Preparation of porous PLGA/Ti biphasic scaffold and osteochondral defect repair
Chaoyong
Zhao
a,
Hongfang
Zhang
b,
Bing
Cai
c,
Guanglin
Wang
b,
Hongsong
Fan
*a and
Xingdong
Zhang
a
aNational Engineering Research Center for Biomaterials, Sichuan University, Chengdu 610064, China. E-mail: hsfan@scu.edu.cn; Fax: +86-28-85410246; Tel: +86-28-85410703
bDepartment of Orthopedics, West China Hospital, Sichuan University, Chengdu 610064, China
cAnalytical and Testing Center, Sichuan University, Chengdu 610064, China
First published on 27th March 2013
Abstract
In this study, a porous PLGA/Ti biphasic scaffold was prepared and its potential to repair osteochondral defects was evaluated. The microstructure and mechanical properties of porous PLGA and porous Ti and their interface were characterized. The as-prepared biphasic scaffolds were implanted into rabbit knees for 1 and 3 months (PLGA/Ti group), and the porous PLGA (PLGA group) or empty defect (Untreated group) was also used. The results showed that both porous PLGA and porous Ti had an interconnected porous structure. The mechanical strength of porous PLGA and porous Ti was similar to that of cartilage and subchondral bone, respectively. The interface analysis revealed that the as-prepared biphasic scaffold had good overall integrity and interface stability. The gross observation and histological evaluation of specimens showed that hyaline-like cartilage filled the defects to a certain extent at 3 months for both PLGA/Ti and PLGA groups while the defects remained in an untreated group. In the PLGA/Ti group, better cartilage and subchondral bone repair was observed, and quantitative macroscopic and histological score evaluations confirmed this result, indicating that the porous biphasic PLGA/Ti scaffold had good biocompatibility and the best osteochondral defect repair ability in this study. The as-prepared porous biphasic scaffold showed the potential to be used in osteochondral tissue engineering for osteochondral defect repair.
1. Introduction
Osteochondral defects resulting from trauma or joint diseases can induce mechanical instability of the joint and run the risk of advancing to severe osteoarthritis without treatment.1 Moreover, mature articular cartilage has a limited self-repairing capacity.2 Although mosaicplasty was reported to yield encouraging results, the clinical use of autologous osteochondral grafts was usually associated with several problems, such as limited availability of material, the donor site morbidity and the conformity of the articular surface.1,3–5 One alternative option came from osteochondral allografts but it involved the risk of disease transmission and an immunogenic response.4Osteochondral tissue engineering provided a promising alternative strategy to overcome these limits,5 and the key issue in regenerating both cartilage and subchondral bone was to seek an appropriate scaffold.6 As osteochondral defects involved two different tissues with different intrinsic healing capacities, a biphasic scaffold was preferred to meet the different mechanical and biological requirements for guiding the regeneration of both tissues.1,5 The chondral phase should allow the cell recruitment, proliferation and/or differentiation of the seeding cells along the chondrogenic pathway and the subsequent deposition of extracellular matrix (ECM). The osseous phase should not only have enough mechanical properties to provide support for the overlying cartilage, but also possess good osteoconductivity to integrate with the host bone for anchoring the scaffold,7–10 as the integration of a bone-to-bone interface was better and faster than that of a cartilage-to-cartilage interface.11
Poly(lactic-co-glycolic acid) (PLGA) was one of the synthetic polymers approved by the FDA for clinical applications.12 At present, it is widely used for various medical applications, such as bone tissue engineering scaffold and drug delivery.13,14 Also, PLGA was used in cartilage tissue engineering, for example, PLGA scaffolds seeded with autologous chondrocytes or cultured mesenchymal stem cells (MSCs) could yield hyaline-like cartilage in vivo.7,15 Compared with natural polymers, such as collagen, hyaluronic acid and chitosan, PLGA had good mechanical properties and an adjustable degradation rate.12 Therefore, it was highly preferred as a chondral phase to regenerate articular cartilage.
Reconstruction of subchondral bone was also important as it provided mechanical support for the regeneration of articular cartilage.6 Lots of publications have already used porous biodegradable and bioresorbable ceramic scaffolds, polymer scaffolds or their composite scaffolds as the osseous phase for the subchondral bone reconstruction.1,2 However, the unsatisfactory mechanical property of these scaffold materials might restrict their applications for the repair of subchondral bone defects under load-bearing conditions. Porous titanium (Ti) scaffolds provided an advantageous alternative as load-bearing bone repair materials. It possessed a good biocompatibility and superior mechanical properties. As well, the elastic modulus of porous titanium scaffold could be adjusted by altering its porosity to match with that of trabecular bone for eliminating stress shielding, thereby preventing bone resorption at the implant interface.16,17 Furthermore, the interconnected porous structure of porous titanium which mimicked the architecture of trabecular bone would permit the transportation of body fluids and be beneficial for bone tissue ingrowth to form biological fixation, thus enhancing the interfacial stability between implants and its surrounding bone tissue.18,19 Many in vitro and in vivo investigations have proved the good biological properties of porous titanium for bone defect repair.18,20–23 Therefore, porous titanium showed a promising potential to repair the defects of subchondral bone, especially under load-bearing conditions.
