Magnetic nanocomposite of hydroxyapatite ultrathin nanosheets/Fe3O4 nanoparticles: microwave-assisted rapid synthesis and application in pH-responsive drug release
Feng
Chen
ab,
Chao
Li
a,
Ying-Jie
Zhu
*b,
Xin-Yu
Zhao
b,
Bing-Qiang
Lu
b and
Jin
Wu
b
aDepartment of Bio-Nano Science and Engineering, Research Institute of Micro/Nano Science and Technology, Shanghai Jiao Tong University, Shanghai 200240, P. R. China
bState Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, P. R. China. E-mail: y.j.zhu@mail.sic.ac.cn
First published on 15th July 2013
Abstract
Synthetic hydroxyapatite (HAP) nanostructured materials have been considered as promising biomaterials due to their excellent biocompatibility. In this study, a magnetic nanocomposite consisting of HAP ultrathin nanosheets (UNs) and Fe3O4 magnetic nanoparticles (MNs) has been prepared using a rapid microwave-assisted route. The Fe3O4 magnetic nanoparticles are hybridized with HAP ultrathin nanosheets, which self-assemble to form a hierarchically nanostructured magnetic nanocomposite (HAPUN/MNs). The as-prepared HAPUN/MNs nanocomposite is characterized and investigated as a drug nanocarrier using hemoglobin (Hb) and docetaxel (Dtxl) as model drugs. The adsorption amount of Hb on the HAPUN/MNs nanocomposite increases with the increasing initial Hb concentration. The release of Hb from the HAPUN/MNs nanocomposite is essentially governed by a diffusion process. The HAPUN/MNs nanocomposite has a good sustained release profile for Dtxl, and shows good pH-responsive drug release properties, which can be explained by the gradual dissolution of HAP in a low pH value environment. The HAPUN/MNs nanocomposite has a high biocompatibility and also a high in vitro anticancer effect after loading Dtxl.
Introduction
Hydroxyapatite (HAP) is abundant in nature, especially in calcified hard tissues of vertebrate.1–3 Synthetic HAP nanostructured materials can mimic the structures and dimensions of the inorganic components of calcified hard tissues, and be utilized as promising biomaterials due to their excellent biocompatibility.4–7 Nowadays, the development of nanotechnology has advanced the synthesis and applications of HAP nanostructures as biomaterials. Therefore, various synthetic HAP materials have been investigated for applications in bone repair, drug/gene delivery and other biomedical areas.8–13 HAP materials with a variety of morphologies including nanorods, plate-like nanocrystals, nanoparticles, nanotubes and three-dimensional structures have been prepared.14–20 The structure, size, morphology and properties of synthetic HAP materials are usually determined by their preparation methods. The facile synthesis of HAP nanostructured materials with a well-defined structure, size and morphology is desirable and remains a big challenge.Fe3O4 magnetic nanoparticles (MNs) have been synthesized using various methods including coprecipitation, thermal decomposition, hydrothermal synthesis, sol–gel synthesis and so on.21–24 The thermal decomposition method has been widely used for the synthesis of magnetic nanoparticles with small particle sizes.25 Recently, a novel and efficient thermal decomposition method has been developed using microwave heating and room temperature ionic liquids (RTILs).26 Magnetic nanoparticles have found wide applications in environmental remediation, bioseparation and other biomedical fields such as magnetic resonance imaging and targeted drug delivery.27–32
Due to the important functions and promising applications of HAP and magnetic nanoparticles, it is desirable to develop facile methods for the preparation of their nanocomposite materials. For instance, the HAP/MNs and laminas have been prepared and applied in the removal of heavy metal ions from water and in gene delivery.33,34 In addition, by using CaCO3 as a template, porous HAP/Fe3O4 structures have been fabricated.35,36 However, efficient preparation methods for hierarchically nanostructured HAP/MNs nanocomposites have been less well reported.
Herein, we report a facile microwave-assisted method for the preparation of a hierarchically nanostructured magnetic nanocomposite consisting of HAP ultrathin nanosheets and Fe3O4 magnetic nanoparticles (HAPUN/MNs). The as-prepared HAPUN/MNs nanocomposite is investigated as a drug nanocarrier using hemoglobin and the anticancer drug docetaxel as model drugs. It is worth emphasizing that HAP has an excellent biocompatibility, thus the as-prepared HAPUN/MNs nanocomposite is promising for application in drug delivery systems with a magnetic function.
