 Open Access Article
Open Access ArticleIonic conductivity of [Li+@C60](PF6−) in organic solvents and its electrochemical reduction to Li+@C60˙−
Hiroshi
Ueno
a,
Ken
Kokubo
*a,
Yuji
Nakamura
a,
Kei
Ohkubo
b,
Naohiko
Ikuma
a,
Hiroshi
Moriyama
c,
Shunichi
Fukuzumi
bd and
Takumi
Oshima
a
aDivision of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan. E-mail: kokubo@chem.eng.osaka-u.ac.jp; Fax: +81-6-6879-4593; Tel: +81-6-6879-4592
bDepartment of Material and Life Science, Graduate School of Engineering, Osaka University, and ALCA, Japan Science and Technology (JST), Suita, Osaka 565-0871, Japan
cDepartment of Chemistry, Faculty of Science, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510, Japan
dDepartment of Bioinspired Science, Ewha Womans University, Seoul, 120-750, Korea
First published on 27th June 2013
Abstract
The ionic conductivity of [Li+@C60](PF6−) was measured in o-dichlorobenzene, and found to be higher than that of TBA+PF6−. Electrochemical reduction of [Li+@C60](PF6−) without any supporting electrolyte gave the monovalent radical anion Li+@C60˙−, as confirmed by the characteristic ESR signal and NIR absorption band.
Lithium-cation-encapsulated [60]fullerene Li+@C60, which was first isolated as the [Li+@C60](SbCl6−) salt, is the only known endohedral metallo[60]fullerene with 100% encapsulation ratio, and its structure has been established by single crystal X-ray analysis.1–3 It has attracted growing attention because of its unique properties such as stronger electron acceptability than pristine C60 and high reactivities in photoinduced electron transfer and regioselective multihydroxylation.4–8 The most remarkable property of the [Li+@C60] salt is its high ionicity resulting from the encapsulated lithium cation. Although this ionic nature of [Li+@C60](PF6−) is stated to be responsible for its rock-salt-type crystal structure,2 no other details of the effects of ionicity have been reported so far.
The fullerene radical anion has been recognised as an intriguing material because of its unique electronic properties such as (super)conductivity and ferromagnetism.9–12 In addition, the radial anion can serve as an important reaction intermediate for various unique chemical modifications of the fullerene cage.13 Although many methods for the preparation of empty C60 radical anion salts have been reported,14 studies on endohedral fullerene radical anions are relatively stagnant.
We herein report the high ionic conductivity of [Li+@C60](PF6−) in organic solvents, which has enabled a facile electrochemical synthesis of the radical anion (Li+@C60˙−) without any supporting electrolyte.
The ionic conductivity measurements were performed in o-dichlorobenzene (o-DCB) and benzonitrile (PhCN) since [Li+@C60](PF6−) showed sufficient solubility and electrochemical stability in these solvents.† The molar conductivity Λ is defined in eqn (1), where κ is the measured conductivity at each concentration (c). The Λ values for various concentrations
| Λ = κ/c | (1) |
 measured in PhCN (red circles) and o-DCB (blue squares) solutions containing various concentrations of [Li+@C60](PF6−) at 298 K. The black line is the result of TBA+PF6− measured as a reference (asterisks). The picture shows the various concentrations of [Li+@C60](PF6−) in o-DCB solutions.](/image/article/2013/CC/c3cc43901a/c3cc43901a-f1.gif) | ||
| Fig. 1 Molar conductivity of [Li+@C60](PF6−) measured in PhCN (red circles) and o-DCB (blue squares) solutions containing various concentrations of [Li+@C60](PF6−) at 298 K. The black line is the result of TBA+PF6− measured as a reference (asterisks). The picture shows the various concentrations of [Li+@C60](PF6−) in o-DCB solutions. | ||
