A CO2-switchable polymer brush for reversible capture and release of proteins†
Surjith
Kumar
ab,
Xia
Tong
a,
Yves L.
Dory
*a,
Martin
Lepage
*b and
Yue
Zhao
*a
aDépartement de chimie, Université de Sherbrooke, Sherbrooke, QC, Canada J1K 2R1. E-mail: yves.dory@usherbrooke.ca; yue.zhao@usherbrooke.ca
bDépartement de médecine nucléaire et radiobiologie and Centre d’imagerie moléculaire de Sherbrooke, Université de Sherbrooke, Sherbrooke, QC, Canada J1H 5N4. E-mail: martin.lepage@usherbrooke.ca
First published on 9th November 2012
Abstract
We report on a polymer brush that can be switched between extended (hydrated) and collapsed (dehydrated) chain conformational states just by passing CO2 and an inert gas like N2 in solution alternately. This conformational change allows for reversible adsorption and release of a protein. In contrast to adding acids and bases for pH change, using gases as the trigger makes it possible to repeat the switching cycle many times without salt accumulation.
The development of functional and responsive surfaces has generated much interest due to their wide range of potential applications,1 including protein binding,2 chemical and biochemical gates,3 enzyme immobilization,4 non-biofouling surfaces,5 and superhydrophilic surfaces.6 A variety of stimuli-responsive polymer surfaces in the form of thin films or coatings has been developed. Among them, the covalent attachment of polymer brushes via surface-initiated polymerization (SIP) has emerged as one of the prominent strategies, offering advantages in terms of control over the polymer chain length and grafting density.7
Brushes of stimuli-responsive polymers are able to reversibly switch their hydrophobicity or hydrophilicity in response to external stimuli such as pH, temperature, light, or redox agents,8 and have potential for applications in selective filtration, sensing, release, actuation, and antifouling surfaces.9 Of the many stimuli exploited, changes in pH are particularly interesting because these can be found physiologically. A number of pH-responsive surfaces exhibit the switchability;10 however, repeated switching cycles require repeated addition of acids and bases into the solution, resulting in salt accumulation that could contaminate the system and diminish the switchability. As a nontoxic and inexpensive gas, carbon dioxide (CO2) can reversibly switch certain types of solvents, surfactants, vesicles and polymer gels.11,12 The use of CO2 as a trigger for switching properties or functions of materials holds promise for applications. Here we report on, for the first time, a CO2-switchable polymer brush and its use in repeated gas-controllable adsorption and release of a protein by simply passing CO2 and an inert gas (N2) through the solution without adding acids and bases.
The reversible capture and release of proteins using a CO2-switchable polymer surface is illustrated in Fig. 1. Brushes of poly(N,N-diethylaminoethyl methacrylate) (PDEAEMA) were chosen. At room (or body) temperature and pH = 7, PDEAEMA brushes are insoluble in water, being in the collapsed state. Upon passing CO2 through the solution, the tertiary amine groups in PDEAEMA react with CO2 in water, forming charged ammonium bicarbonate11 and render the polymer brushes soluble in water,12 thus adopting the chain-extended state. Subsequently, passing N2 through the solution can remove CO2 and bring PDEAEMA brushes back to the insoluble state. In the present study, after the used ultrapure water was saturated with CO2, the pH of the solution decreased from 7 to 4.9; while after N2 bubbling, the initial pH value of 7 was recovered. The underlying mechanism for the conformational transition of the PDEAEMA brush is the switchable polymer solubility governed by the protonation and deprotonation of the tertiary amine groups as a result of pH decrease (with CO2) and pH recovery (after CO2 removal), respectively. However, as shown below, unlike the conventional pH change induced by adding acids and bases, such CO2-switchable water solubility can be repeated many times for reversible adsorption and desorption of a protein without contamination of the solution by accumulated salt. This is the main advantage of using gases as the trigger.
