ||
No Ag was detected throughout all of the EDX analyses of the E. coli from the dynamic contact test, i.e. less than the detection limit (0.1 atom% from an EDX sample volume of approximately 1 μm3). For reference, the Lα emission line of Ag would appear at 2.984 keV. This result may be validated by using the work of Feng et al., as they were able to follow the consumption of similarly sized AgNPs by E. coli using TEM and EDAX analyses.7 Tentatively therefore, the AgNPs are not being consumed during the antibacterial process, but rather are probably being slowly consumed as Ag+ and are therefore not leaching during the antibacterial process.
Next we investigated the antibacterial activity of MPC10/Ag10 films under growing conditions. Films were incubated with E. coli suspended in growth medium containing a starting concentration of 1.35 × 105 CFU mL−1 and the number of colony forming units was determined up to 6 h.
Fig. 7 shows that the negative control (Si) and the MPC10/Ag0 film containing no Ag exhibit a continual increase in CFUs until 4 h (the 6 h value was not countable due to the high amount of CFUs). Meanwhile, the MPC10/Ag10 film containing 10% Ag provides a slight decrease in CFUs over the same time-frame after which growth continues at the 6 h measurement. As this is a test performed in culture medium which supports the exponential growth of E. coli, one can conclude that the MPC10/Ag10 films (5.25 cm2) can suppress the bacterial growth with a starting number of 1.35 × 105 CFU mL−1 for up to 4 h. Conversely, due to the consistent growth of E. coli in solutions containing films of p(HEMA-co-MPC) only, as well as continued growth under the MPC film during the agar diffusion tests, it would indicate that the MPC alone displays no antimicrobial response under these experimental conditions despite containing a quaternary ammonium salt functionality. This effect has been witnessed before32 although is contrary to the well-known antimicrobial behavior of such polymeric functionalities.33,34
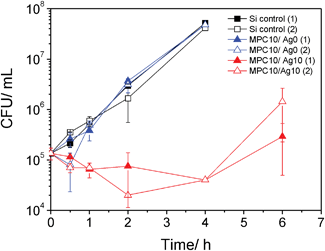 | ||
| Fig. 7 Antibacterial activity of p(HEMA-co-MPC) films of MPC10/Ag0 and MPC10/Ag10 tested under growing conditions. | ||
Conclusions
Dual functional bioinspired hybrid films were extensively tested using E. coli as a suitable bacterial model. The conclusions may be summarized visually in Fig. 8.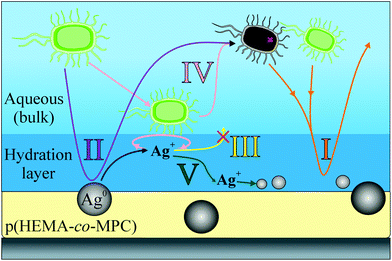 | ||
| Fig. 8 A summary of the mechanisms observed throughout the study of the bioactive and biopassive films of p(HEMA-co-MPC)/Ag films. | ||
The high hydrophilicity of the p(HEMA-co-MPC) acts to significantly hinder non-specific binding of both live and dead E. coli for up to 7 d and concentrations of 2.5 × 106 CFU mL−1 (Fig. 8, path I). Furthermore, the incorporated AgNPs showed antibacterial properties (99.9%) up to 1 × 105 CFU mL−1 after 1 h in non-growing medium and 4 h under growing conditions. We hypothesize that the antibacterial effect occurs mainly by a contact active mechanism with contributions from a slow, non-measurable release of silver ions (Fig. 8, path II). This was supported by agar diffusion tests where no zone of inhibition was witnessed and a lack of any Ag seen in/on the E. coli as measured using EDX analysis. Detachment and diffusion of the Ag out of the films was not seen (Fig. 8, path III), and a change in AgNP size was observed using SEM and EDX analysis. While consumption of AgNPs cannot be ruled out (Fig. 8, path IV), evidence points towards dissolution of AgNPs into the hydration layer followed by a reduction process at the film surface (Fig. 8, path V). In this way, most of the Ag is conserved thus providing a material with long-term antibacterial efficacy against E. coli on a surface highly resistant to biofouling. The film preparation by drop-casting of p(HEMA-co-MPC)/AgNP colloids and subsequent solvent annealing provides a simple coating method and one interesting future aspect will be the investigation of film stability on different clinically relevant surfaces. It is envisioned that these films may prove effective for coating devices such as catheters, stents and dialysis equipment.