Experimental section
Preparation of the hierarchically nanostructured magnetic nanocomposite
The block copolymer monomethoxyl poly(ethylene glycol)-b-polylactide (PLA-mPEG) (Mw = 8000 Da) was purchased from Jinan Daigang Biomaterials Co., Ltd, and the molecular weight of the mPEG segment was 3000 Da. Other chemicals were purchased from Sinopharm Chemical Reagent Co. and used as received without further purification.For the synthesis of the Fe3O4 magnetic nanoparticles, 1.800 g of tris(acetylacetonato) iron(III) (Fe(acac)3) and 0.650 g of bis(acetylacetonato) iron(II) (Fe(acac)2) were mixed by a magnetic stirrer in a 50 mL tube containing 30 mL dibenzyl ether, 3.400 g oleic acid and 0.5 mL of the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM]BF4). The suspension was microwave-heated (Discover, CEM, USA) to 200 °C and maintained at this temperature for 5 min. Then, the reaction system was heated to 230 °C and maintained at this temperature for 10 min. The resulting solution was allowed to cool to room temperature, and diluted and washed with absolute ethanol. The product was separated magnetically.
For the preparation of the hierarchically nanostructured magnetic nanocomposite (HAPUN/MNs), 1 mmol of Ca(CH3COO)2, 0.100 g of PLA-mPEG and 0.050 g of the Fe3O4 nanoparticles obtained by the above method were dissolved in a mixed solvent system containing 15 mL deionized water and 15 mL ethylene glycol. Then 10 mL of aqueous solution containing 1 mmol of NaH2PO4·2H2O was added dropwise to the above solution. The resulting solution was transferred to a Teflon autoclave, sealed, and heated by microwaves to 120 °C and was maintained at this temperature for 30 min. The microwave oven used was a microwave-solvothermal system (MDS-6, Sineo, China). After cooling to room temperature, the product was separated by a magnet, and washed with deionized water and ethanol several times.
Characterization
X-ray powder diffraction (XRD) patterns were recorded using a Rigaku D/max 2550V X-ray diffractometer with a graphite monochromator (Cu Kα radiation, λ = 1.54178 Å). Transmission electron microscopy (TEM) micrographs were recorded with a JEOL JEM 2100 field-emission electron microscope. Scanning electron microscopy (SEM) was performed on a JEOL JSM-6700 field-emission scanning electron microscope. A physical property measurement system (PPMS, Quantum Design, USA) was used to evaluate the magnetic properties at room temperature. Fourier transform infrared (FTIR) spectroscopy was performed with a spectrophotometer (FTIR-7600, Lambda, Australia) with the KBr pellet technique. The nitrogen adsorption–desorption isotherm, the corresponding Barrett–Joyner–Halenda (BJH) pore size distribution and the Brunauer–Emmett–Teller (BET) specific surface area were measured by a surface area and porosity analyzer (V-Sorb 2800P, Gold APP, China).In vitro hemoglobin adsorption and release
Hemoglobin (Hb) was used as a model protein in this study. For the Hb adsorption experiments, the powder of the HAPUN/MNs nanocomposite (2 mg) was immersed in solutions (2 mL each) with variable protein concentrations (0–1000 μg mL−1). Each solution was shaken at a constant rate of 130 rpm for 4 h at 37 °C. Then, the solution was centrifuged and the concentration of the Hb protein in the supernatant was measured by UV-vis absorption analysis at a wavelength of 406 nm.For the Hb release experiments, the powder of the HAPUN/MNs nanocomposite (8 mg) was immersed in 10 mL of Hb aqueous solution with a concentration of 500 μg mL−1, and the solution was shaken at a rate of 130 rpm for 4 h at 37 °C to obtain the HAPUN/MNs nanocomposite drug delivery system. Then, the powder of the HAPUN/MNs nanocomposite drug delivery system (2 mg) was immersed into 2 mL of phosphate buffered saline (PBS) at 37 °C under shaking at a rate of 140 rpm. The Hb release solution (400 μL) was withdrawn for UV-vis absorption analysis at given time intervals, and the withdrawn solution was replaced with the same volume of fresh PBS.
In vitro docetaxel loading and release
The docetaxel (Dtxl) drug loading experiments were performed as follows: the dried powder of the as-prepared HAPUN/MNs nanocomposite (100 mg) was dispersed into 5 mL of ethanol solution with a Dtxl concentration of 20 mg mL−1. The suspension was shaken at a constant rate of 130 rpm in a sealed vessel at 37 °C for 24 h, followed by centrifugation and drying to obtain the HAPUN/MNs nanocomposite drug delivery system. The loading amount of Dtxl in the HAPUN/MNs was measured using thermogravimetric (TG) analysis (STA 409/PC, Netzsch, Germany, heating rate 10 °C min−1, air atmosphere).The in vitro Dtxl drug release experiments were performed as follows: the powder of the HAPUN/MNs nanocomposite drug delivery system (5 mg) was immersed in 8 mL of PBS (pH 7.4 and 4.5) at 37 °C with a constant shaking (140 rpm). The shaking device was a desk-type constant-temperature oscillator (THI-92A, China). The release medium (0.4 mL) was withdrawn for UV-vis absorption analysis at a wavelength of 230 nm at given time intervals and replaced with the same volume of fresh PBS solution.
