Formation and harvesting of thick pancreatic β-cell sheets on a highly O2-permeable plate modified with poly(N-isopropylacrylamide)†
Kikuo
Komori
*,
Mari
Udagawa
,
Marie
Shinohara
,
Kevin
Montagne
,
Tatsuro
Tsuru
and
Yasuyuki
Sakai
Institute of Industrial Science, University of Tokyo, Komaba, Meguro-ku, Tokyo 153-8505, Japan. E-mail: kkomori@iis.u-tokyo.ac.jp; Fax: +81-3-5452-6349; Tel: +81-3-5452-6349
First published on 25th February 2013
Abstract
Producing sheet-like tissues is a promising strategy for implantable engineered tissues, because in vitro pre-vascularization is dispensable in this configuration. We developed a simple methodology for the formation and non-destructive harvesting of a thick pancreatic β-cell sheet consisting of mouse insulinoma MIN6-m9 cells and mouse NIH3T3 fibroblasts using an O2-permeable polydimethylsiloxane plate modified with poly(N-isopropylacrylamide) (O2+/PNIPA–PDMS plate). Owing to the direct oxygenation of the cells through the PNIPA-modified PDMS plate, a viable, metabolically active sheet 5–6 cell layers thick (ca. 60 μm thick) was formed spontaneously; in the absence of direct oxygenation, only a thin cell sheet could be formed consisting of at most 2 layers (ca. 20 μm thick) with mainly anaerobic metabolism. Consequently, the net density of MIN6-m9 cells under direct oxygenation was about twice as high as in the absence of direct oxygenation. Accordingly, the insulin secretion for 10 to 60 min after glucose stimulation was also about 1.5 times higher with oxygenation. Furthermore, the thick cell sheet was successfully harvested from the O2+/PNIPA–PDMS plate surface in a non-destructive manner by inducing a phase transition of PNIPA by lowering the temperature below the lower critical solution temperature. Thus, the present report shows a promising and simple method to produce thick sheet-like engineered tissues for transplantation that could be used as a treatment for type 1 diabetes.
1. Introduction
Engineered cell sheets are one of the most promising cell-based strategies for tissue engineering and regenerative medicine, because in vitro pre-vascularization, which is a main obstacle in tissue engineering, is not essential for cell sheets. Cell sheet-based therapy has recently been presented for several tissues.1,2 Among the potential therapeutic applications, implantation of pancreatic β-cell sheets is particularly expected to be an effective treatment for type 1 diabetes. Although infusion of pancreatic islets into the hepatic portal vein is widely used as a kind of gold standard for semi-permanent treatment thanks to its minimally invasive nature,3,4 such an infusion-based procedure often has a poor engraftment rate, even right after the infusion. To overcome this problem, Okano and his co-workers formed a cell sheet consisting of insulin- and glucagon-positive cells, which were isolated from Lewis rats, and transplanted it under the skin of another Lewis rat.5 The sheet stably produced and secreted insulin in vivo for 7 days after transplantation, laying the basis for a new, minimally invasive surgical treatment for diabetes.To obtain cell sheets, culture plates covered with thermo-responsive polymers such as poly(N-isopropylacrylamide) (PNIPA) are an emerging technology.6–8 The PNIPA-modified surface behaviour switches between hydrophilic and hydrophobic at its lower critical solution temperature (LCST) of 32 °C, due to the phase transition of PNIPA.9,10 Based on this, a cell sheet formed on the hydrophobic surface can spontaneously detach from the hydrophilic surface at temperatures below the LCST. This method dispenses with conventional proteolytic enzymes, such as trypsin and collagenase, and thus the cell sheet totally maintains cell-to-cell contacts and its extracellular matrix. Consequently, tissue-engineered cell sheets thus prepared exhibit rapid engraftment to an implantation site.11 Recently, it has also been reported that a cell sheet could be formed on an Au electrode modified with a self-assembled monolayer of Arg-Gly-Asp peptides with thiol groups and then non-invasively harvested from the electrode surface by reductive desorption of thiols.12 However, in order to increase the number of cells per unit area at an implantation site and enhance the efficacy of the treatment, a three-dimensional cell sheet should preferably be used for implantation. Unfortunately, cell sheets obtained by the above-mentioned techniques are usually one-layer films. Therefore, to obtain a thick cell sheet, thin cell layers have to be stacked prior to implantation.13,14
To successfully form multilayered cell sheets in vitro, oxygenation to cells is crucially important. Since poor solubility of oxygen in the culture medium generally limits the amount of oxygen that can diffuse from the air–liquid interface of the culture medium to the cells, it is comparably difficult to form a thick cell sheet on the bottom of a commercial cell culture plate, such as a tissue culture-treated polystyrene (TCPS) plate, due to low oxygen tension. In contrast, cultivating cells on a highly gas-permeable membrane allows the formation of a thick cell sheet, thanks to the direct oxygenation of the cells through the membrane. Actually, we have succeeded in directly forming thick cell sheets consisting of two cell layers or more15–17 and semispherical cell aggregates18–20 on highly gas-permeable polydimethylsiloxane (PDMS) membranes.21 In the case of human hepatoma Hep G2 cells, the thickness of the cell sheet could exceed six cell layers.16 If a PDMS membrane modified with PNIPA is used, it should be possible to spontaneously form a thick cell sheet and non-invasively harvest it, while maintaining its sheet structure, by just reducing the temperature.
In the present work, we propose a simple methodology enabling the formation of a thick pancreatic β-cell sheet by growing cells on a PNIPA-modified PDMS (O2+/PNIPA–PDMS) membrane at a temperature above the LCST and harvesting it in a non-destructive manner from the membrane surface at a temperature below the LCST. As a model, a mouse pancreatic β-cell line, MIN6-m9, and mouse NIH3T3 fibroblasts were used to form the thick heterogeneous pancreatic β-cell sheet. Fibroblastic cells are known to accelerate the organization of organ parenchymal cells and enhance the mechanical strength of engineered cell sheets.22,23 Cellular growth, histology, respiratory metabolism and insulin secretion of the thick cell sheet formed on the O2+/PNIPA–PDMS-based cell culture plate were also examined. The proposed methodology could play a significant role for further developments in cell therapy and regenerative medicine.