Integration between the chondral phase and the osseous phase was a critical issue.2 One approach was to engineer the two phases independently and combine into an osteochondral composite prior to implantation.1,5 However, it was not easy to achieve the integration, and it was usually approached through suturing or using glues.11,24,25 Another approach was to prepare a well-integrated biphasic scaffold prior to cell culture. The thus-engineered osteochondral tissue showed a better potential for the reconstruction of articular cartilage.7,8,10,26
In view of the good cartilage repairing ability of porous PLGA scaffold7,15,27 and the excellent mechanical and biological properties of porous titanium scaffold, we tried to develop a porous PLGA/titanium biphasic scaffold by using a combination of solvent merging/particulate leaching method and a uniaxially pressing method and evaluated its potential for the repair of osteochondral defects in a rabbit model used in this study. Up to now, few researchers have attempted to develop porous degradable polymer/metal biphasic scaffolds for repairing osteochondral defects.
2. Materials and methods
2.1. Materials
PLGA (molar ratio 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, average molecular weight 200
25, average molecular weight 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Jinan Dai Gang Biotechnology Co., Ltd, China. Silver nitrate was purchased from Aladdin reagent, China. 10 wt% chloral hydrate was provided by Experimental Animal Center of West China, Sichuan University. The type II collagen monoclonal antibody was from Acris, Germany (no. AF5710). Other chemicals and reagents were purchased from Chengdu Kelong Chemical Co., Ltd, China.
000) was purchased from Jinan Dai Gang Biotechnology Co., Ltd, China. Silver nitrate was purchased from Aladdin reagent, China. 10 wt% chloral hydrate was provided by Experimental Animal Center of West China, Sichuan University. The type II collagen monoclonal antibody was from Acris, Germany (no. AF5710). Other chemicals and reagents were purchased from Chengdu Kelong Chemical Co., Ltd, China.
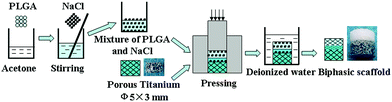 | ||
| Fig. 1 Schematic illustration of the preparation process for the porous biphasic scaffold. | ||
It is described in detail as follows. 5 g PLGA particles were dissolved in 50 ml acetone. NaCl particles with a size of a 40–80 mesh were added to the solution in 85/15 weight ratio and stirred uniformly. After solvent evaporation, the mixture of PLGA and NaCl was placed on the top of the porous Ti inside a mould (5 mm in diameter), and an uniaxial pressure about 4 MPa was applied to force the mixture into the outer pores of porous Ti, and held for 2 min. Then, the received composites were placed in a fume hood for at least 24 h to remove the residual solvent. After that, the specimens were immersed in deionized water to remove the salt, and the water was refreshed every 5 h. 0.1 M L−1 silver nitrate solution was used to examine the remnants of salt in the water until no white deposits were found. After drying in the fume hood, the biphasic scaffold with 5 mm in diameter and 5 mm in height (2 mm in height of the scaffold was PLGA, serving as the chondral phase) was obtained and stored until use. The porous PLGA scaffold was also prepared by using a similar method.
2.2. Materials characterization
The structure of porous Ti and porous PLGA was characterized by scanning electron microscopy (SEM). Their pore size distributions were measured by using Image Analysis Software (Image Pro plus 6.0 software). The microstructure and composition at the interface between porous Ti and porous PLGA were analyzed by SEM with energy dispersive X-ray spectroscopy (EDX). Compressive tests of both porous Ti (∅4 × 10 mm) and porous PLGA (∅5 × 13 mm) were conducted by using a Material Testing Machine (Rising Sun Testing Instruments Co., WDW-50, China) at a loading rate of 1 mm min−1. The stress–strain curves were used to obtain the compressive strength of the porous Ti and porous PLGA scaffolds and calculate their elastic modulus.2.3. Animal experiments