Experimental
Material
Ultrapure water was obtained from a Milli-Q 185 Plus water purification system (Millipore). LB-medium was prepared by dissolving bacteriological peptone (1% w/v, Fluka), yeast extract (0.5% w/v, Roth) and NaCl (1% w/v) in ultrapure water, adjusting the pH with 1 N NaOH to 7.0 and autoclaving the medium for 20 min at 121 °C. For the preparation of agar plates, 1.5% w/v agar (Fluka) was dissolved in LB-medium, sterilized by autoclaving, and poured in 50 mm Petri dishes. Minimal LB-medium was used as a 10 fold dilution of the LB-medium. The LB-medium and agar plates were stored at 4 °C and used within 30 d.Biofilm formation
A fresh shake culture of Escherichia coli (E. coli K-12 wildtype, K-12 DSM 498, ATCC 23716) was prepared as described above and the bacteria were diluted with minimal LB-medium to 2.5 × 106 CFU mL−1. Samples (3.5 cm × 1.5 cm, 5.25 cm2) and silicon wafer control were placed in a 6-well culture plate (Greiner, Germany) and submerged with 2 mL bacteria dilution, covered with a lid, sealed with parafilm and incubated on a horizontal shaker (Innova 44, New Brunswick Scientific) at 37 °C and 50 rpm for the indicated time points (1 h, 24 h, 7 d). Then the samples were placed in a fresh 6-well culture plate and washed 4 times with saline. Bacteria attaching to the films were stained with the Live/Dead BacLight Bacterial Viability Kit (Life Technologies, Germany) according to the supplier's protocol.The bacteria were imaged on an inverted microscope (Olympus IX-70) equipped with a 100 W mercury lamp, a 20× phase contrast objective and a CCD camera (F-View, Olympus) for digital imaging. Living bacteria stained with SYTO 9 (green) were excited with a band pass filter at 470 nm to 490 nm and the emission was detected by 520 nm. Dead bacteria cells stained with propidium iodide were excited with a band pass filter at 530–550 nm and the emission was detected by 590 nm. Image processing was performed with CellSens Dimensions 1.5 (Olympus) and ImageJ (Fiji).
Antimicrobial activity (non-growing conditions)
The antimicrobial activity of the p(HEMA-co-MPC) films with and without AgNPs (Table 1) was assessed by an adapted protocol according to the ‘Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents Under Dynamic Contact Conditions’ (E2149-10; ASTM International) which is designed for non-leaching antimicrobial treated surfaces. Briefly, a fresh shake culture of Escherichia coli (E. coli K-12 wildtype, K-12 DSM 498, ATCC 23716) was prepared in sterile LB-medium by shaking at 37 °C and 200 rpm for 18 h. An exponential growing culture was obtained by dilution with sterile LB-medium to an absorbance of 0.1 at 600 nm and shaking to an absorbance of 0.5 at 600 nm which correspond to a concentration of 1.0 × 108 CFU mL−1 (determined by plating and counting of serial dilutions). The bacteria solutions were further adjusted with DPBS buffer without calcium and magnesium (Life Technologies) to the indicated concentrations (∼2 × 104–2 × 107 CFU mL−1) by serial dilutions. Films (3.5 cm × 1.5 cm, 5.25 cm2) were submerged in 7 mL bacteria dilution. Uncoated silicon wafers (3.5 cm × 1.5 cm, 5.25 cm2) served as control surfaces. The concentration of bacterial dilutions at T0 was determined by serial dilutions (undiluted, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500, 1
1500, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000, 1
3000, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000) by direct plating on agar plates. The samples were shaken for 1 h at 37 °C and 200 rpm on a horizontal incubator (Innova 44, New Brunswick Scientific). Again, serial dilutions at T1 h were plated on Petri dishes and all Petri dishes (T0, T1 h) were incubated for 24 h at 37 °C to give an estimate of viable cell count as colony-forming units per milliliter (CFU mL−1). The mean value and standard deviation (SD) were calculated from the serial dilutions of each sample. The results in Fig. 3b were collected from six independent experiments.