In vitro cell viability
The human gastric carcinoma cells (SGC-7901), cultured in an RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin at 37 °C for 24 h, were used for the cell viability test. Then, the cells were seeded in a 96 well flat-bottom microassay plate at a concentration of 104 cells per mL and cultured for 24 h. The sterilized HAPUN/MNs nanocomposite sample was added into wells at concentrations ranging from 1 to 150 μg mL−1, and was co-cultured with cells for 24 h. The tissue culture plate was used as a control sample. Cell viability was quantified by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. All reagents used in the cell viability experiments were purchased from Sigma-Aldrich. Images of the SGC-7901 cells treated with fluorescein-loaded HAPUN/MNs were obtained using an Olympus IX71 optical microscope.Results and discussion
The schematic illustration for the preparation of the Fe3O4 magnetic nanoparticles and the HAPUN/MNs nanocomposite is shown in Fig. 1. We first synthesized the Fe3O4 magnetic nanoparticles using a microwave-assisted ionic liquid method at 200 °C for 5 min and at 230 °C for 10 min, using Fe(acac)3 and Fe(acac)2 in a mixed solvent system containing dibenzyl ether, oleic acid and an ionic liquid ([BMIM]BF4). Subsequently, the microwave-assisted solvothermal method was adopted for the preparation of the HAPUN/MNs nanocomposite using Ca(CH3COO)2, NaH2PO4·2H2O, the block copolymer PLA-mPEG, and the as-prepared Fe3O4 magnetic nanoparticles in a mixed solvent system containing deionized water and ethylene glycol at 120 °C for 30 min.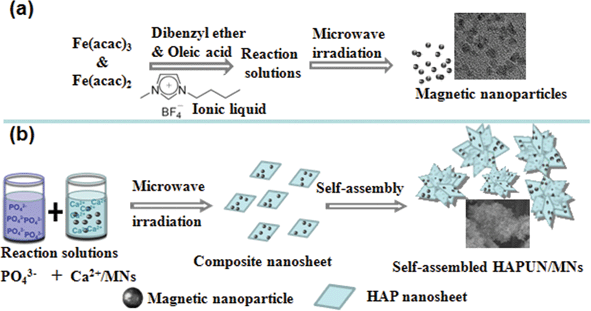 | ||
| Fig. 1 Schematic illustration of the synthesis of Fe3O4 magnetic nanoparticles (a) and the HAPUN/MNs nanocomposite (b). | ||
The morphologies of the as-prepared Fe3O4 magnetic nanoparticles and the HAPUN/MNs nanocomposite were investigated with TEM (Fig. 2). Fig. 2a shows that the as-prepared Fe3O4 magnetic nanoparticles were almost monodisperse with small sizes and a narrow size distribution. The average diameter of the Fe3O4 magnetic nanoparticles measured from the TEM micrographs was 6.25 nm (Fig. 2b). The microwave-assisted solvothermal synthesis of nanomaterials has been recognized as an economical method with a high efficiency. The efficient heating with microwaves can evidently reduce chemical reaction times from hours to minutes, especially in the presence of a room-temperature ionic liquid which acts as an excellent microwave absorber.
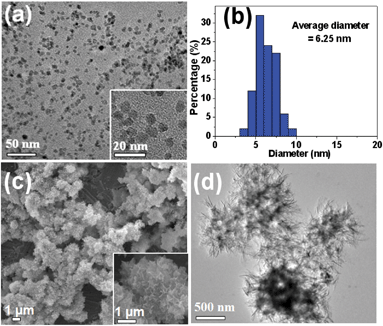 | ||
| Fig. 2 (a) TEM micrograph and (b) the diameter distribution of the Fe3O4 magnetic nanoparticles prepared by a microwave-assisted ionic liquid method; (c) SEM micrograph and (d) TEM micrograph of the HAPUN/MNs nanocomposite obtained by a microwave-assisted solvothermal method at 120 °C for 30 min. | ||
From the SEM and TEM micrographs (Fig. 2c), one can see that the as-prepared HAPUN/MNs nanocomposite consisted of Fe3O4 magnetic nanoparticles which were hybridized with HAP ultrathin nanosheets, and the HAP nanosheets with thicknesses of ∼5 nm were building blocks self-assembled to form a three-dimensional hierarchically nanostructured porous nanocomposite. The structural feature of the HAPUN/MNs nanocomposite is favorable for the potential application in magnetic drug delivery systems. Energy dispersive spectroscopy (EDS) was adopted to investigate the chemical composition of the HAPUN/MNs nanocomposite, and this indicated that the iron element could be found both at the edge and central sites of the HAPUN/MNs nanocomposite (Fig. 3).