This animal study was done with the approval of the Animal Care and Use Committee of Sichuan University. Twelve New Zealand white rabbits weighing 2.5–3.0 kg (3–4 months old), which were supplied by Experimental Animal Center of West China, Sichuan University, were used in this experiment. Male and female animals were used randomly. The rabbits were kept in good ventilated and laboratory animal facility rooms, maintained singly in cages and fed at regular intervals. All rabbits received bilateral knee joint surgery. The rabbits were anesthetized by an intraperitoneal injection of 10 wt% chloral hydrate with a dose of 3 ml kg−1 body weight, and then penicillin was administered by intramuscular injection. The hind limbs of rabbits were shaved, sterilized and covered with a sterile towel. Under general sterile conditions, the knee joint was opened with a medial parapatellar approach. The patella was dislocated laterally and the articular surface of the femoropatellar groove was exposed. The osteochondral defect 5.2 mm in diameter and 5 mm in depth was created using an electric drill equipped with a 5.2 mm diameter drill bit. After irrigating the defect with sterile saline, it was randomly treated with porous biphasic scaffold (PLGA/Ti group), porous PLGA scaffold (PLGA group) or empty defect (Untreated group), as shown in Fig. 2. Four animals per treatment group were used. Joint capsule and skin were carefully sutured separately. After operation, the rabbits were allowed to move freely in cages without immobilization. They were sacrificed at 1 and 3 months and the distal femurs were retrieved. The maintenance and handling of experimental animals were performed according to the guidelines for animal experiments of Experimental Animal Center of West China, Sichuan University.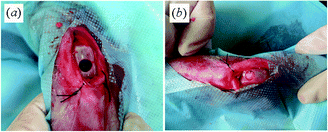 | ||
| Fig. 2 Surgical procedures. (a) Osteochondral defect (∅5.2 mm, depth 5 mm) in the patella groove of the femoral articular cartilage. (b) Scaffold was inserted into the osteochondral defect. Bleeding from the subchondral bone was visible shortly after implantation. | ||
2.4. Macroscopic and histological evaluation
The retrieved distal femurs were examined macroscopically and photographed with a digital camera for evaluation.In order to analyze cartilage regeneration on articular surface and bone ingrowth into and around the porous Ti implants, the gross non-decalcified histological sections were performed along the direction of osteochondral defect. The retrieved samples were fixed in 4% paraformaldehyde solution for 7 days, half of the samples were dehydrated in increasing concentrations of ethanol from 70 to 100%, embedded in methyl methacrylate that polymerized at 37 °C for a week. The non-decalcified histological sections of about 100 μm thickness were made by using a diamond saw (Leica microtome, Germany) and stained with toluidine blue.
In order to further analyze cartilage regeneration on articular surfaces, decalcified sections of the regenerated cartilage were made. After being fixed in 4% phosphate-buffered paraformaldehyde solution for 7 days, the regenerated cartilage was decalcified in a sodium citrate–formic acid solution for about 2 weeks, embedded in paraffin and sectioned at 5 μm thickness for hematoxylin and eosin (HE), Safranin O, toluidine blue and immunohistological staining for collagen type II. Both non-decalcified histological sections and decalcified sections were observed by using an optical microscope (Olympus B×60, Japan) equipped with a digital camera.
The macroscopic appearance and histological findings of the repair tissue were also assessed blindly using an 8-point (Table 1) and 28-point (Table 2) grading scale proposed by Niederauer et al.,30 respectively.
| Edge integration (new tissue relative to native cartilage) | Full | 2 |
| Partial | 1 | |
| None | 0 | |
| Smoothness of the cartilage surface | Smooth | 2 |
| Intermediate | 1 | |
| Rough | 0 | |
| Cartilage surface, degree of filling | Flush | 2 |
| Slight depression | 1 | |
| Depressed/overgrown | 0 | |
| Color of cartilage, opacity or translucency of the neocartilage | Transparent | 2 |
| Translucent | 1 | |
| Opaque | 0 |
| Characteristic | Score | |
|---|---|---|
| I. Nature of predominant tissue | Hyaline cartilage | 4 |
| Mostly hyaline cartilage | 3 | |
| Mixed hyaline and fibrocartilage | 2 | |
| Mostly fibrocartilage | 1 | |
| Some fibrocartilage, mostly non-chondrocytic cells | 0 | |
| II. Structural characteristics | ||
| A. Surface regularity | Smooth and intact | 3 |