![Electrostatic potential of the [Li+@C60] cation and the distance between encapsulated Li+ and outer PF6− obtained by the theoretical calculation (B3LYP/6-31G* method), the distances between Li+ and P for Li+@C60 and LiPF6, and the Mulliken charges on C and Li+ atoms of the [Li+@C60] cation.](/image/article/2013/CC/c3cc43901a/c3cc43901a-f2.gif) | ||
| Fig. 2 Electrostatic potential of the [Li+@C60] cation and the distance between encapsulated Li+ and outer PF6− obtained by the theoretical calculation (B3LYP/6-31G* method), the distances between Li+ and P for Li+@C60 and LiPF6, and the Mulliken charges on C and Li+ atoms of the [Li+@C60] cation. | ||
| c/μM | κ/mS m−1 | Λ/mS m−2 mol−1 |
|---|---|---|
| a Values in parentheses are measured in PhCN. b Not determined due to the solubility limitation. | ||
| [Li+@C60](PF6−) | ||
| 1.6 | 0.006 (0.008)a | 3.75 (5.00)a |
| 8 | 0.023 (0.029)a | 2.88 (3.63)a |
| 40 | 0.107 (0.133)a | 2.68 (3.33)a |
| 200 | 0.466 (0.646)a | 2.33 (3.23)a |
| 1000 | 1.688 (—b)a | 1.69 (—b)a |
| TBA+PF6− | ||
| 1.6 | 0.005 | 3.13 |
| 8 | 0.022 | 2.75 |
| 40 | 0.078 | 1.95 |
| 200 | 0.238 | 1.19 |
| 1000 | 0.649 | 0.50 |
| Pristine C60 | ||
| Any | 0.000 | 0.00 |
To discuss the ionizability of [Li+@C60](PF6−), the electrostatic potential of the [Li+@C60] cation and the distance between the cation and the PF6− anion at the most stable position were calculated by the density functional theory (DFT) at the B3LYP/6-31G* level. The result showed that the positive charge of the [Li+@C60] cation was not only located on the encapsulated Li+ but also strongly delocalised on the carbon atoms of the C60 cage. Because of the thermal motion of the encapsulated Li+ in the C60 cage, the charge delocalization might be more dynamically significant.2 Furthermore, the Li–P distance of [Li+@C60](PF6−) was calculated to be 5.65 Å, which was much longer than that in LiPF6 (2.63 Å). This difference implied that the positional relationship observed between Li+ and PF6− in [Li+@C60](PF6−) is unusual in common ion pairs, indicating that the electrostatic attractive force between the [Li+@C60] cation and PF6− is weak due to the delocalised positive charge on the C60 cage and the distance limitation by this cage. This result is consistent with the previous report that the encapsulated Li+ is in thermal motion at around room temperature even if the [Li+@C60] cation forms an ion pair with PF6−.2
Moreover, the solvation effect is also important for the ion pair formation.15 Because C60 is a π-conjugated molecule, there must be strong π–π interaction between the cage and the aromatic solvent molecules, and the solvation can stabilize the ionized [Li+@C60] cation.16 Thus, [Li+@C60](PF6−) tends to ionize, showing ionic conductivity, to be used as a unique electrolyte which, despite its high ionicity, can be used in low-polarity aromatic solvents. Incidentally, the higher value of Λ in PhCN than that in o-DCB was explained by the difference in the relative permittivity (εr) between PhCN (εr = 25.2) and o-DCB (9.93).17
Based on the above results, we attempted at synthesis of the Li+-encapsulated fullerene radical anion electrochemically without using any supporting electrolyte. All the synthetic process was carried out under N2 atmosphere. The synthetic scheme is shown in Fig. 3. An o-DCB solution of [Li+@C60](PF6−) (0.25 mg mL−1, 0.29 μM) was set in an H-type cell, cooled to 278 K, and electrolyzed using a Pt electrode at a constant current (0.3 μA) for 3–4 days. The homogeneous purple solution gradually became gradated because of ion conduction by the [Li+@C60] cation and PF6−. The cation was electrochemically reduced at the cathode, and a monovalent radical anion of Li+-encapsulated fullerene Li+@C60˙− was selectively formed.