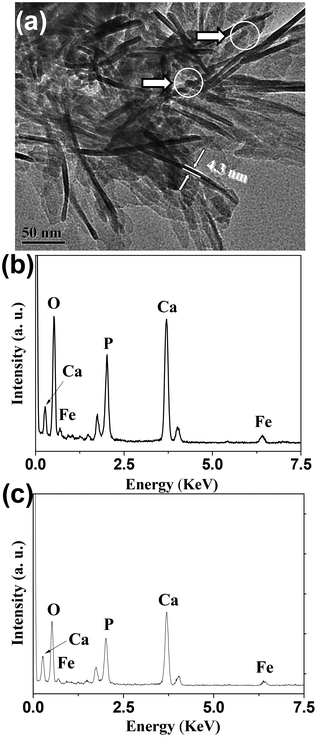 | ||
| Fig. 3 (a) TEM micrograph and (b,c) energy dispersive spectroscopy patterns of the as-prepared HAPUN/MNs nanocomposite. (b) and (c) represent EDS patterns taken at the regions in TEM micrograph (a) labelled by the upper and lower circles, respectively. | ||
In the preparation process, a biocompatible amphiphilic block copolymer of PLA-mPEG was used. The block copolymer could form spherical micelles through self-assembly in an aqueous solution. In this reaction system, PLA-mPEG was favorable for dispersing the Fe3O4 magnetic nanoparticles through hydrophobic interactions between the PLA chains and the hydrophobic surfaces of the Fe3O4 magnetic nanoparticles. After mixing the Ca2+ ions and PO43− ions together with the Fe3O4 magnetic nanoparticles, the temperature of the reaction system rapidly increased under the microwave solvothermal conditions, and a large number of HAP nuclei formed. Then, HAP ultrathin nanosheets formed through crystal growth of the HAP nuclei under microwave solvothermal conditions. In this process, the PLA-mPEG micelles became unstable due to the microwave irradiation and the enhanced temperature. Therefore, the hydrophobic surfaces of the Fe3O4 magnetic nanoparticles were exposed in the solution. Through the hydrophobic force, the small sized Fe3O4 magnetic nanoparticles were hybridized with HAP ultrathin nanosheets. Thereafter, with the increasing reaction time, the HAP ultrathin nanosheets self-assembled to reduce their surface energy in solution. In this way, the HAPUN/MNs nanocomposite was formed.
The XRD pattern (Fig. 4a) indicates that the as-prepared magnetic nanoparticles can be indexed to a single-phase of Fe3O4 (JCPDS No. 19-0629). A series of characteristic peaks at 2θ = 30.095°, 35.422°, 43.052°, 53.391°, 56.942°, and 62.515° correspond to the (220), (311), (400), (422), (511), and (440) crystal faces of Fe3O4, respectively. The low intensity and broadening of the XRD peaks are due to the very small sizes of the as-prepared magnetic nanoparticles. These diffraction peaks of Fe3O4 also appeared in the XRD pattern of the HAPUN/MNs nanocomposite, and additional peaks are related to HAP with a hexagonal structure (JCPDS No. 09-0432). The diffraction peaks at 2θ = 25.8°, 31.7° and 40.5° correspond to the (002), (211), (221) crystal faces of HAP. These results indicate that the as-prepared product was a nanocomposite consisting of Fe3O4 and HAP.
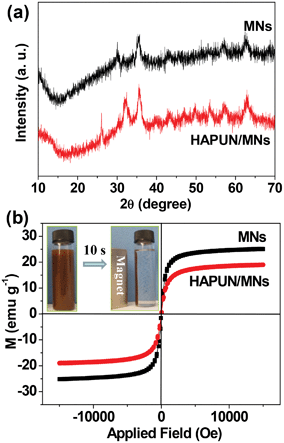 | ||
| Fig. 4 (a) XRD patterns of the Fe3O4 magnetic nanoparticles and the HAPUN/MNs nanocomposite; (b) hysteresis loops of the Fe3O4 magnetic nanoparticles and the HAPUN/MNs nanocomposite measured at room temperature. The inset of (b) shows the good magnetic separation performance of the HAPUN/MNs nanocomposite by using a magnet for only 10 seconds in an aqueous solution. | ||
Fig. 4b shows the hysteresis loops of the as-synthesized Fe3O4 magnetic nanoparticles and the HAPUN/MNs nanocomposite at room temperature. It can be seen that the Fe3O4 magnetic nanoparticles exhibited a superparamagnetic behavior with a saturation magnetization of 25.1 emu g−1. The HAPUN/MNs nanocomposite also showed a superparamagnetic behavior, and the saturation magnetization was measured to be 18.9 emu g−1, which is relatively high for a good magnetic response. The inset of Fig. 4b shows the magnetic separation behavior of the HAPUN/MNs nanocomposite. A good magnetic separation performance of the HAPUN/MNs nanocomposite was observed by using a magnet for only 10 seconds in an aqueous solution.