| Superficial horizontal lamination | 2 | |
| Fissures | 1 | |
| Severe disruption, including fibrillation | 0 | |
| B. Structural integrity, homogeneity | Normal | 2 |
| Slight disruption, including cysts | 1 | |
| Severe disintegration, disruptions | 0 | |
| C. Thickness | 100% of normal adjacent cartilage | 2 |
| 50–100% of normal cartilage | 1 | |
| 0–50% of normal cartilage | 0 | |
| D. Bonding to adjacent cartilage | Bonded at both ends of graft | 2 |
| Bonded at one end or partially at both ends | 1 | |
| Not bonded | 0 | |
| III. Freedom from cellular changes of degeneration | ||
| A. Hypocellularity | Normal cellularity | 2 |
| Slight hypocellularity | 1 | |
| Moderate hypocellularity or hypercellularity | 0 | |
| B. Chondrocyte clustering | No clusters | 2 |
| <25% of the cells | 1 | |
| 25–100% of the cells | 0 | |
| IV. Freedom from degenerative changes in adjacent cartilage | Normal cellularity, no clusters, normal staining | 3 |
| Normal cellularity, mild clusters, moderate staining | 2 | |
| Mild hypocellularity, slight staining | 1 | |
| Severe hypocellularity, poor or no staining | 0 | |
| V. Subchondral bone | ||
| A. Reconstruction of subchondral bone | Normal | 3 |
| Reduced subchondral bone reconstruction | 2 | |
| Minimal subchondral bone reconstruction | 1 | |
| No subchondral bone reconstruction | 0 | |
| B. Inflammatory response in subchondral bone | None/mild | 2 |
| Moderate | 1 | |
| Severe | 0 | |
| VI. Safranin-O staining | Normal or near normal | 3 |
| Moderate | 2 | |
| Slight | 1 | |
| None | 0 |
2.5. Statistical analysis
The unpaired one-tailed Student's t-test was used to investigate the differences in score for the macroscopic appearance and histological findings of the repair tissue between the groups, and p < 0.05 was considered significant.3. Results
3.1. Scaffold characterization
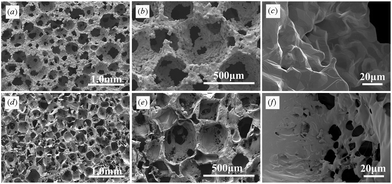 | ||
| Fig. 3 SEM photographs of (a–c) porous Ti and (d–f) porous PLGA at magnifications of (a, d) 35×, (b, e) 100× and (c, f) 1000×. The pores of both porous titanium and porous PLGA were highly interconnected and were almost spherical. Macropore walls contained micropores (c, f). | ||
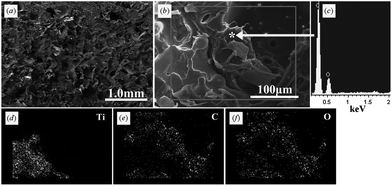 | ||
| Fig. 4 Microstructure and composition at the interface between porous titanium and porous PLGA. (a) and (b) SEM image of the interface. (c) EDX analysis of substance within the outer pores of porous titanium. (d–f) SEM-EDX mapping images of the interface. Ti, titanium; C, carbon; O, oxygen. PLGA was successfully inserted into the outer pores of porous titanium. | ||
3.2. Evaluation for the osteochondral defect repair ability of the biphasic scaffold
At 1 month after implantation, the gross appearance of the defects in PLGA/Ti and PLGA groups was quite similar. Their surfaces were filled with thin translucent tissue and the margins of the defects could be clearly recognized. Most of the scaffolds in both groups were not degraded and could be clearly distinguished (Fig. 5a and b). The defects in the untreated group were almost empty, and the bottoms of the defects were covered with a thin layer of white tissue (Fig. 5c).
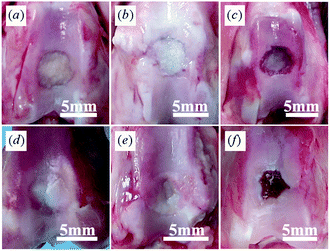 | ||
| Fig. 5 Gross appearance of (a, d) PLGA/Ti group, (b, e) PLGA group and (c, f) Untreated group at (a–c) 1 month and (d–f) 3 months after operation. PLGA/Ti group showed the best repairing ability compared with both PLGA and untreated groups at 3 months after operation. | ||
At 3 months, the defects in the PLGA/Ti group were almost completely covered with an opaque white cartilage-like tissue (Fig. 5d). The smoothness of the surface was obviously improved. The newly formed tissue integrated well with the host cartilage and the margins between the regenerated tissue and the host cartilage became obscured. In the PLGA group, small portions of surfaces were still not filled with thick tissue, and the scaffolds remained visible (Fig. 5e). In the untreated group, the defects showed some tissue regeneration in the peripheral areas, but the regenerated tissue was still irregular and concave (Fig. 5f). The macroscopic scores indicated better results in the PLGA/Ti group (4.0 ± 0.8) and the PLGA group (2.5 ± 0.6) compared with the control group (1.3 ± 0.5), and the PLGA/Ti group showed the best gross appearance (p < 0.05).