6000) by direct plating on agar plates. The samples were shaken for 1 h at 37 °C and 200 rpm on a horizontal incubator (Innova 44, New Brunswick Scientific). Again, serial dilutions at T1 h were plated on Petri dishes and all Petri dishes (T0, T1 h) were incubated for 24 h at 37 °C to give an estimate of viable cell count as colony-forming units per milliliter (CFU mL−1). The mean value and standard deviation (SD) were calculated from the serial dilutions of each sample. The results in Fig. 3b were collected from six independent experiments.
The percent of bacterial reduction was calculated from treated sample (A) directly compared to untreated control (B) at T1 h.
| Reduction, % (CFU mL−1) = (BCFU mL−1 − ACFU mL−1)/BCFU mL−1 × 100 |
Antimicrobial activity (growing conditions)
The antimicrobial activity of the p(HEMA-co-MPC) films with and without AgNPs (Table 1) under growing conditions was assessed in LB-medium. A fresh shake culture of Escherichia coli (E. coli K-12 wildtype, K-12 DSM 498, ATCC 23716) was prepared as described above. Duplicate samples (2 cm × 2 cm, 4 cm2) were placed in a 6-well culture plate (Greiner, Germany) and submerged with 2 mL bacteria dilution (∼ 1 × 105 CFU mL−1), covered with a lid, sealed with parafilm and incubated on a horizontal shaker (Innova 44, New Brunswick Scientific) at 37 °C and 50 rpm for 30 min, 1 h, 2 h, 4 h and 6 h. The bacteria concentrations were determined at T0 and the indicated time points by serial dilutions in saline (undiluted, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250, 1
250, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), plating of a 50 μL aliquot on agar plates and incubation for 24 h at 37 °C to give an estimate of viable cell count as CFU mL−1. The mean value and standard deviation (SD) were calculated from the serial dilutions of each sample.
1000), plating of a 50 μL aliquot on agar plates and incubation for 24 h at 37 °C to give an estimate of viable cell count as CFU mL−1. The mean value and standard deviation (SD) were calculated from the serial dilutions of each sample.
Scanning electron microscope (SEM)
SEM images were acquired on a LEO Gemini 1530 (Carl Zeiss AG, Germany) under accelerating voltages between 0.30–0.70 keV using an In-lens SE detector. Images of the films were acquired (after washing three times with water) before and after both dynamic contact assays and biofilm formation assays and were performed in duplicate.Energy dispersive X-ray (EDX)
EDX analysis was performed on a Hitachi SU8000 SEM coupled with a Bruker XFlash Detector 5010 (SSD) using an accelerating voltage of 6.0 keV. Again, images of the films were acquired before and after dynamic contact assays and were also performed in duplicate.Acknowledgements
We thank Caroline Hogl for skillful technical support and Gunnar Glasser for backscattered SEM and EDX measurements.Notes and references
- X. Khoo and M. W. Grinstaff, MRS Bull., 2011, 36, 357–366 CrossRef CAS; D. Neut, J. G. E. Hendriks, J. R. van Horn, R. S. Z. Kowalski, H. C. van der Mei and H. J. Busscher, J. Orthop. Res., 2006, 24, 291–299 CrossRef; A. Roosjen, W. Norde, H. C. van der Mei and H. J. Busscher, Prog. Colloid Polym. Sci., 2006, 132, 138–144 CrossRef; A. Vertes, V. Hitchins and K. S. Phillips, Anal. Chem., 2012, 84, 3858–3866 CrossRef.
- I. Banerjee, R. C. Pangule and R. S. Kane, Adv. Mater., 2011, 23, 690–718 CrossRef CAS.