2. Experimental
2.1. Preparation of 2-aminoethanethiol–PNIPA24,25
PNIPA terminated at one end with 2-aminoethanethiol (AESH) was synthesized from N,N-dimethylformamide containing 2.34 M NIPA, 22.5 mM AESH and 2.4 mM N,N′-azobisisobutyronitrile as an initiator at 70 °C for 15 h in a nitrogen atmosphere. The resulting polymer was precipitated with diethyl ether. The LCST was determined to be about 28.2 °C by a transmittance measurement in an aqueous solution containing 700 mM PNIPA–AESH (monomer unit). The weight-average molecular weight (Mw) of PNIPA–AESH was estimated to be about 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 by gel permeation chromatography (GPC). The GPC system consisted of a PU714 HPLC pump, a DG660B degassing device, a CO705 column oven and an RI704 refractive index detector (GL Sciences Inc., Japan). Polyethylene oxide molecular weight standards (Polymer Laboratories, USA) and the EZ Chrom Elite software (Scientific Software International Inc., USA) were used to determine Mw of PNIPA–AESH.
000 by gel permeation chromatography (GPC). The GPC system consisted of a PU714 HPLC pump, a DG660B degassing device, a CO705 column oven and an RI704 refractive index detector (GL Sciences Inc., Japan). Polyethylene oxide molecular weight standards (Polymer Laboratories, USA) and the EZ Chrom Elite software (Scientific Software International Inc., USA) were used to determine Mw of PNIPA–AESH.
2.2. Preparation of cell culture plates
A mixture of PDMS prepolymer and curing agent (Silpot 184 and Catalyst Silpot 184, both from Toray-Dow Corning, Japan) at a mass ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was poured into a polystyrene template and then degassed in a vacuum chamber. The template was subsequently heated to 75 °C for 2 h in an electric oven to form a PDMS membrane (ca. 1.0 mm thick). The PDMS membrane was fixed by screws between a polycarbonate frame with through-holes and a pierced stainless steel blade to obtain an oxygen permeable PDMS (O2+/PDMS) plate (ca. 1.9 cm2 in area).16,18
1 was poured into a polystyrene template and then degassed in a vacuum chamber. The template was subsequently heated to 75 °C for 2 h in an electric oven to form a PDMS membrane (ca. 1.0 mm thick). The PDMS membrane was fixed by screws between a polycarbonate frame with through-holes and a pierced stainless steel blade to obtain an oxygen permeable PDMS (O2+/PDMS) plate (ca. 1.9 cm2 in area).16,18
The PDMS membrane was first exposed to O2 plasma for 5 s at 75 W in a Reactive Ion Etching machine (RIE-10NR, Samco, Japan) to generate hydroxyl groups; an aliquot of a 1.0% acetic acid aqueous solution containing 2.0% 3-aminopropyltriethoxysilane (APTES) was then added to the O2+/PDMS plate and incubated for 45 min at 75 °C in the oven. After cooling at room temperature, the PDMS surface was treated with a 200 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer solution containing 0.5 mM SAND (Pierce, P-C21549, Rockford, IL) as a photocross-linker. The SAND-treated PDMS surface was further treated with a 700 mM PNIPA–AESH (monomer unit) aqueous solution at 25 °C for 12 h after UV light irradiation (8.5 mW cm−2). After each treatment, the PDMS plate was thoroughly rinsed with distilled water and 200 mM HEPES buffer solution. PNIPA–AESH was covalently linked to the PDMS surface via APTES and SAND. Consequently, an oxygen permeable PNIPA-modified PDMS (O2+/PNIPA–PDMS) plate was obtained.
In parallel, a PDMS membrane embedded in a TCPS (O2−/PDMS) plate was also prepared as an oxygen impermeable PDMS plate. An aliquot of the mixture of PDMS prepolymer and curing agent was poured into a commercial TCPS plate, degassed in a vacuum chamber, and then cured at 75 °C for 2 h in an oven, generating a PDMS membrane approximately 1.0 mm thick.
2.3. Cell culture
MIN6-m9 cells were a gift from Prof. Seino (Kobe University, Japan)26 and NIH3T3 cells were purchased from the Japanese Collection of Research Bioresources. MIN6-m9 and NIH3T3 cells were mixed and inoculated together at cell densities of 1.2 × 105 and 3.0 × 104 cells cm−2, respectively. These cells were cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM, Wako Pure Chem. Ind., Ltd, Japan) supplemented with 10% fetal bovine serum (Gemini Bio-Products, USA), 50 μM 2-mercaptoethanol, 100 units mL−1 penicillin (Wako), 100 μg mL−1 streptomycin (Wako), and 0.25 μg mL−1 amphotericin B (Sigma-Aldrich, USA) in a CO2 incubator (5% CO2, 37 °C) for 9 days. The culture medium was changed every 1 or 2 days and samples were collected for biochemical assays. The total number of MIN6 and NIH3T3 cells was counted after trypsin/EDTA treatment.2.4. Biochemical assays
To determine the distribution and ratios of MIN6-m9 and NIH3T3 cells in the formed cell sheets, MIN6-m9 and NIH3T3 cells were labeled with the respectively green and red fluorescent lipophilic tracers PKH67 (λex = 490 nm and λem = 504 nm) and PKH26 (λex = 551 nm and λem = 567 nm) (both from Sigma-Aldrich). These cells were seeded and incubated according to the above-described procedure. At the end of the culture period, the resulting cell-loaded plates were treated with PBS containing 0.125% trypsin and 0.01% EDTA and the dispersed PKH67-labeled MIN6-m9 and PKH26-labeled NIH3T3 cells were collected. These fluorescent lipophilic tracers are maintained in both cell membranes even after cell division, although the quantity of tracers per cell decreases. The collected cells were separated and counted by using a fluorescence activated cell sorting (FACS) system (MoFloTM XDP, Beckman Coulter, Inc.).For histochemical staining, cell-loaded PDMS membranes were fixed in phosphate buffered saline (PBS, pH 7.3) containing 4% paraformaldehyde for 12 h at the end of the culture period. The samples embedded in paraffin were transversely sliced in 5 μm sections and then stained with hematoxylin and eosin (HE). The macroscopic morphologies were observed with a transmitted light microscope (MX50, Olympus, Japan).
