Nanosheet-assembled hierarchical nanostructures of hydroxyapatite: surfactant-free microwave-hydrothermal rapid synthesis, protein/DNA adsorption and pH-controlled release
Xin-Yu
Zhao
,
Ying-Jie
Zhu
*,
Feng
Chen
,
Bing-Qiang
Lu
and
Jin
Wu
State Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai, 200050, P. R. China. E-mail: y.j.zhu@mail.sic.ac.cn; Fax: +86 21-52413122
First published on 24th October 2012
Abstract
In this paper, a surfactant-free rapid microwave-assisted hydrothermal synthesis of hydroxyapatite nanosheet-assembled flower-like hierarchical nanostructures (NFHNs) is reported. The effects of the experimental conditions on the morphology and crystal phase of the product are investigated. A possible formation mechanism of hydroxyapatite NFHNs is proposed. The morphology of the product can vary from flower-like to polyhedra by adjusting the microwave heating temperature. The protein and DNA adsorption properties of the as-prepared hydroxyapatite NFHNs are studied. The loading capacities of the as-prepared hydroxyapatite NFHNs for bovine serum albumin (BSA), hemoglobin (Hb) and fish sperm DNA are determined to be 165, 164 and 112 mg g−1, respectively. The protein release process is conducted at different pH values (pH 7.2, 5.5 and 4.8) in phosphate buffer saline (PBS), and the pH-controlled protein release behavior has been found. Thus, the as-prepared hydroxyapatite NFHNs are promising for protein drug delivery applications.
1 Introduction
Hydroxyapatite (HA) is the most stable calcium phosphate phase under physiological conditions and it exists all over the human body as the main inorganic constituent of bone and tooth.1 Due to its outstanding biocompatibility and high stability, synthetic HA nanostructures have been considered as excellent candidates for biomedical applications. Synthetic HA nanostructures with different morphologies such as nanorods, nanowires, nanotubes and three-dimensional structures have been prepared2–6 and they have been investigated for applications in bone repair, tissue engineering, drug and gene delivery, and other biomedical areas.7–10 Recently, this research group synthesized flower-like nanostructured hydroxyapatite hollow microspheres assembled with nanosheets using NaH2PO4, Na2HPO4, Ca(CH3COO)2 and block copolymer poly(lactide)-block-poly(ethylene glycol) (PLA-PEG) by a rapid microwave-assisted hydrothermal route at 120 °C for a short period of time (5 min), and the hydroxyapatite hollow microspheres were explored as anticancer drug carriers for cellular delivery of mitoxantrone, which exhibited sustained drug release behavior in vitro.5 The DNA-templated hydrothermal synthesis of HA nanosheet-assembled hollow microspheres with a nanoporous structure was also reported.11HA generally exists in the hexagonal structure and provides two different binding sites (positively charged C sites and negatively charged P sites) on its surface.12 Thus, HA has a high affinity for many substances such as proteins due to its surface properties and has been studied as an adsorbent for blood purification13,14 and a carrier for protein drugs.11 High adsorption capacity for proteins and sustained protein release property are expected for HA nanostructures as drug carriers. HA nanostructures are also investigated as promising carriers for gene transfection.15–20
The solubility of HA increases with the decrease in the pH value of solution, and HA can be dissolved to form nontoxic ions (Ca2+, PO43−) in aqueous solution with a low pH value. It is well-known that the pH values in tumors and inflammatory tissues are lower than those in blood and normal tissues, and the acidic cellular environments exhibit even lower pH values (e.g. endosomes (pH ≈ 5.0) and lysosomes (pH ≈ 4.5)).21,22 Thus, HA nanostructures can be designed and used as pH-sensitive drug nanocarriers that can respond to physiopathological pH signals to control the drug release process.
Herein, we report a surfactant-free rapid microwave-assisted hydrothermal synthesis of hydroxyapatite NFHNs. The effects of the reaction conditions on the morphology and crystal phase of the product were investigated and a possible formation mechanism was proposed. The protein loading and pH-controlled release properties of hydroxyapatite NFHNs were studied. Bovine serum albumin (BSA) and hemoglobin (Hb) were used as model proteins and fish sperm DNA was used as model DNA to evaluate the loading and release properties of as-prepared hydroxyapatite NFHNs. The protein loading capacity of as-prepared hydroxyapatite NFHNs for BSA and Hb were determined to be 165 mg g−1 and 164 mg g−1, respectively. The protein drug release tests were conducted at different pH values (pH = 7.2, 5.5, and 4.8) in phosphate buffer saline (PBS) and the pH-controlled release behaviors were investigated. The experimental results indicate that the as-prepared hydroxyapatite NFHNs have a high protein and DNA loading capacity and excellent pH-controlled protein release behavior in PBS.