Fig. 5 shows the nitrogen adsorption–desorption isotherms and the corresponding BJH pore size distribution of the as-prepared HAPUN/MNs nanocomposite obtained by a microwave-assisted solvothermal method at 120 °C for 30 min. According to the International Union of Pure and Applied Chemistry, it can be classified as a type-IV isotherm loop. The BET specific surface area was measured to be 92.8 m2 g−1, and the BJH desorption cumulative pore volume and the average pore size were 0.60 cm3 g−1 and 16.2 nm, respectively. The high specific surface area of the as-prepared HAPUN/MNs nanocomposite can be explained by the self-assembled structure of the HAP ultrathin nanosheets and the very small sizes of the Fe3O4 nanoparticles. The relatively large specific surface area, pore volume and nanoporous structure of the as-prepared HAPUN/MNs nanocomposite are favorable for the application in protein adsorption and drug delivery.
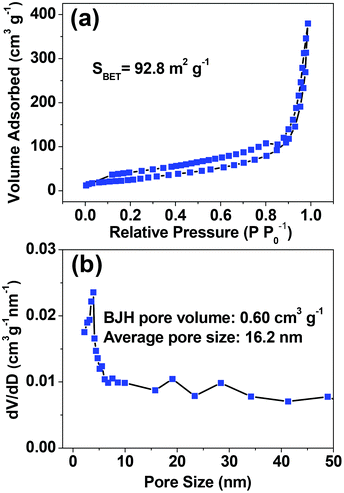 | ||
| Fig. 5 (a) Nitrogen adsorption–desorption isotherms and (b) pore size distribution of the as-prepared HAPUN/MNs nanocomposite. | ||
The adsorption of Hb using the HAPUN/MNs nanocomposite as the adsorbent at different initial concentrations of Hb ranging from 50 to 1000 μg mL−1 was studied. The Hb adsorption capacity on the HAPUN/MNs nanocomposite was relatively high even at a low initial concentration. The Hb adsorption amount on the HAPUN/MNs nanocomposite increased with increasing the initial Hb concentration (Fig. 6b), and the adsorption values reached 133 and 150 mg g−1 at the concentrations of 500 and 1000 μg mL−1, respectively. Actually, the UV-vis absorption spectra (Fig. 6a) shows that almost all the Hb dissolved in the solution had been adsorbed at a Hb concentration of 50 μg mL−1. These results can be explained by the three-dimensional porous nanosheet-assembled HAP nanostructures which have abundant active sites for protein adsorption.
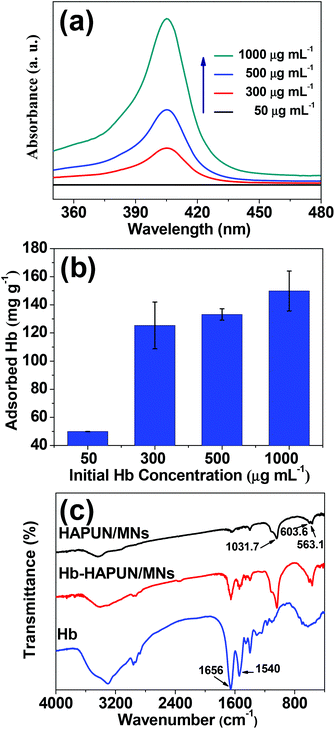 | ||
| Fig. 6 (a) UV-vis absorption spectra of Hb solutions with different concentrations after Hb adsorption in the HAPUN/MNs nanocomposite; (b) Hb adsorption amount by the HAPUN/MNs nanocomposite versus initial Hb concentration; (c) FTIR spectra of the HAPUN/MNs nanocomposite, Hb-loaded HAPUN/MNs nanocomposite and pure Hb. | ||
In the FTIR spectrum of the HAPUN/MNs nanocomposite (Fig. 6c), the absorption bands corresponding to PO43− were observed at about 1031.7, 603.6 and 563.1 cm−1. As shown in the FTIR spectrum of pure Hb, there was an obvious absorption peak at about 1656 cm−1, which is assigned to the amide (I) group consisting of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N. In addition, the peak at about 1540 cm−1 is assigned to the amide (II) band resulting from a combination of N–H in-plane bending and C–N stretching of the peptide groups. These characteristic absorption peaks also appeared in the FTIR spectrum of the Hb-adsorbed HAPUN/MNs nanocomposite, indicating that the Hb molecules in solution could be adsorbed by the HAPUN/MNs nanocomposite. The FTIR result is consistent with the Hb adsorption measurements.