Glucose consumption and lactate production based on cellular respiration in the culture medium were determined with a YSI 7100 Multiparameter Bioanalytical System (YSI Inc., USA). Insulin secretion was determined as follows: the cell sheet was preincubated in low-glucose (5 mM) DMEM for 30 min and then further incubated in high-glucose (25 mM) DMEM for 2 h. The Insulin concentration in each collected supernatant was measured by a sandwich-type enzyme-linked immuno-sorbent assay (ELISA) using anti-mouse insulin/proinsulin (D3E7) and horseradish peroxidase-conjugated anti-mouse insulin/proinsulin (D6C4) monoclonal antibodies (both from HyTest Ltd).
2.5. Oxygen tension in the culture medium
The oxygen tension (pO2) near the cell layers was measured with a fluorescent oxygen probe (PreSens GmbH, Germany). The probe tip was located near the bottom of the plate next to the cells. The pO2 change on culture days 2 and 10 was monitored for at least 60 min.2.6. Statistical analysis
Unpaired Student's t-test was performed for statistical evaluation. Differences with P < 0.05 (*), P < 0.01 (**), or P < 0.001 (***) were considered significant. The number of replicates is indicated in each figure caption.3. Results and discussion
3.1. Overall cell growth on the PNIPA-modified PDMS membrane
First, to check the effects of direct oxygenation of cells through the PNIPA-modified PDMS membrane, we compared overall growth of heterogeneous cell layers consisting of MIN6-m9 and NIH3T3 cells on the TCPS, O2−/PDMS, O2+/PDMS, and O2+/PNIPA–PDMS plates (Fig. 1A). All heterogeneous cell layers obtained were stably attached to the bottom of the plates for at least 9 days at 37 °C. As expected, the total cell numbers in the O2+/PDMS and O2+/PNIPA–PDMS plates, which ensure direct oxygenation through the bottom of the PDMS membrane, were approximately 2.5 times higher than for the TCPS and O2−/PDMS plates, which do not allow direct oxygenation from the bottom (Fig. 1B). This result shows a similar tendency to our previous report showing the spontaneous growth of human hepatoma Hep G2 cells into a thick cell sheet.16 Note that the number of cells after 9 days of culture had increased about twofold in the TCPS and O2−/PDMS plates, 5.6-fold in the O2+/PDMS plates and 4.9-fold in the O2+/PNIPA–PDMS plates compared with the initial cell number after seeding (the total cell number was about 1.5 × 105 cells cm−2). Additionally, PNIPA showed negligible effects on cell growth as evidenced from the comparison between O2+/PDMS and O2+/PNIPA–PDMS plates. Hence, the cell sheets formed in the O2+/PNIPA–PDMS and TCPS plates were subsequently used for detailed comparison of cell growth, metabolic capacity and insulin secretion.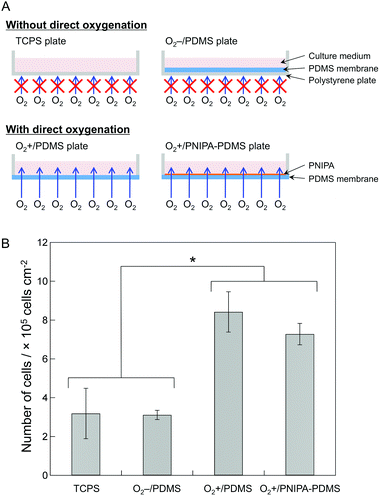 | ||
| Fig. 1 (A) Schematic illustration of the TCPS, O2−/PDMS, O2+/PDMS and O2+/PNIPA–PDMS plates. (B) The total number of cells in the TCPS, O2−/PDMS, O2+/PDMS and O2+/PNIPA–PDMS plates after 9 days of culture. The bars show the mean ± SD of six plates from two independent experiments. Student's t-test was performed, with P < 0.05 indicated by an asterisk (*). | ||
3.2. Morphology of the thick pancreatic β-cell sheets
The thickness of the cell sheets on the O2+/PNIPA–PDMS (with direct oxygenation) and TCPS plates (without direct oxygenation) was compared. According to transverse cross sections, the thickness of the cell sheet in the O2+/PNIPA–PDMS plate was about 60 μm with 5–6 cell layers (Fig. 2A), whereas that in the TCPS plate was about 20 μm, at most, with 2 cell layers (Fig. 2B). This difference was attributed to the number of cells. In addition, the morphology of the cell sheets varied between the different types of plates; in the O2+/PNIPA–PDMS plate, the cell sheet consisted of cuboidal cells, with a viability of about 90%, whereas in the TCPS plate, the cell sheet consisted of flattened cells. Cells grown in the O2+/PNIPA–PDMS plate were therefore similar to in vivo pancreatic β-cells in islets. This can partly explain the difference in the thickness of each type of cell sheet.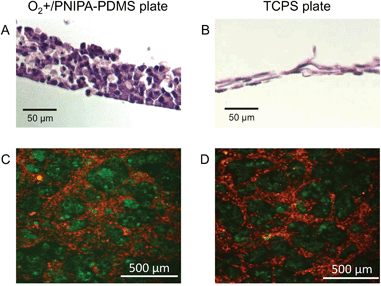 | ||
| Fig. 2 (A and B) Cross section views and (C and D) horizontal cell distribution in the cell sheet on day 10 in the (A and C) O2+/PNIPA–PDMS and (B and D) TCPS plates, respectively. In the horizontal distribution views, MIN6-m9 and NIH3T3 cells were labeled with PKH67 (green) and PKH26 (red), respectively. | ||
Next, the horizontal distribution of fluorophore-labeled MIN6-m9 and NIH3T3 cells in the cell sheet on day 10 was observed by fluorescence microscopy (see the Experimental section). In both plates, conspecific cells gathered and formed separate domains (Fig. 2C and D). More specifically, MIN6-m9 cells tended to form round aggregates similar to pancreatic islets. In contrast, NIH3T3 cells tended to form a network-like structure and filled the interstitial spaces between the MIN6-m9 aggregates, indicating that cells organized themselves in a manner partly mimicking the in vivo tissue structure over the 10 day culture period,27,28 although dispersed, single MIN6-m9 and NIH3T3 cells had been mixed and seeded together onto the TCPS and O2+/PNIPA–PDMS plates on day 1.