- H. H. Lara, E. N. Garza-Trevino, L. Ixtepan-Turrent and D. K. Singh, J. Nanobiotechnol., 2011, 9, 30 CrossRef CAS.
- S. Kittler, C. Greulich, J. Diendorf, M. Köller and M. Epple, Chem. Mater., 2010, 22, 4548–4554 CrossRef CAS; C.-N. Lok, C.-M. Ho, R. Chen, Q.-Y. He, W.-Y. Yu, H. Sun, P. Tam, J.-F. Chiu and C.-M. Che, J. Biol. Inorg. Chem., 2007, 12, 527–534 CrossRef; Z.-M. Xiu, J. Ma and P. J. J. Alvarez, Environ. Sci. Technol., 2011, 45, 9003–9008 CrossRef.
- R. D. Glover, J. M. Miller and J. E. Hutchison, ACS Nano, 2011, 5, 8950–8957 CrossRef CAS.
- N. Lubick, Environ. Sci. Technol., 2008, 42, 8617 CrossRef CAS; S. F. Hansen and A. Baun, Nat. Nanotechnol., 2012, 7, 409–411 CrossRef; B. Nowack, C. Brouwer, R. E. Geertsma, E. H. Heugens, B. L. Ross, M. C. Toufektsian, S. W. Wijnhoven and R. J. Aitken, Nanotoxicology, 2012 DOI:10.3109/17435390.2012.711863; S. W. P. Wijnhoven, W. Peijnenburg, C. A. Herberts, W. I. Hagens, A. G. Oomen, E. H. W. Heugens, B. Roszek, J. Bisschops, I. Gosens, D. Van de Meent, S. Dekkers, W. H. De Jong, M. Van Zijverden, A. Sips and R. E. Geertsma, Nanotoxicology, 2009, 3, 109–U178 CrossRef; J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramirez and M. J. Yacaman, Nanotechnology, 2005, 16, 2346–2353 CrossRef.
- Q. L. Feng, J. Wu, G. Q. Chen, F. Z. Cui, T. N. Kim and J. O. Kim, J. Biomed. Mater. Res., 2000, 52, 662–668 CrossRef CAS.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76–83 CrossRef CAS.
- M. C. Stensberg, Q. Wei, E. S. McLamore, D. M. Porterfield, A. Wei and M. S. Sepulveda, Nanomedicine-UK, 2011, 6, 879–898 CrossRef CAS.
- I. Sondi and B. Salopek-Sondi, J. Colloid Interface Sci., 2004, 275, 177–182 CrossRef CAS.
- ECDC, Stockholm, 2010.
- J. Dankert, A. H. Hogt and J. Feijen, CRC Crit. Rev. Biocompat., 1986, 2, 219–301 Search PubMed; P. J. Nowatzki, R. R. Koepsel, P. Stoodley, K. Min, A. Harper, H. Murata, J. Donfack, E. R. Hortelano, G. D. Ehrlich and A. J. Russell, Acta Biomater., 2012, 8, 1869–1880 CrossRef CAS.
- M. Yamanaka, K. Hara and J. Kudo, Appl. Environ. Microbiol., 2005, 71, 7589–7593 CrossRef CAS.
- Z.-m. Xiu, Q.-b. Zhang, H. L. Puppala, V. L. Colvin and P. J. J. Alvarez, Nano Lett., 2012, 12, 4271–4275 CrossRef CAS.
- H. Xu, F. Qu, H. Xu, W. Lai, Y. Andrew Wang, Z. Aguilar and H. Wei, BioMetals, 2012, 25, 45–53 CrossRef CAS.
- J. A. Hayward and D. Chapman, Biomaterials, 1984, 5, 135–142 CrossRef CAS.
- N. Nakabayashi and Y. Iwasaki, BioMed. Mater. Eng., 2004, 14, 345–354 CAS.
- N. Nakabayashi, Macromol. Symp., 2006, 245/246, 591–598 CrossRef CAS.