N. In addition, the peak at about 1540 cm−1 is assigned to the amide (II) band resulting from a combination of N–H in-plane bending and C–N stretching of the peptide groups. These characteristic absorption peaks also appeared in the FTIR spectrum of the Hb-adsorbed HAPUN/MNs nanocomposite, indicating that the Hb molecules in solution could be adsorbed by the HAPUN/MNs nanocomposite. The FTIR result is consistent with the Hb adsorption measurements.
The Hb release behavior of the Hb-adsorbed HAPUN/MNs nanocomposite prepared at an initial Hb concentration of 500 μg mL−1 was investigated in PBS at 37 °C. As shown in Fig. 7a and b, the Hb release was rapid in the first several hours. After the initial rapid release stage, the Hb release rate reduced gradually. The cumulative Hb release percentages in PBS reached 38.7%, 59.6%, 74.9% and 84.1% at a release time of 1, 2, 4 and 8 h, respectively. Furthermore, an approximately linear relationship between the cumulative amount of released Hb and the square root of the release time has been found (Fig. 7c). This result indicates that the Hb release from the Hb-adsorbed HAPUN/MNs nanocomposite was essentially governed by a diffusion process according to the Higuchi model.37,38
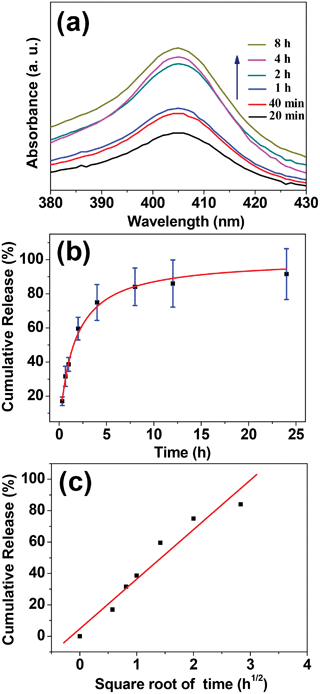 | ||
| Fig. 7 (a) UV-vis absorption spectra of the Hb release medium for different Hb release times; (b) cumulative Hb release percentages from the HAPUN/MNs nanocomposite in PBS at different times; (c) cumulative Hb release percentage as a function of square root of the release time. The sample used for the in vitro Hb release was prepared at an initial Hb concentration of 500 μg mL−1. | ||
We also investigated the drug release performance of the as-prepared HAPUN/MNs nanocomposite using a typical antitumor drug, Dtxl. Thermal analysis of the HAPUN/MNs nanocomposite without and with Dtxl loading was employed to estimate the drug loading capacity. The Dtxl loading capacity of the HAPUN/MNs nanocomposite was about 56 mg g−1. The Dtxl release behavior of the HAPUN/MNs nanocomposite drug delivery system in PBS was studied at different pH values of 7.4 and 4.5 at 37 °C. As shown in Fig. 8a, Dtxl was gradually released from the HAPUN/MNs nanocomposite drug delivery system at pH 7.4 and 4.5 in a time period of 24 h. Then, the Dtxl-loaded HAPUN/MNs nanocomposite exhibited a stable cumulative Dtxl release level (about 30%) at a high pH value (pH 7.4) after the initial drug release stage. In contrast, the cumulative Dtxl release percentages in PBS with a pH value of 4.5 reached a higher level of about 80% and 98% at the release time of 24 h and 108 h, respectively. The higher cumulative Dtxl release percentage at a lower pH value may be attributed to the increased dissolution of the nanocarrier in an acidic environment. In the PBS with a lower pH value (pH 4.5), the nanocarrier could gradually dissolve, and then Dtxl adsorbed on the nanocarrier would release into the solution. The experimental results indicate that the as-prepared HAPUN/MNs nanocomposite has favourable pH-controlled drug release properties, and is promising for the application as a pH-responsive drug nanocarrier.
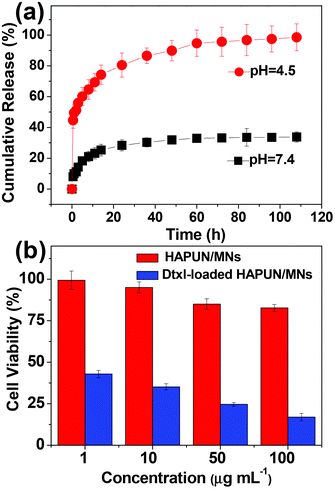 | ||
| Fig. 8 (a) The cumulative drug release percentages of docetaxel from the HAPUN/MNs nanocomposite drug delivery system in PBS with different pH values of 7.4 and 4.5; (b) cell viability tests of the HAPUN/MNs without and with docetaxel drug loading. | ||
The cytotoxicity tests of the HAPUN/MNs nanocomposite without and with Dtxl drug loading were performed using SGC-7901 cells. The results of the MTT assays showed little toxicity when the cells were co-cultured with the HAPUN/MNs nanocomposite at concentrations in the range of 1–100 μg mL−1 (Fig. 8b). The high biocompatibility may be explained by the chemical nature of the as-prepared HAPUN/MNs nanocomposite. Furthermore, in the presence of the Dtxl-loaded HAPUN/MNs nanocomposite, the cell viability decreased with increasing concentration of the Dtxl-loaded HAPUN/MNs drug delivery system. The cell viabilities were only 43% and 17% when the concentrations of the sample were 1 and 100 μg mL−1, respectively.