The ratio of fluorophore-labeled MIN6-m9 and NIH3T3 cells in the cell sheet on day 10 was further determined by FACS analysis to be about 85.0 ± 2.0 and 15.0 ± 2.0% in the TCPS plate and about 67.0 ± 5.7 and 33.0 ± 5.7% in the O2+/PNIPA–PDMS plate, respectively. In the former, the values were similar to those at cell seeding (80 and 20% for MIN6-m9 and NIH3T3, respectively) because cells did not proliferate much due to lack of oxygen. In the latter, however, the proportion of NIH3T3 cells increased during cell culture, likely due to the fact that the proliferation rate for NIH3T3 cells is higher than for MIN6-m9 cells. Actually, MIN6-m9 and NIH3T3 cells reached 80–90% confluence on days 7 and 4, respectively, when cells were seeded on the TCPS plate at 1.0 × 104 cells cm−2. Thus, the direct oxygenation to cells might give rise to the observed difference in the ratio of MIN6-m9 and NIH3T3 cells in the cell sheet. From these results, the numbers of MIN6-m9 and NIH3T3 cells in the cell sheet on day 10 were estimated to be about 2.7 × 105 and 4.8 × 104 cells cm−2 in the TCPS plate and about 4.9 × 105 and 2.4 × 105 cells cm−2 in the O2+/PNIPA–PDMS plate, respectively. Thus, growth of MIN6-m9 cells in the O2+/PNIPA–PDMS plate was roughly twice as high as that in the TCPS plate.
3.3. Harvest of the thick pancreatic β-cell sheet
We attempted to non-destructively harvest the thick cell sheets from the O2+/PNIPA–PDMS plate surface by changing the temperature. After the O2+/PNIPA–PDMS plate was filled with culture medium at 4.0 °C, the cell sheet detached from the plate surface within 1 min (Fig. 3). In contrast, the cell sheet did not detach from the O2+/PDMS and TCPS plates without PNIPA coating after addition of the culture medium at 4 °C. These results apparently indicate that the hydration of PNIPA below the LCST contributed to the detachment of the cell sheet. However, the retrieved cell sheet was smaller than the bottom surface area of the O2+/PNIPA–PDMS plate. It is very likely that the released cell sheet shrank due to intracellular contraction forces. This shrinkage might make it possible to further increase the number of cells at the grafted area and to produce sheets with even higher cell densities.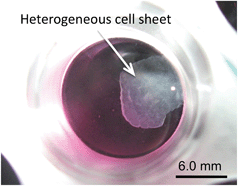 | ||
| Fig. 3 A picture of the cell sheet detached from the bottom surface of the O2+/PNIPA–PDMS plate after filling with the culture medium at 4 °C. | ||
Cell sheets consisting of only MIN6-m9 cells were also formed on the O2+/PNIPA–PDMS plate. However, those homogeneous cell sheets could not be successfully harvested from the plate as the sheets disintegrated after the plate was filled with the culture medium at 4.0 °C (Fig. S1, ESI†). This result suggests that the cell-to-cell adhesion between MIN6-m9 cells was considerably weaker due to the lack of fibroblast NIH3T3 cells, which produce a connective tissue and enhance the mechanical strength of such sheet-like tissues. If such fragile cell sheets need to be harvested from the O2+/PNIPA–PDMS plate, a support membrane, commonly used to harvest single-layer cell sheets,5 should be placed on the cell sheet to maintain its structure; this process, however, is laborious. In the present work, fibroblast cells were needed to form mechanically strong pancreatic β-cell sheets.
3.4. Oxygen tension in the O2+/PNIPA–PDMS plate
Direct oxygenation to cells through the PDMS membrane is important to form a thick cell sheet, as described above. To examine the accurate oxygen tension, pO2, near the cell-layer surface on the TCPS and O2+/PNIPA–PDMS plates, we used a fluorescent oxygen probe. Fig. 4A and B show the time course changes in the pO2 value on days 2 and 10. On day 2 (Fig. 4A), the pO2 value remained constant at about 2% for the TCPS plate and 16% for the O2+/PNIPA–PDMS plate. On day 10 (Fig. 4B), the pO2 value also remained constant at about 5% for the TCPS plate and 17% for the O2+/PNIPA–PDMS plate. The pO2 values on day 2 were particularly lower than on day 10 in the TCPS plate. This is discussed later (section 3.5). In any case, the pO2 was maintained above 15% near the bottom surface of the O2+/PNIPA–PDMS plate after day 2.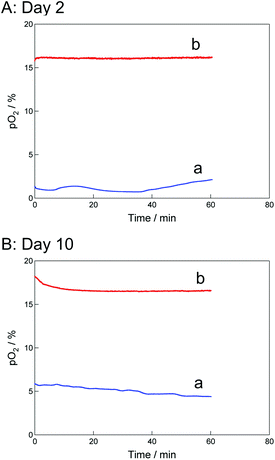 | ||
| Fig. 4 Time course changes in pO2 in the (a) TCPS and (b) O2+/PNIPA–PDMS plates with cultured cell sheets on days (A) 2 and (B) 10. | ||
The theoretical oxygen demand for a cell sheet on the O2+/PNIPA–PDMS plate was estimated to show the need for direct oxygenation to cells.29 Here, a cell sheet consisting of about 7.2 × 105 cells cm−2 with a 2 to 1 ratio of MIN6-m9 and NIH3T3 cells was used for the theoretical calculation. Since the oxygen consumption rate is known to be about 25 amol s−1 cell−1 for pancreatic islet cells30 and 23 amol s−1 cell−1 for fibroblasts,31 the theoretical oxygen demand for the cell sheet was estimated at about 18 pmol s−1 cm−2. At the same time, assuming that the oxygen concentration was nil at the surface of the O2+/PNIPA–PDMS or TCPS plate, we roughly determined the oxygen supply to the cells using Fick's first law. Constant parameters used here are as follows. The oxygen diffusion coefficients in PDMS and the culture medium are 3.4 × 10−5 cm2 s−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 and 2.1 × 10−5 cm2 s−1,33 respectively. The oxygen concentrations in PDMS and the culture medium are 2 mM and 0.204 mM,34,35 respectively. Based on Fick's first equation and the above values, the direct oxygen supply to cells through the PDMS membrane (ca. 1 mm thick) was determined to be about 680 pmol s−1 cm−2. This value was about 37 times larger than the theoretical oxygen demand. This can explain how the pO2 value in the O2+/PNIPA–PDMS plate could be maintained above 15% even after day 2. In contrast, the oxygen supply only through the culture medium was determined to be about 8.6 pmol s−1 cm−2, in the case where the depth of the culture medium was 0.5 cm. The estimated oxygen supply was about half the theoretical oxygen demand, explaining how the pO2 value in the TCPS plate consequently decreased to 5–6% on day 10. Even if the depth of the culture medium was 0.2 cm, which is the minimum depth of the culture medium in conventional protocols, the oxygen supply, estimated to be about 20 pmol s−1 cm−2, would be about 34 times lower than that in the O2+/PNIPA–PDMS plate. Although this estimated oxygen supply may barely fulfill the theoretical oxygen demand, the lower volume of the culture medium might then not supply enough nutrients to the cells.