2 Experimental section
Materials
Bovine serum albumin (BSA) was purchased from Aladdin Chemistry Co., Ltd. Hemoglobin (Hb) and fish sperm DNA (FS-DNA) were purchased from Sangon Biotech (Shanghai) Co., Ltd. Other chemicals were purchased from Sinopharm Chemical Reagent Co. All chemicals were used as received without further purification.Preparation of NFHNs of hydroxyapatite
In a typical experiment, 0.140 g Ca(CH3COO)2·H2O was dissolved in 10 mL deionized water under magnetic stirring to form Solution A. 0.125 g NaH2PO4·2H2O was dissolved in 20 mL deionized water under magnetic stirring to form Solution B. Then, Solution A was added into Solution B and the pH value of the resulting solution was adjusted to 4.5 using 1 M HCl. The transparent precursor solution was transferred to a Teflon autoclave (70 mL), sealed, and microwave-heated to 100 °C and kept at this temperature for 5 min. The microwave oven used was a microwave-solvothermal synthesis system (MDS-6, Sineo, China). The product was collected by centrifugation, washed with deionized water twice and absolute ethanol twice, and dried at 60 °C in air.Protein loading in hydroxyapatite NFHNs
Bovine serum albumin (BSA) and hemoglobin (Hb) were used as model proteins in this study. The powder of hydroxyapatite NFHNs (5 mg) was immersed in aqueous solutions (2 mL) containing variable protein concentrations (0–2000 μg mL−1). Each solution was shaken at a constant rate for 4 h at 37 °C. Then, the solution was centrifuged and the amount of protein in the supernatant was measured by the UV-vis absorption analysis at 406 nm for Hb and BCA (bicinchoninic acid) protein assay (Beyotime, China) at 562 nm for BSA.DNA loading in hydroxyapatite NFHNs
Fish sperm DNA (FS-DNA) was used as a model DNA. The powder (1 mg) of hydroxyapatite NFHNs was immersed in aqueous solutions (1 mL) containing variable DNA concentrations (0–1000 μg mL−1). Each solution was shaken at a constant rate for 4 h at room temperature. Then, the solution was centrifuged and the amount of DNA in the supernatant was measured by the UV-vis absorption analysis at 260 nm.pH-controlled protein release
The powder (50 mg) of hydroxyapatite NFHNs was immersed in 20 mL hemoglobin (Hb) aqueous solution with a concentration of 2000 μg mL−1, and the solution was shaken at a constant rate for 4 h at 37 °C to obtain the Hb–HA drug delivery system. The in vitro protein release experiments were performed as follows: the Hb–HA drug delivery system (4 mg) was immersed into 2 mL phosphate buffer saline (PBS) at 37 °C under shaking at a rate of 140 rpm. The protein release solution (400 μL) was withdrawn for UV-vis absorption analysis at given time intervals, and the withdrawn solution was replaced with the same volume of fresh PBS. PBS solutions with different pH values (7.2, 5.5 and 4.8) were used as the release media. Three parallel experiments were performed for each measurement and the result was presented as the mean and standard deviation.Characterization