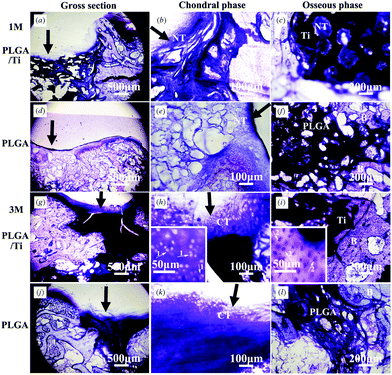 | ||
| Fig. 6 Non-decalcified histological sections of (a–c, g–i) PLGA/Ti group and (d–f, j–l) PLGA group at 1 month (a–f) and 3 months (g–l) after operation. The photos of (b, e, h, k) were the local magnification of chondral phase (200×) in the photos of (a, d, g, j), respectively, and the photos of (c, f, i, l) were the local magnification of osseous phase (100×) in the photos of (a, d, g, j), respectively. Insertions were enlarged views of the cartilage-like area and subchondral bone area. Stain: Toluidine-blue. NT: Newly formed tissue, CT: Cartilage-like tissue, B: bone. The black arrow pointed to the repair interface of cartilage, arrow 1 pointed to the chondrocytes and arrow 2 pointed to the osteocytes. | ||
Fig. 7 shows the decalcified sections to further analyze the cartilage regeneration on the articular surface at 3 months after operation. In the PLGA/Ti group, HE staining for structural details and cell appearance showed that the regenerated cartilage-like tissue in the margin of the defect was more mature than that in the center and chondrocytes with well demarcated lacunae presented in the margin of regenerated areas (Fig. 7a and b), but some areas in the center of the cartilage defect were not well regenerated (Fig. 7a). Toluidine blue staining for cartilaginous matrix showed metachromasia in the margin of the defect (Fig. 7c). The strong positive staining of Safranin-O for sulfated glycosaminoglycan (Fig. 7g and h) and immunohistochemical staining for collagen type II (Fig. 7i) revealed the secretion of cartilage extracellular matrix in the margin of the defect, indicating the formation of hyaline-like cartilage. While in the PLGA group, HE staining showed that the regenerated cartilage-like tissue was inferior to that in the PLGA/Ti group (Fig. 7d and e). Toluidine blue staining showed that less lacunae were observed (Fig. 7f), indicating that the regenerated cartilage-tissue was not mature. Safranin-O staining for sulfated glycosaminoglycan and immunohistochemical staining for collagen type II was weakly positive (Fig. 7j–l), indicating less secretion of cartilage extracellular matrix. In general, although the regenerated tissue in the PLGA group still showed metachromasia and positive staining for the cartilage extracellular matrix, the staining was obviously weaker than that in the PLGA/Ti group, indicating the poor cartilage defect repair ability of the PLGA scaffold alone. The total histological scores in the PLGA/Ti group (12.3 ± 3.0) were significantly higher than those in the PLGA group (8.8 ± 1.7) (p < 0.05). The histological score evaluation confirmed the result of the macroscopic score evaluation.
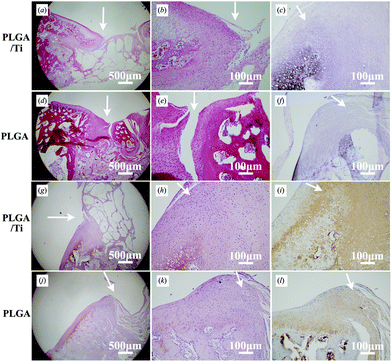 | ||
| Fig. 7 Decalcified histological sections of (a–c, g–i) the PLGA/Ti group and (d–f, j–l) the PLGA group at 3 months after operation at magnifications of (a, d, g, j) 40× and (b, c, e, f, h, i, k, l) 200×. The defects were stained with HE (a, b, d, e), toluidine-blue (c, f), safranin-O (g, h, j, k) and immunohistochemically stained with collagen type II (i, l). The arrows pointed to the repair interface of cartilage. | ||
In all, both non-decalcified and decalcified histological observations demonstrated that the regenerated tissue in the margin of the defect was hyaline-like cartilage, but the PLGA/Ti group showed a better repair ability for osteochondral defects than the PLGA group.
4. Discussion
As natural osteochondral tissue had distinctly mechanical and biological properties, it was advantageous to prepare a biphasic scaffold to regenerate both cartilage and bone tissue.1,5 In this study, we designed a biphasic scaffold with porous PLGA as the chondral phase for cartilage regeneration and porous Ti as the osseous phase for bone reconstruction. As shown in this study, porous PLGA had a low mechanical strength similar to cartilage and porous Ti had a relatively high mechanical strength similar to subchondral bone. Especially, their elastic modulus (14.18 MPa for porous PLGA and 3.77 GPa for porous Ti) was optimized to be close to that of natural cartilage (0.1–2 MPa) or subchondral bone (1–15 GPa),32 which would be beneficial for tissue reconstruction. Furthermore, the porous structure and the optimized pore size range of porous PLGA and Ti would allow for cell invasion and tissue ingrowth (Fig. 3).33 However, the integration at the interface between the chondral and osseous phases was still a challenging problem. We successfully integrated the chondral phase and osseous phase to form a porous PLGA/Ti biphasic scaffold by using a combination of a solvent merging/particulate leaching method and an uniaxially pressing method. The good interface stability and overall integrity of the porous PLGA/Ti biphasic scaffold was achieved by partially pressing the PLGA into the outer pores of porous Ti to form a mechanical interlock (Fig. 4).As expected, the as-prepared porous PLGA/Ti biphasic scaffold showed the best repair ability for osteochondral defects among the three groups. This phenomenon might be explained by the role of scaffolds in the process of chondrogenesis and osteogenesis as described in Fig. 8. The graphical illustration in Fig. 8 was adapted from the work of Ho et al.34 In our experimental models, there was no pre-addition of any chondrogenic or osteogenic cells in the scaffolds. Therefore, the cells supporting the following osteochondral repair most likely came from the bone marrow around the defects, in which many native progenitor cells, chondrogenic or osteogenic growth factors and other nutrition were present and could migrate into the scaffolds,35,36 as shown in Fig. 8. This was also demonstrated by Fig. 2b that bleeding from the subchondral bone marrow into the