32 and 2.1 × 10−5 cm2 s−1,33 respectively. The oxygen concentrations in PDMS and the culture medium are 2 mM and 0.204 mM,34,35 respectively. Based on Fick's first equation and the above values, the direct oxygen supply to cells through the PDMS membrane (ca. 1 mm thick) was determined to be about 680 pmol s−1 cm−2. This value was about 37 times larger than the theoretical oxygen demand. This can explain how the pO2 value in the O2+/PNIPA–PDMS plate could be maintained above 15% even after day 2. In contrast, the oxygen supply only through the culture medium was determined to be about 8.6 pmol s−1 cm−2, in the case where the depth of the culture medium was 0.5 cm. The estimated oxygen supply was about half the theoretical oxygen demand, explaining how the pO2 value in the TCPS plate consequently decreased to 5–6% on day 10. Even if the depth of the culture medium was 0.2 cm, which is the minimum depth of the culture medium in conventional protocols, the oxygen supply, estimated to be about 20 pmol s−1 cm−2, would be about 34 times lower than that in the O2+/PNIPA–PDMS plate. Although this estimated oxygen supply may barely fulfill the theoretical oxygen demand, the lower volume of the culture medium might then not supply enough nutrients to the cells.
3.5. Respiratory metabolism of the thick pancreatic β-cell sheet
Next, as an indicator of metabolic capacity, we examined the glucose consumption of the cell sheets in the TCPS and O2+/PNIPA–PDMS plates to characterize their respiratory metabolism. In general, the glycolytic pathway is widely known to be formulated using the following two reactions:36| Glucose + 2ADP + 2Pi → 2lactate + 2ATP | (1) |
| Glucose + 6O2 + 38ADP + 38Pi → 6CO2 + 6H2O + 38ATP | (2) |
Fig. 5A shows changes in glucose consumption over time. The glucose consumption for the cell sheet in both TCPS and O2+/PNIPA–PDMS plates increased up to day 6 and then levelled off. This result indicates cell proliferation. On the other hand, Fig. 5B shows changes in L-lactate production during the same period. Lactate is produced through anaerobic glycolysis, reaction (1). L-Lactate production in the TCPS plate increased during the first 5 days of incubation and then levelled off, whereas that in the O2+/PNIPA–PDMS plate increased for 3 days before levelling off. The final L-lactate production per unit volume in the O2+/PNIPA–PDMS plate was nearly three-quarters that measured in the TCPS plate. This result likely suggests that aerobic and anaerobic glycolyses occurred together in the O2+/PNIPA–PDMS plate. Thus, ATP was efficiently produced, thanks to the direct oxygenation to cells through the PDMS membrane.
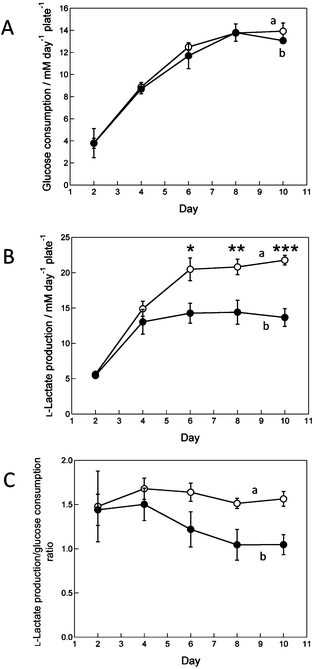 | ||
| Fig. 5 Time course changes in (A) glucose consumption per plate, (B) L-lactate production per plate and (C) the ratio of L-lactate production/glucose consumption in the (a) TCPS and (b) O2+/PNIPA–PDMS plates. The graphs A and B represent the mean ± SD of six plates from two independent experiments. Student's t-test was performed in (A) and (B); asterisks indicate P < 0.05 (*), P < 0.001 (**) or P < 0.0001 (***). | ||
To further characterize the glycolytic pathway, we calculated the molar ratio of L-lactate production to glucose consumption (shown in Fig. 5C). Theoretically, that ratio should be 2 when completely anaerobic glycolysis (reaction (1)) occurs, and 0 when completely aerobic glycolysis (reaction (2)) takes place. The ratio in the TCPS plate was nearly constant at about 1.6 throughout the incubation period, but decreased and then levelled off at about 1.0 in the O2+/PNIPA–PDMS plate. From these ratios, we can deduce that anaerobic and aerobic glycolyses respectively represent about 78.0 and 22.0% in the TCPS plate and 52.5 and 47.5% in the O2+/PNIPA–PDMS plate.
Regarding the relation between respiratory metabolism and pO2 values (Fig. 4), as described above, the pO2 values were almost the same on days 2 and 10 in the O2+/PNIPA–PDMS plate, whereas those on day 2 were slightly lower than on day 10 in the TCPS plate. This difference can be attributed to the respiratory metabolism. On day 2, the cells required large quantities of ATP for their attachment, spreading and proliferation. ATP mainly produced by anaerobic respiration was insufficient, so that the cells’ aerobic respiration pathway was partially enhanced to efficiently produce ATP in both plates. Note that cells seeded in the present work mainly used anaerobic respiration since culture and harvest were performed in commercial TCPS dishes (without direct oxygenation). On day 10, the aerobic respiration of cells in the O2+/PNIPA–PDMS plate further increased and could be maintained, thanks to the higher oxygenation from the bottom of the plate to cells. In fact, the daily glucose consumption per cell was about 13.3 and 9.5 nM cell−1 day−1 on days 2 and 10, whereas the daily L-lactate production per cell was about 19.1 and 9.9 nM cell−1 day−1 on days 2 and 10, respectively, when the number of cells on day 2 is assumed to be 1.5 × 105 cells cm−2. This value is the initial cell density at cell seeding. Based on the glucose consumption and L-lactate production, the molar ratio of produced L-lactate to consumed glucose was estimated at about 1.4 and 1.0 on days 2 and 10, respectively. In the TCPS plate, since ATP mainly produced by anaerobic respiration was enough for cells on day 10 because they had reached a growth plateau, the aerobic respiration decreased due to the lower oxygenation to cells. The glucose consumption was about 13.3 and 23.0 nM cell−1 day−1 on days 2 and 10, whereas the L-lactate production was about 19.7 and 35.9 nM cell−1 day−1 on days 2 and 10, respectively. The produced L-lactate/consumed glucose ratio was about 1.5 and 1.6 on days 2 and 10, respectively. The difference between the two values was low, so that the plots in Fig. 5C show no dramatic change. Thus, after the growth plateau, both anaerobic and aerobic glycolyses took place in the O2+/PNIPA–PDMS plate, whereas in the TCPS plate, glycolysis was mainly anaerobic.