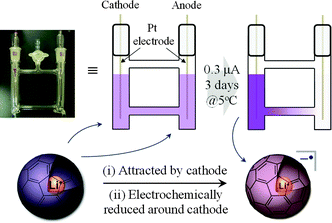 | ||
| Fig. 3 Pattern diagram of the electrochemical synthesis of Li+@C60˙−. | ||
The generated Li+@C60˙− was characterised by UV-vis-NIR and ESR spectroscopy. 7Li and 13C NMR, dynamic light scattering (DLS), and ζ potential distribution measurements did not furnish meaningful signals/results, probably because of the low concentration of the target species, in addition to paramagnetic relaxation. The UV-vis-NIR spectra of the solution collected from the cathode side after 3 days are shown in Fig. 4, along with the spectrum of the starting [Li+@C60](PF6−) solution. The absorption maximum observed at 1035 nm was clearly assignable to the monovalent radical anion of the Li+-encapsulated C60.7,8 The generation of Li+@C60˙− was also confirmed by the ESR spectrum (Fig. 5). The observed g value (2.00058) clearly indicated that the monovalent radical anion was exclusively produced through one-electron reduction. At 77 K, the thermal motion of the encapsulated Li+ may stop, and thus, the spectrum showed a sharp signal. The calculated spin density of Li+@C60˙− for the optimised structure indicated that the delocalised spin density is somewhat close to the encapsulated Li+. However, because of the small hyperfine coupling constant of the Li species, the interaction between the encapsulated Li+ and the spin centre could not be evidenced from the spectrum. Although the ζ potentials of the starting [Li+@C60](PF6−) and the resulting Li+@C60˙− could not be compared because of the above reasons, the two species could be clearly distinguished from each other on the basis of the calculated electrostatic potential (Fig. 6). This result indicated that the chemical reactivities of the two types of Li+-encapsulated fullerenes, the external-counter-anion-type [Li+@C60](PF6−) and the cation-encapsulated-anion-type Li+@C60˙−, are much different, and hence, Li+@C60˙− is a potential nucleophilic reactant for unique chemical transformations.
 (red line) and starting [Li+@C60](PF6−) solution (blue line) in o-DCB. The inset shows the expanded view of the NIR region of the spectrum of the product.](/image/article/2013/CC/c3cc43901a/c3cc43901a-f4.gif) | ||
| Fig. 4 UV-vis-NIR absorption spectra of the product solution by electrochemical reduction of [Li+@C60](PF6−) (red line) and starting [Li+@C60](PF6−) solution (blue line) in o-DCB. The inset shows the expanded view of the NIR region of the spectrum of the product. | ||
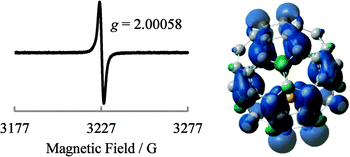 | ||
| Fig. 5 ESR spectrum of the product measured at 77 K (left) and calculated spin density (B3LYP/6-31G* method) of Li+@C60˙− in the optimised structure (right). | ||
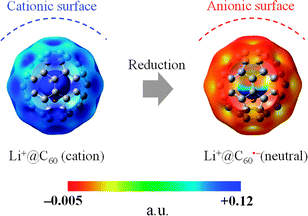 | ||
| Fig. 6 Calculated electrostatic potential (B3LYP/6-31G* method) of Li+@C60 (left) and Li+@C60˙− (right). The left figure is different from Fig. 2 only in the potential range. Li+@C60˙− had a slightly negative potential on the C60 cage (∼0.022). In order to simplify the map, the range was set in +0.002 to +0.12. | ||
In conclusion, the ionic conductivity of Li+-encapsulated [60]fullerene [Li+@C60](PF6−) was measured in two organic solvents, o-DCB and PhCN. [Li+@C60](PF6−) showed higher ionic conductivity than TBA+PF6−, indicating the possibility of electrochemical applications even in the absence of any supporting electrolyte. Furthermore, the Li+-encapsulated [60]fullerene monovalent radical anion Li+@C60˙− was synthesised selectively by a very facile electrochemical method. Further studies on the use of anion-exchanged [Li+@C60], application of [Li+@C60](PF6−) as a unique electrolyte, and derivatisation via the anion species are now in progress, in addition to the synthesis and characterization of multivalent anions.
This work was supported by Grant-in-Aid for Exploratory Research (No. 23651111 to K.K.; No. 23750014 to K.O.; No. 23550211 to H.M. & 23108010 to S.F.) from JSPS, Japan, Health Labour Sciences Research Grants from MHLW, Japan, and a WCU project (R31-2008-000-10010-0) through KOSEF/MEST, Korea.
References
- S. Aoyagi, E. Nishibori, H. Sawa, K. Sugimoto, M. Takata, Y. Miyata, R. Kitaura, H. Shinohara, H. Okada, T. Sakai, Y. Ono, K. Kawachi, K. Yokoo, S. Ono, K. Omote, Y. Kasama, S. Ishikawa, T. Komuro and H. Tobita, Nat. Chem., 2010, 2, 678 CrossRef CAS.