scaffolds during operation, was entrapped within the pores of the biphasic scaffold. The growth factors within it would signal for the migration of the native progenitor cells.34 However, the following fate of the migrated native progenitor cells was different due to the different models among the three groups. In the untreated osteochondral defects, the absence of a scaffold as a temporary artificial ECM to accommodate the recruited native progenitor cells made the cells difficult to receive proper stimuli to guide cell proliferation and differentiation along the targeted cells.37 Hence, the recruited native progenitor cells could not reach the articular surface due to the lack of scaffolds for cell migration and attachment and thus physiological stress could not transfer to these cells (Fig. 8a). Accordingly, after 3 months operation, the defects did not heal (Fig. 5f). While in the other two groups with scaffolds filling the defects, the osteochondral defects were repaired to a certain extent, which demonstrated that scaffolds play an important role in accommodating the recruited native progenitor cells and providing a good microenvironment for osteogenic and chondrogenic differentiation of these cells. The scaffolds in both PLGA/Ti and PLGA groups had good interconnectivity, which not only supplied stable substrates for cell attachment, but also allowed the native progenitor cells to migrate to the inner pores of scaffolds and the articular surface.9 Then the scaffolds and the native growth factors might regulate the differentiation of recruited cells along a chondrogenic or osteogenic pathway (Fig. 8b and c). However, because of the varied biomimetic structure of both scaffolds, the cell fate might be different, and thus the osteochondral defect repair ability would be varied. It is known that osteochondral tissue is composed of two parts, the articular cartilage and the underlying subchondral bone.32 As mentioned above, the properties of porous PLGA are more similar to that of cartilage tissue and the properties of porous Ti more similar to that of bone tissue. As illustrated in Fig. 8b and c, due to the multilineage potential of native progenitor cells,38 the cells migrated into the scaffolds might have the potential to differentiate along a chondrogenic or an osteogenic pathway, depending on both the growth factors and the scaffold property. This cell differentiation function which depended on the properties of materials has been reported in many materials both in vitro and in vivo.9,10,24 Therefore, a more osteo-biomimetic scaffold of porous Ti might be able to stimulate higher osteogenic differentiation than PLGA did, while the upper PLGA phase might be more beneficial for chondrogenic differentiation.15 Consequently, a better bone repair was achieved by porous Ti which was proved in this study (Fig. 6). This better reconstruction of subchondral bone in PLGA/Ti group provided mechanical support for the regenerated cartilage tissue and in turn promoted the repair of its upper cartilage.6 Therefore, the PLGA/Ti group showed the best repair ability for cartilage defects in this study.
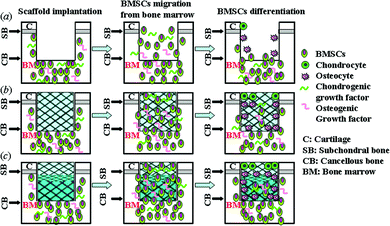 | ||
| Fig. 8 Schematic illustration of the possible mechanism of osteochondral defect repair by scaffolds. (a) Untreated group. (b) PLGA group and (c) PLGA/Ti group. | ||
Although the osteochondral defects of rabbits were partly repaired by using a porous PLGA/Ti biphasic scaffold alone for 3 months after surgery and no adverse tissue reactions were observed, it should be noted that some areas at the center of the cartilage defect were not well regenerated, indicating that a biphasic scaffold alone in this study was insufficient for an effective osteochondral defect repair before 3 months. Nagura et al. reported that cartilage and subchondral bone was regenerated treated by a porous PLGA scaffold in a similar rabbit model after 24 weeks implantation without cultured cells and growth factors.27 Uematsu et al. seeded cultured mesenchymal stem cells (MSCs) on a three-dimensional special PLGA scaffold and implanted into large defects in rabbit knees. Smooth hyaline-like cartilage was formed after 12 weeks postoperatively.15 Jiang et al. implanted a porous PLGA/PLGA-TCP biphasic osteochondral scaffold into osteochondral defects of femoral condyles of mini-pigs for 6 months and found that only fibrous tissue formed on the surface of defects, while the hyaline cartilage was regenerated in the scaffold seeded with the autologous chondrocytes group.7 As the osteochondral defects of rabbits were partly repaired by using a porous PLGA/Ti biphasic scaffold in a short period and good repairing effects of porous PLGA scaffold were reported by prolonging the implantation time or pre-seeding of mesenchymal stem cells or chondrocytes in the scaffolds, we infer that prolonging the implantation time or pre-seeding of mesenchymal stem cells or chondrocytes in the scaffolds might further improve the repairing ability of the thus-prepared PLGA/titanium biphasic scaffold in this study. To date, many researchers have developed different scaffolds for the repair of osteochondral defect and most of them have gained good outcomes in their studies by using the scaffold alone.1,2 However, most of the current investigations used different animal species of different defect size, age, implantation sites and implantation time, so it was difficult to compare the results of this study with other similar studies. As the as-prepared porous PLGA/Ti biphasic scaffold in this study had good biocompatibility and osteochondral defect repair ability, especially good mechanical properties which could be used for the osteochondral defect repair under load-bearing conditions, it showed some clinical potential.