3.6. Insulin secretion capacity of the thick pancreatic β-cell sheet
The insulin secretion capacity of the cell sheets on day 10 was examined after an increase in glucose concentration from 5 mM (generally equivalent to fasting glucose levels) to 25 mM (representing an abnormally high glucose level, e.g. hyperglycemia) in the culture medium. The insulin secretion pathway has been widely investigated.37–39In vivo, pancreatic islets secrete insulin within 10 min after glucose stimulation.Fig. 6A shows time course changes in the insulin secretion per plate after addition of 25 mM glucose. Since the insulin concentration just before addition of 25 mM glucose was almost the same in both TCPS and O2+/PNIPA–PDMS plates (ca. 12.3 ± 4.8 ng cm−2), the concentration was normalized to 1. During the first 30 min, the normalized insulin secretion by the cell sheet in both TCPS and O2+/PNIPA–PDMS plates sharply increased once then decreased; after 30 min, the insulin secretion slightly increased again and then decreased. However, the insulin secretion curve for the O2+/PNIPA–PDMS plate was roughly 1.5 times higher than for the TCPS plate between 10 and 60 min. This difference might be attributed to the total number of MIN6-m9 cells in the plate. However, insulin secretion per MIN6-m9 cell in the O2+/PNIPA–PDMS plate was slightly lower than that in the TCPS plate (Fig. 6B). This result is likely due to the fact that the diffusion coefficient of insulin in islet tissue (5.0 × 10−3 cm2 s−1) is roughly one order of magnitude larger than that of glucose (3.0 × 10−4 cm2 s−1).40 Therefore, insulin secretion is limited by glucose diffusion. Based on this, the insulin secretion per MIN6-m9 cell might depend on the ratio of MIN6-m9 cells per unit volume. Another reason could be that insulin released from MIN6-m9 cells might be rapidly consumed by the MIN6-m9 cells themselves and/or NIH3T3 cells. The consumption rate of insulin in the O2+/PNIPA–PDMS plate was likely higher than in the TCPS plate due to the approximately 2.5 times higher number of cells in the former. Therefore, the net normalized insulin secretion per MIN6-m9 cell in the O2+/PNIPA–PDMS plate might be underestimated.
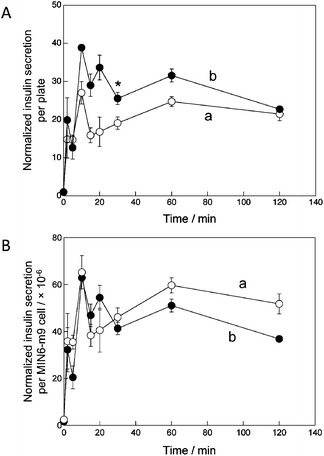 | ||
| Fig. 6 Time course changes in the normalized insulin secretion (A) per plate and (B) per MIN6-m9 cell for the cell sheet on day 10 in the (a) TCPS and (b) O2+/PNIPA–PDMS plates after exposure to 25 mM glucose for 2 h. The graph shows the mean ± SD of three plates. Student's t-test was performed, with P < 0.05 indicated by an asterisk (*). | ||
To solve the problem of mass transport, a vascular network is definitely needed in the thick cell sheet. Vascularization has been previously achieved in layered cell sheets within a few weeks after transplantation in vivo.41,42 If the thick cell sheets obtained here were transplanted in the body, e.g. in the mesentery or subcutaneous layer, active vascularization of the thick cell sheets might occur. Eventually, the vascular network may cause rapid release of insulin from all pancreatic cells into the blood after glucose stimulation without the limitation in mass transport that occurs in vitro culture as discussed above. Hence, future implantation studies are necessary to address these issues.
3.7. Feasibility of the present methodological approach for tissue engineering
In the present paper, we reported that thick cell sheets consisting of both MIN6-m9 and NIH3T3 cells could be formed on O2+/PNIPA–PDMS plates and non-destructively harvested from the plate surface by decreasing the temperature below the LCST. The present method should contribute to the simple formation of thick cell sheets without the need for repeatedly stacking thin cell sheets, which has been one of the major obstacles in cell sheet-based regenerative medicine. In addition, this method can be applied to various types of cells other than pancreatic β-cells, as partially evidenced in our latest reports showing spontaneous multilayered cell growth of hepatic cells under direct oxygenation.16,43We roughly estimated the feasibility of thick pancreatic β-cell sheets for implantation therapy in humans. Assuming that the number of islets in the pancreas and cells in one islet are 1.0 × 106 islets and 2.5 × 102 cells,44 respectively, and considering that the proportion of β-cells in one islet is 54%45 and the cell diameter is 10 μm, the total volume of β-cells in the body is about 0.71 cm3. If a 50 μm high cell sheet is formed from β-cells and fibroblasts with a β-cell to fibroblast ratio of 2 to 1, its area should be about 210 cm2 to reproduce the in vivo volume of β-cells. For comparison, this estimated value is much lower than that of the mesentery (ca. 900 cm2). Thus, the present methodology may be useful for the development of a transplantable thick tissue for diabetes therapy. In addition, this methodology may overcome the initial islet loss occurring during the commonly used islet infusion.