Fig. 9 shows the cell morphology after treatment with the fluorescein-loaded HAPUN/MNs nanocomposite. From the images, one can see that the cells could maintain a spindle morphology which stands for a good physiological state after treatment with the fluorescein-loaded HAPUN/MNs nanocomposite. Furthermore, the cells were labelled with a green colour which was generated by the fluorescein-loaded HAPUN/MNs nanocomposite observed under the fluorescence microscope. These results indicate that the as-prepared HAPUN/MNs nanocomposite can enter into the cells through a process of phagocytosis. The amplified images also indicate that the HAPUN/MNs nanocomposite is favourable to deliver the fluorescein into the cells.
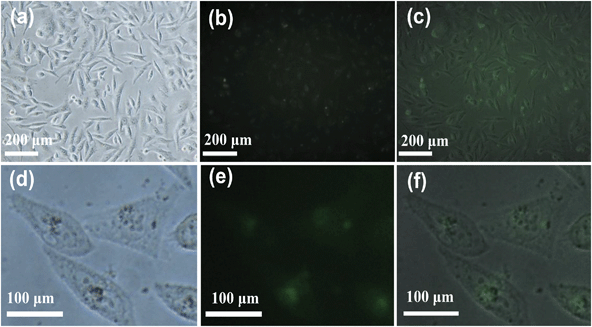 | ||
| Fig. 9 Images of human gastric carcinoma cells (SGC-7901) treated with the fluorescein-loaded HAPUN/MNs nanocomposite obtained using an Olympus IX71 optical microscope. | ||
Conclusions
A facile microwave-assisted liquid phase method has been developed for the preparation of a magnetic nanocomposite consisting of HAP ultrathin nanosheets and Fe3O4 magnetic nanoparticles. Fe3O4 magnetic nanoparticles are hybridized with HAP ultrathin nanosheets, which self-assemble to form a porous nanocomposite under microwave-solvothermal conditions. The as-prepared HAPUN/MNs nanocomposite is investigated as a drug nanocarrier using Hb and Dtxl as a model protein and an anticancer drug. The HAPUN/MNs nanocomposite has a good sustained drug release profile, and shows good pH-controlled drug release behavior, which can be explained by the gradually dissolution of the nanocarrier in a low pH environment. Due to the high biocompatibility, magnetic response property, pH-controlled sustained drug release and the ability to enter cells, the as-prepared HAPUN/MNs nanocomposite is promising for the application in drug delivery.Acknowledgements
The financial support from the National Basic Research Program of China (973 Program, No. 2012CB933600), the National Natural Science Foundation of China (51172260, 51102258, 51121064), the Science and Technology Commission of Shanghai (11nm0506600), and Fund for Youth Scholar of State Key Laboratory of High Performance Ceramics and Superfine Microstructure is gratefully acknowledged.Notes and references
- L. C. Palmer, C. J. Newcomb, S. R. Kaltz, E. D. Spoerke and S. I. Stupp, Chem. Rev., 2008, 108, 4754–4783 CrossRef CAS.
- S. Weiner and H. D. Wagner, Annu. Rev. Mater. Sci., 1998, 28, 271–298 CrossRef CAS.
- J. E. Salk, Science, 1945, 101, 122–124 CAS.
- Y. R. Cai and R. K. Tang, J. Mater. Chem., 2008, 18, 3775–3787 RSC.
- P. P. Yang, Z. W. Quan, C. X. Li, X. J. Kang, H. Z. Lian and J. Lin, Biomaterials, 2008, 29, 4341–4347 CrossRef CAS.
- C. Qi, Y. J. Zhu, B. Q. Lu, X. Y. Zhao, J. Zhao and F. Chen, J. Mater. Chem., 2012, 22, 22642–22650 RSC.
- Z. Y. Hou, P. P. Yang, H. Z. Lian, L. L. Wang, C. M. Zhang, C. X. Li, R. T. Chai, Z. Y. Cheng and J. Lin, Chem.–Eur. J., 2009, 15, 6973–6982 CrossRef CAS.
- S. I. Stupp and P. V. Braun, Science, 1997, 277, 1242–1248 CrossRef CAS.