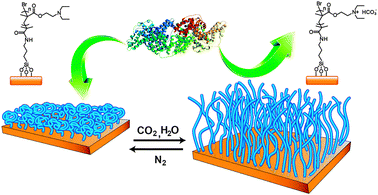 | ||
| Fig. 1 Schematic of protein capture and release using CO2-responsive polymer brushes. Collapsed polymer brushes (before exposure to CO2 or upon N2 bubbling that removes CO2) form a surface favorable for protein attachment, while polymer brushes in chain-extended conformation (upon exposure to CO2) form a surface unfavorable for protein adsorption, leading to the release of adsorbed proteins. The chemical structure of CO2-responsive brushes is also shown. | ||
PDEAEMA brushes grafted onto either a silicon or a gold surface were obtained by using surface-initiated atom transfer radical polymerization (SI-ATRP) (for synthesis and characterization details see ESI,† Scheme S1).13 Unless otherwise stated, the PDEAEMA brush has a thickness of ∼21 nm at the collapsed state and a grafting density of ∼0.42 chains nm−2. The reversible conformational changes of PDEAEMA were investigated in different ways. Firstly, static water contact angle measurements were carried out with PDEAEMA brushes on a silicon wafer. As seen in Fig. 2a, upon exposure to CO2-purged aqueous solution for 30 min, the water contact angle shows a drastic decrease from 90° to 21°, indicative of polymer brush hydration. After passing N2 gas for 10 min, the initial contact angle was recovered, suggesting the loss of water from the polymer brushes and their collapse upon CO2 removal. The experiments were repeated several times; the switching of the water contact angle on alternating bubbling with CO2 and N2 is complete. This gas-controlled polymer brush water solubility was further studied by using a quartz crystal microbalance (QCM), with which the adsorption process can be detected through a frequency shift (Δf) that is sensitive to the wet mass including the mass of trapped water. For this experiment, PDEAEMA brushes were grafted onto a gold-coated QCM crystal by SI-ATRP. The polymer brush sample was clamped within a flow cell through which ultrapure water was flowed by means of a peristaltic pump at a rate of 81 μL min−1. At first, the changes in frequency of the PDEAEMA brush immersed in water were set to zero at equilibrium. As seen in Fig. 2b, when CO2 was bubbled through the solution, the Δf value decreased until a plateau (Δf ≈ −125) was reached after ∼30 min. The significant frequency shift indicates a high water uptake by the polymer brush. Upon passing N2 through the solution, Δf increased to the initial level, indicating dehydration of the polymer brush and release of water molecules bound to the brush. Again, the change in the apparent amount of water on the substrate surface is reversible for several cycles of alternating presence and absence of CO2 in the solution. Finally, the gas-induced change between extended and collapsed states of the PDEAEMA brush was directly observed using in situ atomic force microscopy (AFM) with the sample in a liquid environment. Fig. 2c shows that when imaged under water in the absence of CO2, the height of PDEAEMA brushes was about 20 nm (collapsed state), the brush surface being essentially flat. The apparent height fluctuations are likely from the underlying roughness of the silica substrate and/or the inherent polydispersity of the grafted polymer chain lengths. After exposure to CO2 in water for 30 min, the PDEAEMA brushes were clearly swollen, resulting in an increase in the height from 20 to 40 nm. The change between the swollen and collapsed states was reversible.
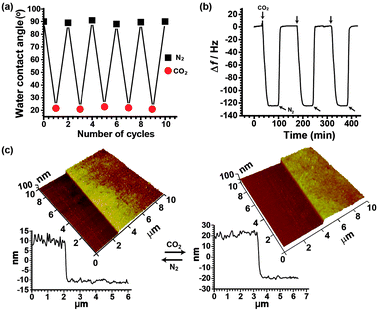 | ||
| Fig. 2 Switching of (a) advancing water contact angles, (b) frequency of quartz crystal microbalance (on a Au resonator), (c) 3D aqueous AFM height images (on a Si wafer) and corresponding cross-sectional profiles obtained for the surface grafted PDEAEMA brush upon treatment with CO2 (chain-extended) and collapse upon treatment with N2. | ||
To demonstrate possible applications of this gas-controllable polymer surface, we investigated its use in reversible adsorption and release of a model protein: bovine serum albumin (BSA), and compared the result with conventional pH control. The BSA adsorption on PDEAEMA surfaces was evaluated by using QCM, with which the adsorbed mass of proteins could be analyzed in situ through the frequency shift (Δf). The QCM analysis setup (Fig. S3, ESI†) consisted of a PDEAEMA brush grafted onto an AT cut quartz crystal resonator that was placed in a flow cell and had one side exposed to the solution. A peristaltic pump was used to drive the protein solution from a reservoir bottle to the flow cell (81 μL min−1). A feed solution purged with either CO2 or N2 was used for switching the PDEAEMA brush using two gases, while another feed solution with its pH adjusted to either 5 or 7 by adding acids or bases was used for comparison. Fig. 3 shows results obtained with BSA (1 mg mL−1) incubated in phosphate buffered saline (PBS, 10 mM) with both stimuli. In the case of CO2-triggered switching, with PDEAEMA initially in the collapsed state, the adsorption of BSA increased over time, with the decreasing Δf reaching a plateau after ∼40 min. Protein not firmly adsorbed was removed by