The physiological activity and cellular configuration of tissue-engineered thick cell sheets are not important before but after implantation. Before realizing a cell sheet-based diabetes treatment, we have to address several remaining issues. The cell sheet structure, including cell–cell and cell–extracellular matrix contacts, will have to be investigated in detail after implantation, as vascularization and remodeling of the tissue may occur, as described above. In addition, glucose stimulation and insulin secretion via the neovasculature will also have to be assessed. Instead of insulinoma cells, this method will also have to be applied to normal pancreatic β-cells. In particular, the cell source is a major issue for implantation. Although polymer-encapsulated (and, therefore, immunoisolated) animal islet cells have previously been used for xenograft transplantation,46–48 their insulin secretion gradually decreased over time likely due to cell necrosis because of internal oxygen mass transfer limitation. Recently, insulin-producing cells were generated from stem cells, such as embryonic49,50 and induced pluripotent51 stem cells. Ultimately, these cells should enable cell-based therapy in humans without the need for immunoisolation. The present method might help to form and organize thick cell sheets consisting of such progenitor pancreatic β-cells and harvest them non-destructively for implantation therapy.
Conclusions
We proposed a simple methodology to grow and non-destructively harvest thick cell sheets, thanks to direct oxygenation to the cells through a PNIPA-modified PDMS membrane and the phase transition of PNIPA based on temperature change. Here, thick heterogeneous cell sheets (ca. 60 μm thick) consisting of MIN6-m9 and NIH3T3 cells were successfully obtained and exhibited in vivo-like cuboidal cells. In contrast, the cell sheets grew to only a 20 μm thickness, at most, in the TCPS plate without direct oxygenation from the bottom. The net density of MIN6-m9 cells in the O2+/PNIPA–PDMS plate was about twice as high as in the TCPS plate, allowing the sheet, and, therefore, the transplantation site, to have a higher number of cells per unit area without the need to stack cell monolayers. In addition, the thick cell sheet could be rapidly harvested from the O2+/PNIPA–PDMS plate by lowering the temperature below the LCST. Although vascularization upon implantation is necessary to maintain cell viability and function, the present methodology may contribute to the development of easy-to-handle thick cell sheets for engineered tissue-based therapies.Acknowledgements
We are grateful to Prof. S. Seino (Kobe University) for providing MIN6-m9 cells, Prof. T. Fujii and Dr F. Evenou for their help with the preparation of the PDMS membrane, Prof. T. Tatsuma and Mr H. Karasawa for their help with GPC measurements and Dr N. Kojima for his support during FACS measurements. This work was partially supported by a Grant-in-Aid for Scientific Research (A) (No. 22246101 for YS) and Young Scientist (B) (No. 22710062 for KK) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan.Notes and references
- N. Matsuda, T. Shimizu, M. Yamato and T. Okano, Adv. Mater., 2007, 19, 3089–3099 CrossRef CAS.
- J. Yang, M. Yamato, T. Shimizu, H. Sekine, K. Ohashi, M. Kanzaki, T. Ohki, K. Nishida and T. Okano, Biomaterials, 2007, 28, 5033–5043 CrossRef CAS.
- J. Shapiro, J. R. T. Lakey, E. A. Ryan, G. S. Korbutt, E. Toth, G. L. Warnock, N. M. Kuneteman and R. V. Rajotte, N. Engl. J. Med., 2000, 343, 230–238 CrossRef.
- J. Shapiro, C. Ricordi, B. H. Hering, H. Auchincloss, R. Lindblad, R. P. Robertson, A. Secchi, M. D. Brendel, T. Berney, D. C. Brennan, E. Cagliero, R. Alejandro, E. A. Ryan, B. DiMercurio, P. Morel, K. S. Polonsky, J.-A. Reems, R. G. Bretzel, F. Bertuzzi, T. Froud, R. Kandaswamy, D. E. R. Sutherland, G. Eisenbarth, M. Segal, J. Preiksaitis, G. S. Korbutt, F. B. Barton, L. Viviano, V. Seyfert-Margolis, J. Bluestone and J. R. T. Lakey, N. Engl. J. Med., 2006, 355, 1318–1330 CrossRef.
- H. Shimizu, K. Ohashi, R. Utoh, K. Ise, M. Gotoh, Y. Yamato and T. Okano, Biomaterials, 2009, 30, 5943–5949 CrossRef CAS.
- T. Takezawa, Y. Mori and K. Yoshizato, Bio/Technology, 1990, 8, 854–856 CrossRef CAS.
- T. Okano, N. Yamada, H. Sakai and Y. Sakurai, J. Biomed. Mater. Res., 1993, 27, 1243–1251 CrossRef CAS.
- O. H. Kwon, A. Kikuchi, M. Yamato, Y. Sakurai and T. Okano, J. Biomed. Mater. Res., 2000, 50, 82–89 CrossRef CAS.
- Y. G. Takei, T. Aoki, K. Sanui, N. Ogata, Y. Sakurai and T. Okano, Macromolecules, 1994, 27, 6163–6166 CrossRef CAS.
- T. Yakushiji, K. Sakai, A. Kikuchi, T. Aoyagi, Y. Sakurai and T. Okano, Langmuir, 1998, 14, 4657–4662 CrossRef CAS.
- J. Yang, M. Yamato, C. Kohno, A. Nishimoto, H. Sekine, F. Fukai and T. Okano, Biomaterials, 2005, 26, 6415–6422 CrossRef CAS.
- R. Inaba, A. Khademhosseini, H. Suzuki and J. Fukuda, Biomaterials, 2009, 30, 3573–3579 CrossRef CAS.
- M. Yamato, M. Utsumi, A. Kushida, C. Konno, A. Kikuchi and T. Okano, Tissue Eng., 2001, 7, 473–480 CrossRef CAS.
- J. Lee, R. Nishimura, H. Sakai, N. Sasaki and T. Kenmochi, Cell Transplant., 2008, 17, 51–59 CrossRef.
- M. Nishikawa, N. Kojima, K. Komori, T. Yamamoto, T. Fujii and Y. Sakai, J. Biotechnol., 2008, 133, 253–260 CrossRef CAS.
- F. Evenou, T. Fujii and Y. Sakai, Tissue Eng., Part C, 2010, 16, 311–318 CrossRef CAS.
- F. Evenou, M. Hamon, T. Fujii, S. Takeuchi and Y. Sakai, Biotechnol. Prog., 2011, 27, 1146–1153 CrossRef CAS.
- M. Nishikawa, T. Yamamoto, N. Kojima, K. Komori, T. Fujii and Y. Sakai, Biotechnol. Bioeng., 2008, 99, 1472–2481 CrossRef CAS.