- W. Suchanek and M. Yoshimura, J. Mater. Res., 1998, 13, 94–117 CrossRef CAS.
- M. Y. Ma, Y. J. Zhu, L. Li and S. W. Cao, J. Mater. Chem., 2008, 18, 2722–2727 RSC.
- A. Maitra, Expert Rev. Mol. Diagn., 2005, 5, 893–905 CrossRef CAS.
- F. Chen, Y. J. Zhu, J. Wu, P. Huang and D. X. Cui, Nano Biomed. Eng., 2012, 4, 41–49 CrossRef CAS.
- F. Chen, P. Huang, Y. J. Zhu, J. Wu, C. L. Zhang and D. X. Cui, Biomaterials, 2011, 32, 9031–9039 CrossRef CAS.
- M. Jevtić, M. Mitrić, S. Škapin, B. Jančar, N. Ignjatović and D. Uskoković, Cryst. Growth Des., 2008, 8, 2217–2222 Search PubMed.
- H. B. Zhang, K. C. Zhou, Z. Y. Li and S. P. Huang, J. Phys. Chem. Solids, 2009, 70, 243–248 CrossRef CAS.
- C. Lai, S. Q. Tang, Y. J. Wang and K. Wei, Mater. Lett., 2005, 59, 210–214 CrossRef CAS.
- K. L. Lin, J. Chang, Y. J. Zhu, W. Wu, G. F. Cheng, Y. Zeng and M. L. Ruan, Cryst. Growth Des., 2009, 9, 177–181 CAS.
- M. G. Ma, Y. J. Zhu and J. Chang, J. Phys. Chem. B, 2006, 110, 14226–14230 CrossRef CAS.
- F. Chen, Q. L. Tang, Y. J. Zhu, K. W. Wang, M. L. Zhang, W. Y. Zhai and J. A. Chang, Acta Biomater., 2010, 6, 3013–3020 CrossRef CAS.
- F. Chen, Y. J. Zhu, K. W. Wang and K. L. Zhao, CrystEngComm, 2011, 13, 1858–1863 RSC.
- J. P. Jolivet, C. Chanéac and E. Tronc, Chem. Commun., 2004, 481–487 RSC.
- S. H. Sun and H. Zeng, J. Am. Chem. Soc., 2002, 124, 8204–8205 CrossRef CAS.
- T. Hyeon, S. S. Lee, J. Park, Y. Chung and H. Bin Na, J. Am. Chem. Soc., 2001, 123, 12798–12801 CrossRef CAS.
- W. Cai and J. Q. Wan, J. Colloid Interface Sci., 2007, 305, 366–370 CrossRef CAS.
- J. Liu, S. Z. Qiao, Q. H. Hu and G. Q. Lu, Small, 2011, 7, 425–443 CrossRef CAS.
- H. Y. Hu, H. Yang, P. Huang, D. X. Cui, Y. Q. Peng, J. C. Zhang, F. Y. Lu, J. Lian and D. L. Shi, Chem. Commun., 2010, 46, 3866–3868 RSC.
- M. Mahmoudi, S. Sant, B. Wang, S. Laurent and T. Sen, Adv. Drug Delivery Rev., 2011, 63, 24–46 CrossRef CAS.
- A. H. Lu, E. L. Salabas and F. Schuth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS.
- S. Yean, L. Cong, C. T. Yavuz, J. T. Mayo, W. W. Yu, A. T. Kan, V. L. Colvin and M. B. Tomson, J. Mater. Res., 2005, 20, 3255–3264 CrossRef CAS.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995–4021 CrossRef CAS.
- T. K. Jain, M. A. Morales, S. K. Sahoo, D. L. Leslie-Pelecky and V. Labhasetwar, Mol. Pharmaceutics, 2005, 2, 194–205 CrossRef CAS.
- P. Huang, Z. M. Li, J. Lin, D. P. Yang, G. Gao, C. Xu, L. Bao, C. L. Zhang, K. Wang, H. Song, H. Y. Hu and D. X. Cui, Biomaterials, 2011, 32, 3447–3458 CrossRef CAS.
- X. Wang, Clean: Soil, Air, Water, 2011, 39, 13–20 CrossRef CAS.
- G. Zuo, Y. Wan and Y. Zhang, Mater. Lett., 2012, 68, 225–227 CrossRef CAS.
- N. Tran and T. J. Webster, Acta Biomater., 2011, 7, 1298–1306 CrossRef CAS.
- Y. Guo, Y. Zhou, D. Ha and Q. Meng, Acta Biomater., 2008, 4, 923–931 CrossRef CAS.
- T. Higuchi, J. Pharm. Sci., 1963, 52, 1145–1149 CrossRef CAS.
- J. Andersson, J. Rosenholm, S. Areva and M. Lindén, Chem. Mater., 2004, 16, 4160–4167 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