The prepared PLGA/titanium biphasic scaffold had one more advantage. Its osteochondral repairing ability could be optimized in many aspects. The chondral phase (Porous PLGA) of the as-prepared PLGA/titanium biphasic scaffold could be modified by adding biomacromolecules (Protein, polysaccharides etc.), seeding mesenchymal stem cells or autologous chondrocytes or loading growth factors to further improve its cartilage repairing ability, and the osseous phase (Porous titanium) of the as-prepared PLGA/titanium biphasic scaffold could be modified by using chemical treatments, electrochemical treatments or bioactive coating to improve its bioactivity, which could accelerate osteointegration and even form bone-bonding with the host bone.22,28,31,39,40 Therefore, the prepared PLGA/titanium biphasic scaffold in this study was meaningful for the repair of osteochondral defects and showed some clinical potential.
5. Conclusions
A porous PLGA/Ti biphasic scaffold that exhibited promising properties for osteochondral applications was successfully prepared by using a combination of a solvent merging/particulate leaching method and an uniaxially pressing method. This biphasic scaffold possessed good biocompatibility and showed osteochondral defect repair ability to a certain extent without the addition of cells or growth factors. The further optimization of the porous PLGA/Ti biphasic scaffold might enhance its osteochondral defect repair ability and be promising for clinical use.Acknowledgements
This work was funded by National Basic Research Program of China (Grant Number: 2011CB606201) and Key Technologies R&D Program of Sichuan Province, China (Grant Number: 2010SZ0121, 2012FZ0007). The authors were grateful for Ms Xiuqun Li from Institute of Stem Cell and Tissue Engineering of Sichuan University for the analysis of the histological sections.Notes and references
- I. Martin, S. Miot, A. Barbero, M. Jakob and D. Wendt, J. Biomech., 2007, 40, 750–765 CrossRef.
- W. L. Grayson, P. H. Chao, D. Marolt, D. L. Kaplan and G. Vunjak-Novakovic, Trends Biotechnol., 2008, 26, 181–189 CrossRef CAS.
- A. Getgood, T. P. S. Bhullar and N. Rushton, Orthop. Trauma, 2009, 23, 189–200 CrossRef.
- A. M. Haleem and C. R. Chu, Oper. Tech. Orthop., 2010, 20, 76–89 CrossRef.
- J. Mano and R. Reis, J. Tissue Eng. Regener. Med., 2007, 1, 261–273 CrossRef CAS.
- X. Huang, D. Yang, W. Yan, Z. Shi, J. Feng, Y. Gao, W. Weng and S. Yan, Biomaterials, 2007, 28, 3091–3100 CrossRef CAS.
- C. C. Jiang, H. Chiang, C. J. Liao, Y. J. Lin, T. F. Kuo, C. S. Shieh, Y. Y. Huang and R. S. Tuan, J. Orthop. Res., 2007, 25, 1277–1290 CrossRef CAS.
- J. K. Sherwood, S. L. Riley, R. Palazzolo, S. C. Brown, D. C. Monkhouse, M. Coates, L. G. Griffith, L. K. Landeen and A. Ratcliffe, Biomaterials, 2002, 23, 4739–4751 CrossRef CAS.
- T. Gotterbarm, W. Richter, M. Jung, S. Berardi Vilei, P. Mainil-Varlet, T. Yamashita and S. J. Breusch, Biomaterials, 2006, 27, 3387–3395 CrossRef CAS.
- J. M. Oliveira, M. T. Rodrigues, S. S. Silva, P. B. Malafaya, M. E. Gomes, C. A. Viegas, I. R. Dias, J. T. Azevedo, J. F. Mano and R. L. Reis, Biomaterials, 2006, 27, 6123–6137 CrossRef CAS.
- D. Schaefer, I. Martin, P. Shastri, R. F. Padera, R. Langer, L. Freed and G. Vunjak-Novakovic, Biomaterials, 2000, 21, 2599–2606 CrossRef CAS.
- H. Chiang and C. C. Jiang, J. Formosan Med. Assoc., 2009, 108, 87–101 CrossRef CAS.
- R. A. Jain, Biomaterials, 2000, 21, 2475–2490 CrossRef CAS.
- C. M. Agrawal and R. B. Ray, J. Biomed. Mater. Res., 2001, 55, 141–150 CrossRef CAS.
- K. Uematsu, K. Hattori, Y. Ishimoto, J. Yamauchi, T. Habata, Y. Takakura, H. Ohgushi, T. Fukuchi and M. Sato, Biomaterials, 2005, 26, 4273–4279 CrossRef CAS.