- F. Evenou, T. Fujii and Y. Sakai, Biomed. Microdevices, 2010, 12, 465–475 CrossRef.
- F. Evenou, S. Couderc, B. Kim, T. Fujii and Y. Sakai, J. Biomater. Sci., Polym. Ed., 2011, 22, 1509–1522 CrossRef CAS.
- S. G. Charati and S. A. Stern, Macromolecules, 1998, 31, 5529–5535 CrossRef CAS.
- A. Rabinovitch, T. Russell and D. H. Mintz, Diabetes, 1979, 28, 1108–1113 CAS.
- A. Miki, M. Narushima, T. Okitsu, Y. Takeno, A. Soto-Gutierrez, J. D. Rivas-Carrillo, N. Navarro-Alvarez, Y. Chen, K. Tanaka, H. Noguchi, S. Matsumoto, M. Kohara, J. R. Lakey, E. Kobayashi, N. Tanaka and N. Kobayashi, Cell Transplant., 2006, 15, 325–334 CrossRef.
- Y. Kaneko, K. Sakai, A. Kikuchi, R. Yoshida, Y. Sakurai and T. Okano, Macromolecules, 1995, 28, 7717–7723 CrossRef CAS.
- K. Komori, K. Takada and T. Tatsuma, Anal. Sci., 2005, 21, 351–353 CrossRef CAS.
- K. Minami, H. Yano, T. Miki, K. Nagashima, C.-Z. Wang, H. Tanaka, J. Miyazaki and S. Seino, Am. J. Physiol. Endocrinol. Metab., 2000, 279, E773–E781 CAS.
- N. Kojima, S. Takeuchi and Y. Sakai, Biomaterials, 2011, 32, 6059–6067 CAS.
- N. Kojima, S. Takeuchi and Y. Sakai, Biomaterials, 2012, 33, 4508–4514 CrossRef CAS.
- Y. Sakai, M. Nishikawa, F. Evenou, M. Hamon, H. Huang, K. P. Montagne, N. Kojima, T. Fujii and T. Niino, in Liver Stem Cells, ed. T. Ochiya, Hamana Press, New York, 2011, pp. 189–216 Search PubMed.
- N. E. Mukundan, P. Christopher Flanders, I. Constantinidis, K. K. Papas and A. Sambanis, Biochem. Biophys. Res. Commun., 1995, 210, 113–118 CrossRef CAS.
- P. C. Thomas, M. Halter, A. Tona, S. R. Raghavan, A. L. Plant and S. P. Forry, Anal. Chem., 2009, 81, 9239–9246 CrossRef CAS.
- T. C. Merkel, V. I. Bondar, K. Nagai, B. D. Freeman and I. Pinnau, J. Polym. Sci., Part B: Polym. Phys., 2000, 38, 415–434 CrossRef CAS.
- G. Mehta, K. Mehta, D. Sud, J. W. Song, T. Bersano-Begey, N. Futai, Y. S. Heo, M. A. Mycek, J. J. Linderman and S. Takayama, Biomed. Microdevices, 2007, 9, 123–134 CrossRef CAS.
- T. Kamiya, T. Naito, T. Hirose and K. Mizoguchi, J. Polym. Sci., Part B: Polym. Phys., 1990, 28, 1297–1308 CrossRef.
- H. Shiku, T. Saito, C.-C. Wu, T. Yasukawa, M. Yokoo, H. Abe, T. Matsue and H. Yamada, Chem. Lett., 2006, 35, 234–235 CrossRef CAS.
- K. G. Alberti, J. Clin. Pathol., 1977, s3–11, 14–20 CrossRef.
- J.-C. Henquin, Diabetes, 2000, 49, 1751–1760 CrossRef CAS.
- S. G. Straub and G. W. G. Sharp, Diabetes/Metab. Res. Rev., 2002, 18, 451–463 CrossRef CAS.
- P. E. MacDonald, J. W. Joseph and P. Rorsman, Philos. Trans. R. Soc. London, Ser. B, 2005, 360, 2211–2225 CrossRef CAS.
- P. Buchwald, Theor. Biol. Med. Modell., 2011, 8, 20 CrossRef CAS.
- T. Shimizu, M. Yamato, Y. Isoi, T. Akutsu, T. Setomaru, K. Abe, A. Kikuchi, M. Umezu and T. Okano, Circ. Res., 2002, 90, e40–e48 CrossRef CAS.
- S. Sekiya, T. Shimizu, M. Yamato, A. Kikuchi and T. Okano, Biochem. Biophys. Res. Commun., 2006, 341, 573–582 CrossRef CAS.
- M. Hamon, S. Hanada, T. Fujii and Y. Sakai, Cell Transplantation, 2012, 21, 401–410 CrossRef.
- G. C. Weir and S. Bonner-Weir, J. Clin. Invest., 1990, 85, 983–987 CrossRef CAS.
- M. Brissova, M. J. Fowler, W. E. Nicholson, A. Chu, B. Hirshberg, D. M. Harlan and A. C. Powers, J. Histochem. Cytochem., 2005, 53, 1087–1097 CrossRef CAS.
- M. Qi, Y. Gu, N. Sakata, D. Kim, Y. Shirouzu, C. Yamamoto, A. Hiura, S. Sumi and K. Inoue, Biomaterials, 2004, 25, 5885–5892 CrossRef CAS.
- A. Murua, A. Portero, G. Orive, R. M. Hernández, M. de Castro and J. L. Pedraz, J. Controlled Release, 2008, 132, 76–83 CrossRef CAS.
- N. Sakata, S. Sumi, G. Yoshimatsu, M. Goto, S. Egawa and M. Unno, World Journal of Gastrointestinal Pathophysiology, 2012, 3, 19–26 CrossRef.
- S. Assady, G. Maor, M. Amit, J. Itskovitz-Eldor, K. L. Skorecki and M. Tzukerman, Diabetes, 2001, 50, 1691–1697 CrossRef CAS.
- Y. Shi, Methods Mol. Biol., 2010, 636, 79–85 Search PubMed.
- K. Tateishi, J. He, O. Taranova, G. Liang, A. C. D'Alessio and Y. Zhang, J. Biol. Chem., 2008, 283, 31601–31607 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3bm00123g |
| This journal is © The Royal Society of Chemistry 2013 |