- M. Charnley, M. Textor and C. Acikgoz, React. Funct. Polym., 2011, 71, 329–334 CrossRef CAS; A. M. Klibanov, J. Mater. Chem., 2007, 17, 2479–2482 RSC.
- P. Dallas, V. K. Sharma and R. Zboril, Adv. Colloid Interface Sci., 2011, 166, 119–135 CAS.
- M. C. Coll Ferrer, N. J. Hickok, D. M. Eckmann and R. J. Composto, Soft Matter, 2012, 8, 2423–2431 RSC; I. Sawada, R. Fachrul, T. Ito, Y. Ohmukai, T. Maruyama and H. Matsuyama, J. Membr. Sci., 2012, 387–388, 1–6 CrossRef CAS; H. Stará, Z. Starý and H. Münstedt, Macromol. Mater. Eng., 2011, 296, 423–427 CrossRef; V. Sambhy, M. M. MacBride, B. R. Peterson and A. Sen, J. Am. Chem. Soc., 2006, 128, 9798–9808 CrossRef.
- Y.-C. Chung, I. H. Chen and C.-J. Chen, Biomaterials, 2008, 29, 1807–1816 CrossRef CAS; Q. Jin, X. Liu, J. Xu, J. Ji and J. Shen, Sci. China, Ser. B., 2009, 52, 64–68 CrossRef.
- A. V. Fuchs, C. Walter, K. Landfester and U. Ziener, Langmuir, 2012, 28, 4974–4983 CrossRef CAS.
- S.-i. Sawada, S. Sakaki, Y. Iwasaki, N. Nakabayashi and K. Ishihara, J. Biomed. Mater. Res., Part A, 2003, 64, 411–416 Search PubMed.
- K. Ishihara, H. Oshida, Y. Endo, T. Ueda, A. Watanabe and N. Nakabayashi, J. Biomed. Mater. Res., 1992, 26, 1543–1552 CrossRef CAS.
- K. Fujii, H. N. Matsumoto, Y. Koyama, Y. Iwasaki, K. Ishihara and K. Takakuda, J. Vet. Med. Sci., 2008, 70, 167–173 CrossRef CAS.
- H. Han, J. Wu, C. W. Avery, M. Mizutani, X. Jiang, M. Kamigaito, Z. Chen, C. Xi and K. Kuroda, Langmuir, 2011, 27, 4010–4019 CrossRef CAS.
- C. Marambio-Jones and E. Hoek, J. Nanopart. Res., 2010, 12, 1531–1551 CrossRef CAS.
- A. Agarwal, K. M. Guthrie, C. J. Czuprynski, M. J. Schurr, J. F. McAnulty, C. J. Murphy and N. L. Abbott, Adv. Funct. Mater., 2011, 21, 1863–1873 CrossRef CAS.
- A. Agarwal, T. L. Weis, M. J. Schurr, N. G. Faith, C. J. Czuprynski, J. F. McAnulty, C. J. Murphy and N. L. Abbott, Biomaterials, 2010, 31, 680–690 CrossRef CAS.
- C. Baker, A. Pradhan, L. Pakstis, D. J. Pochan and S. I. Shah, J. Nanosci. Nanotechnol., 2005, 5, 244–249 CrossRef CAS.
- K. Bertal, J. Shepherd, C. Douglas, J. Madsen, A. Morse, S. Edmondson, S. Armes, A. Lewis and S. MacNeil, J. Mater. Sci., 2009, 44, 6233–6246 CrossRef CAS.
- H. Murata, R. R. Koepsel, K. Matyjaszewski and A. J. Russell, Biomaterials, 2007, 28, 4870–4879 CrossRef CAS.
- G. Kim, S. Park, J. Jung, K. Heo, J. Yoon, H. Kim, I. J. Kim, J. R. Kim, J. I. Lee and M. Ree, Adv. Funct. Mater., 2009, 19, 1631–1644 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional SEM and cLSM images of the films. See DOI: 10.1039/c2bm00155a |
| ‡ Shared authorship. |
| This journal is © The Royal Society of Chemistry 2013 |