- G. Ryan, A. Pandit and D. P. Apatsidis, Biomaterials, 2006, 27, 2651–2670 CrossRef CAS.
- K. Alvarez and H. Nakajima, Materials, 2009, 2, 790–832 CrossRef CAS.
- J. P. Li, P. Habibovic, M. van den Doel, C. E. Wilson, J. R. de Wijn, C. A. van Blitterswijk and K. de Groot, Biomaterials, 2007, 28, 2810–2820 CrossRef CAS.
- C. Wen, Y. Yamada, K. Shimojima, Y. Chino, T. Asahina and M. Mabuchi, J. Mater. Sci.: Mater. Med., 2002, 13, 397–401 CrossRef CAS.
- J. P. St-Pierre, M. Gauthier, L. P. Lefebvre and M. Tabrizian, Biomaterials, 2005, 26, 7319–7328 CrossRef CAS.
- A. Bandyopadhyay, F. Espana, V. K. Balla, S. Bose, Y. Ohgami and N. M. Davies, Acta Biomater., 2010, 6, 1640–1648 CrossRef CAS.
- P. Habibovic, J. P. Li, C. M. van der Valk, G. Meijer, P. Layrolle, C. A. van Blitterswijk and K. de Groot, Biomaterials, 2005, 26, 23–36 CrossRef CAS.
- J. P. Li, S. H. Li, C. A. Van Blitterswijk and K. de Groot, J. Biomed. Mater. Res., Part A, 2005, 73A, 223–233 CrossRef CAS.
- J. Gao, J. E. Dennis, L. A. Solchaga, A. S. Awadallah, V. M. Goldberg and A. I. Caplan, Tissue Eng., 2001, 7, 363–371 CrossRef CAS.
- B. Kreklau, M. Sittinger, M. B. Mensing, C. Voigt, G. Berger, G. Burmester, R. Rahmanzadeh and U. Gross, Biomaterials, 1999, 20, 1743–1749 CrossRef CAS.
- G. Chen, T. Sato, J. Tanaka and T. Tateishi, Mater. Sci. Eng., C, 2006, 26, 118–123 CrossRef CAS.
- I. Nagura, H. Fujioka, T. Kokubu, T. Makino, Y. Sumi and M. Kurosaka, J. Bone Jt. Surg., Br. Vol., 2007, 89–B, 258–264 Search PubMed.
- C. Y. Zhao, X. D. Zhu, K. L. Liang, J. T. Ding, Z. Xiang, H. S. Fan and X. D. Zhang, J. Biomed. Mater. Res., Part B, 2010, 95, 387–396 CrossRef.
- C. J. Liao, C. F. Chen, J. H. Chen, S. F. Chiang, Y. J. Lin and K. Y. Chang, J. Biomed. Mater. Res., 2002, 59, 676–681 CrossRef CAS.
- G. G. Niederauer, M. A. Slivka, N. C. Leatherbury, D. L. Korvick, H. H. Harroff, W. C. Ehler, C. J. Dunn and K. Kieswetter, Biomaterials, 2000, 21, 2561–2574 CrossRef CAS.
- M. Takemoto, S. Fujibayashi, M. Neo, J. Suzuki, T. Kokubo and T. Nakamura, Biomaterials, 2005, 26, 6014–6023 CrossRef CAS.
- W. Swieszkowski, B. H. Tuan, K. J. Kurzydlowski and D. W. Hutmacher, Biomol. Eng., 2007, 24, 489–495 CrossRef CAS.
- J. D. Bobyn, R. M. Pilliar, H. U. Cameron and G. C. Weatherly, Clin. Orthop. Relat. Res., 1980, 150, 263–270 Search PubMed.
- S. T. Ho, D. W. Hutmacher, A. K. Ekaputra, D. Hitendra and J. H. Hui, Tissue Eng. A, 2010, 16, 1123–1141 CrossRef CAS.
- F. Shapiro, S. Koide and M. J. Glimcher, J. Bone. Joint. Surg. Am., 1993, 75, 532 CAS.
- T. J. Gill, P. D. Asnis and E. M. Berkson, J. Orthop. Sports. Phys. Ther., 2006, 36, 728–738 Search PubMed.
- X. Zhang, Z. Zheng, P. Liu, Y. Ma, L. Lin, N. Lang, X. Fu, J. Zhang, K. Ma, P. Chen, C. Zhou and Y. Ao, Biomaterials, 2008, 29, 4616–4629 CrossRef CAS.
- M. F. Pittenger, A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simonetti, S. Craig and D. R. Marshak, Science, 1999, 284, 143 CrossRef CAS.
- S. Fujibayashi, M. Neo, H. M. Kim, T. Kokubo and T. Nakamura, Biomaterials, 2004, 25, 443–450 CrossRef CAS.
- J. Sun, Y. Han and K. Cui, Surf. Coat. Technol., 2008, 202, 4248–4256 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