- S. Aoyagi, Y. Sado, E. Nishibori, H. Sawa, H. Okada, H. Tobita, Y. Kasama, R. Kitaura and H. Shinohara, Angew. Chem., Int. Ed., 2012, 51, 3377 CrossRef CAS.
- H. Okada, T. Komuro, T. Sakai, Y. Matsuo, Y. Ono, K. Omote, K. Yokoo, K. Kawachi, Y. Kasama, S. Ono, R. Hatakeyama, T. Kaneko and H. Tobita, RSC Adv., 2012, 2, 10624 RSC.
- H. Ueno, Y. Nakamura, N. Ikuma, K. Kokubo and T. Oshima, Nano Res., 2012, 6, 558 CrossRef.
- H. Ueno, K. Kokubo, E. Kwon, Y. Nakamura, N. Ikuma and T. Oshima, Nanoscale, 2013, 5, 2317 RSC.
- Y. Matsuo, H. Okada, M. Maruyama, H. Sato, H. Tobita, Y. Ono, K. Omote, K. Kawachi and Y. Kasama, Org. Lett., 2012, 14, 3784 CrossRef CAS.
- S. Fukuzumi, K. Ohkubo, Y. Kawashima, D. S. Kim, J. S. Park, A. Jana, V. M. Lynch, D. Kim and J. L. Sessler, J. Am. Chem. Soc., 2011, 133, 15938 CrossRef CAS.
- (a) K. Ohkubo, Y. Kawashima and S. Fukuzumi, Chem. Commun., 2012, 48, 4314 RSC; (b) Y. Kawashima, K. Ohkubo and S. Fukuzumi, J. Phys. Chem. A, 2012, 116, 8942 CrossRef CAS.
- K. Tanigaki, I. Hirosawa, T. W. Ebbesen, J. Mizuki, Y. Shimakawa, Y. Kubo, J. S. Tsai and S. Kuroshima, Nature, 1992, 356, 419 CrossRef CAS.
- A. Y. Ganin, Y. Takabayashi, P. Jeglič, D. Arčon, A. Potočnik, P. J. Baker, Y. Ohishi, M. T. McDonald, M. D. Tzirakis, A. Mclennan, G. R. Darling, M. Takata, M. J. Rosseinsky and K. Prassides, Nature, 2010, 466, 221 CrossRef CAS.
- (a) H. Moriyama, H. Kobayashi, A. Kobayashi and T. Watanabe, J. Am. Chem. Soc., 1993, 115, 1185 CrossRef CAS; (b) H. Kobayashi, H. Tomita, H. Moriyama, A. Kobayashi and T. Watanabe, J. Am. Chem. Soc., 1994, 116, 3153 CrossRef CAS; (c) H. Moriyama, M. Abe, H. Motoke, T. Watanabe, S. Hayashi and H. Kobayashi, Synth. Met., 1998, 94, 167 CrossRef CAS.
- P. W. Stephens, D. Cox, J. W. Lauher, L. Mihaly, J. B. Wiley, P.-M. Allemand, A. Hirsch, K. Holczer, Q. Li, J. D. Thompson and F. Wudl, Nature, 1992, 355, 331 CrossRef CAS.
- K. Kokubo, R. S. Arastoo, T. Oshima, C. C. Wang, Y. Gao, H. L. Wang, H. Geng and L. Y. Chiang, J. Org. Chem., 2010, 75, 4574 CrossRef CAS.
- C. A. Reed and R. D. Bolskar, Chem. Rev., 2010, 100, 1075 CrossRef.
- Y. Marcus and G. Hefter, Chem. Rev., 2006, 106, 4585 CrossRef CAS.
- T. Oshima, T. Mikie, N. Ikuma and H. Yakuma, Org. Biomol. Chem., 2010, 10, 1730 Search PubMed.
- H. F. Friedman and B. Larsen, J. Chem. Phys., 1979, 70, 92 CrossRef CAS.
Footnote |
| † General procedure: [Li+@C60](PF6−) was obtained from Idea International Corporation and purified by recrystallization from chlorobenzene/acetonitrile. All other reagents were commercially available and used without further purification. Ionic conductivity measurement was performed using a HORIBA ES-51 under N2 atmosphere. UV-vis-NIR spectra were recorded on a Shimadzu UV-1800 spectrometer. The ESR spectrum was measured on a JEOL JES-FA100. A YAZAWA CS-12Z constant current regulator was used for electrochemical synthesis of Li+@C60˙−. |
| This journal is © The Royal Society of Chemistry 2013 |