rinsing with PBS buffer solution (10 mM). After rinsing, the adsorption of BSA showed Δf of ∼568 Hz, corresponding to an apparent adsorption quantity (Δm) of about 0.11 μg cm−2. By bubbling CO2 in the PBS solution flowing through the cell, the frequency rose quickly, indicating release of adsorbed protein as a result of the conformational transition of the polymer brush from the collapsed to the extended state. Release of ∼95% BSA occurred after 30 min. It is known that hydrophilic swollen polymer brushes are effective for preventing the binding of proteins.14 Subsequently, upon passing N2 through the solution, which switched the PDEAEMA brush back to the collapsed sate, protein adsorption occurred again when a fresh PBS solution flowed through the cell. Similarly, pH-responsive protein adsorption–desorption was measured at different pH values (pH 5 and 7). As seen in Fig. 3a, the first pH change cycle resulted in BSA adsorption and desorption similar to the CO2/N2 stimuli. At pH 7, PDEAEMA chains in the collapsed state gave rise to a similar frequency decrease, while upon exposure to pH 5 solution making PDEAEMA chains hydrated, the frequency increased, indicating desorption of BSA. Interestingly over repeated cycles of switching, while the frequency shift corresponding to the BSA adsorption remained almost the same within the error range (±42 Hz) in the case of CO2 switching, repeated pH switching resulted in a dramatic decrease in the change in frequency as the cycle number increased from 2 to 7, with the PDEAEMA brush appearing to be inactive upon further pH changes. For example, at the 7th cycle, a frequency change of ∼572 Hz was observed with the gases, compared to a frequency change of ∼128 Hz with the pH change. Here we mention that in order to observe the effect of accumulated salt, the same BSA solution after releasing at pH 5 was used for adsorption at pH 7. Fig. 3b shows the repeated switching of the protein capture and release under the effect of the two stimuli. The cycle as controlled by passing alternately CO2 and N2 in the solution could be repeated many times with no degradation of the brushes in terms of performance. By contrast, switching using the pH effect underwent continuous degradation. In fact, repeated pH switch will increase the salinity of incubated medium and leads to a reduction in the attractive electrostatic interaction and consequently, a marked reduction in the amount of protein adsorbed. It was reported that the adsorption of proteins on the polyelectrolytes surface can be changed by controlling the electrostatic interactions between them.15 Also, no protein adsorption takes place when the ionic strength of the system is high where the electrostatic attractions were screened.16 These results show that repeated capture and release of BSA can readily be achieved using gases, but cannot be obtained with changing pH due to accumulated salt in solution. Finally, we mention that control experiments upon the adsorption of BSA on the bare gold surface and on non-stimuli-responsive hydrophilic and hydrophobic polymer brushes were carried out. The results (Fig. S7 and S8, ESI†) confirm that the responsiveness of the PDEAEMA brush to CO2 is the origin of the reversible adsorption and desorption of the protein.
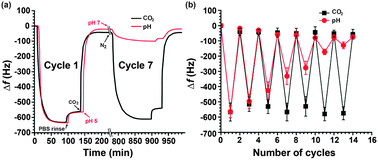 | ||
| Fig. 3 Quartz crystal microbalance response: (a) time-dependent frequency changes for the adsorption of BSA on a PDEAEMA brush surface upon incubation with alternating CO2/N2 bubbling or pH change (between 5 and 7); arrows indicate where gas was injected into or pH change was made to the solution; (b) frequency changes (Δf) for the adsorption of BSA over repeated cycles of switching triggered by gases or pH change. | ||
In conclusion, we have demonstrated a novel stimuli-responsive polymer surface that exhibits gas-controllable switching between extended and collapsed chain conformations. We have further investigated its use in gas-controlled reversible capture and release of a protein. Unlike switching induced by adding acids and bases to change the pH of the solution, the use of CO2-switchable polymer brushes allows the protein adsorption and desorption cycles to be repeated many times at room temperature without salt accumulation that can deteriorate the switching behavior.
We acknowledge the financial support from Le Fonds Quebecois de la Recherche sur la Nature et les Technologies of Quebec (FQRNT) and the Natural Sciences and Engineering Research Council of Canada (NSERC). We also thank Mrs Sonia Blais for help with the XPS measurements and analyses, and Prof. Hugues Menard for QCM. M.L. is the Canada Research Chair in Magnetic Resonance Imaging and a member of the FRSQ-funded Centre de recherche clinique Etienne-Le Bel. Y.L.D. and Y.Z. are members of the FQRNT-funded Center for Self-Assembled Chemical Structures (CSACS) and Centre québécois sur les matériaux fonctionnels (CQMF).
Notes and references
-
(a) M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101 CrossRef
; (b) T. Chen, R. Ferris, J. Zhang, R. Ducker and S. Zauscher, Prog. Polym. Sci., 2010, 35, 94 CrossRef CAS
.
- Z. Zhang, S. Chen and S. Jiang, Biomacromolecules, 2006, 7, 3311 CrossRef CAS
.
- I. Tokarev and S. Minko, Adv. Mater., 2009, 21, 241 CrossRef CAS
.
- K. Christian, H. Andreas and S. Wolfgang, Anal. Chem., 2002, 74, 355 CrossRef
.
-
(a) H. Ma, J. Hyun, P. Stiller and A. Chilkoti, Adv. Mater., 2004, 16, 338 CrossRef CAS
; (b) J. L. Dalsin, B.-H. Hu, B. P. Lee and P. B. Messersmith, J. Am. Chem. Soc., 2003, 125, 4253 CrossRef CAS
.
- R. Blossey, Nat. Mater., 2003, 2, 301 CrossRef CAS
.
-
(a) R. Barbey, L. Lavanant, D. Paripovic, N. Schower, C. Sugnaux, S. Tugulu and H. A. Klok, Chem. Rev., 2009, 109, 5437 CrossRef CAS
; (b) S. Edmondson, V. L. Osborne and W. T. S. Huck, Chem. Soc. Rev., 2004, 33, 14 RSC
.
-
(a) E. Stratakis, A. Mateescu, M. Barberoglou, M. Vamvakaki, C. Fotakisae and S. H. Anastasiadis, Chem. Commun., 2010, 46, 4136 RSC
; (b) N. Schuwer and H. A. Klok, Langmuir, 2011, 27, 4789 CrossRef CAS
; (c) D. M. Jones, J. Smith, W. T. S. Huck and C. Alexander, Adv. Mater., 2002, 14, 1130 CrossRef CAS
; (d) N. Lomadze, A. Kopyshev, J. Rühe and S. Santer, Macromolecules, 2011, 44, 7372 CrossRef CAS
; (e) T. K. Tam, M. Ornatska, M. Pita, S. Minko and E. Katz, J. Phys. Chem. C, 2008, 112, 8438 CrossRef CAS
.
-
(a) P. Jain, M. K. Vyas, J. H. Geiger, G. L. Baker and M. L. Bruening, Biomacromolecules, 2010, 11, 1019 CrossRef CAS
; (b) I. Banerjee, R. C. Pangule and R. S. Kane, Adv. Mater., 2011, 23, 690 CrossRef CAS
; (c) S. Kumar, M. Dory, Y. L. Lepage and Y. Zhao, Macromolecules, 2011, 44, 7385 CrossRef CAS
.
-
(a) M. Motornov, R. Sheparovych, E. Katz and S. Minko, ACS Nano, 2008, 2, 41 CrossRef CAS
; (b) L. A. Fielding, S. Edmondson and S. P. Armes, J. Mater. Chem., 2011, 21, 11773 RSC
.
-
(a) P. G. Jessop, D. J. Heldebrant, X. Li, C. A. Eckert and C. L. Liotta, Nature, 2005, 436, 1102 CrossRef CAS
; (b) Q. Yan, R. Zhou, C. Fu, H. Zhang, Y. Yin and J. Yuan, Angew. Chem., Int. Ed., 2011, 50, 4923 CrossRef CAS
; (c) S. S. Satav, S. Bhat and S. Thayumanavan, Biomacromolecules, 2010, 11, 1735 CrossRef CAS
; (d) Z. Guo, Y. Feng, Y. Wang, J. Wang, Y. Wu and Y. Zhang, Chem. Commun., 2011, 47, 9348 RSC
.
-
(a) D. Han, X. Tong, O. Boissière and Y. Zhao, ACS Macro Lett., 2012, 1, 57 CrossRef CAS
; (b) D. Han, O. Boissiere, S. Kumar, X. Tong, L. Tremblay and Y. Zhao, Macromolecules, 2012, 45, 7440 CrossRef CAS
.
- R. Barbey and H.-A. Klok, Langmuir, 2010, 26, 18219 CrossRef CAS
.
- S. Herrwerth, W. Eck, S. Reinhardt and M. Grunze, J. Am. Chem. Soc., 2003, 125, 9359 CrossRef CAS
.
- M. Malmsten, J. Colloid Interface Sci., 1998, 207, 186 CrossRef CAS
.
- A. Wittemann and M. Ballauff, Anal. Chem., 2004, 76, 2813 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Polymer brush synthesis details, XPS data, FTIR spectra, QCM studies, zeta potential measurements, HPLC analysis and circular dichroism of proteins. See DOI: 10.1039/c2cc36284h |
| This journal is © The Royal Society of Chemistry 2013 |