X-ray powder diffraction (XRD) patterns were recorded using a Rigaku D/max 2550V X-ray diffractometer with a graphite monochromator and a high-intensity Cu Kα radiation (λ = 1.54178 Å). Fourier transform infrared (FT-IR) spectra were recorded on a spectrophotometer (FT-IR-7600, Lambda Scientific, Australia). Scanning electron microscopy (SEM) micrographs were performed on a field-emission scanning electron microscope (S-4800, Hitachi, Japan). UV-vis absorption spectra were taken on a spectrophotometer (UV-2300, Techcomp). The Brunauer–Emmett–Teller (BET) specific surface area was measured by a surface area and pore size analyzer (V-Sorb 2800P, Gold APP, China). The zeta potential values were measured using a zeta potential analyzer (ZetaPlus, Brookhaven Instruments).3 Results and discussion
The fabrication procedure of hydroxyapatite NFHNs and protein loading and release processes are illustrated in Scheme 1. A surfactant-free rapid microwave-assisted hydrothermal synthetic strategy is employed to prepare hydroxyapatite NFHNs. Under the condition of microwave heating, hydroxyapatite nanosheets are firstly formed. Then these nanosheets are self-assembled to form flower-like hierarchical nanostructures of hydroxyapatite.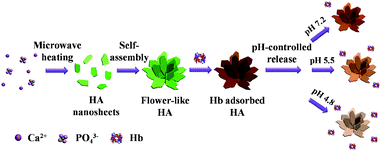 | ||
| Scheme 1 Schematic illustration of the preparation, protein (Hb) loading and pH-controlled release processes of hydroxyapatite NFHNs. | ||
Fig. 1 shows the XRD patterns of the samples prepared using Ca(CH3COO)2·H2O and NaH2PO4·2H2O in aqueous solution by a microwave-assisted hydrothermal method at different temperatures for different microwave heating times. It has been found that the samples prepared at 100 °C for 1 and 5 min consisted of a single phase of hydroxyapatite with a hexagonal structure (JCPDS 09-0432) (Fig. 1a and b). However, the products obtained for a longer period of time (30 min) or at a higher temperature (130–200 °C) were composed of a mixture of hydroxyapatite and β-tricalcium phosphate (β-TCP) (JCPDS 09-0169) (Fig. 1c–f).
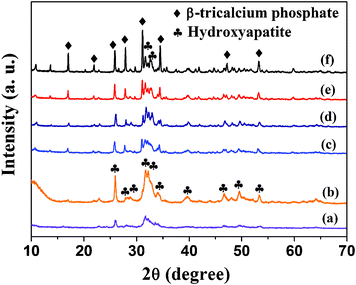 | ||
| Fig. 1 XRD patterns of the samples prepared using Ca(CH3COO)2·H2O and NaH2PO4·2H2O by a microwave-assisted hydrothermal method at different temperatures for different microwave heating times: (a) 100 °C, 1 min; (b) 100 °C, 5 min; (c) 100 °C, 30 min; (d) 130 °C, 5 min; (e) 160 °C, 5 min; (f) 200 °C, 5 min. | ||
The morphology of the as-prepared hydroxyapatite samples were characterized by SEM. Fig. 2 shows that the hydroxyapatite sample prepared using Ca(CH3COO)2·H2O and NaH2PO4·2H2O by a microwave-assisted hydrothermal method at 100 °C for 5 min was composed of nanosheet-assembled flower-like hierarchical nanostructures (NFHNs). The sizes of hydroxyapatite NFHNs ranged from 1 to 3 μm, and the thicknesses of the nanosheets as the building blocks were less than 50 nm. These hydroxyapatite nanosheets self-assembled to form three-dimensional flower-like hierarchical nanostructures under microwave hydrothermal conditions.
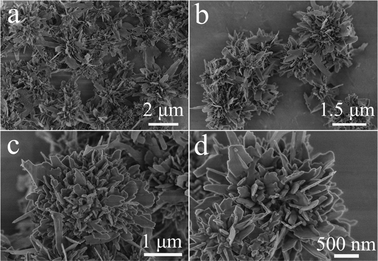 | ||
| Fig. 2 SEM micrographs of hydroxyapatite NFHNs prepared using Ca(CH3COO)2·H2O and NaH2PO4·2H2O by a microwave-assisted hydrothermal method at 100 °C for 5 min. | ||
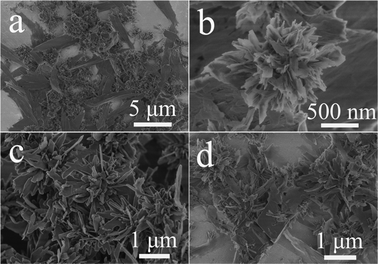
The effects of microwave heating temperature and heating time on the morphology of the product were investigated. Fig. 3 shows the SEM micrographs of the samples prepared using Ca(CH3COO)2·H2O and NaH2PO4·2H2O by a microwave-assisted hydrothermal method at different temperatures of 130, 160 and 200 °C for 5 min. As discussed above, the product prepared at 100 °C for 5 min was composed of hydroxyapatite NFHNs (Fig. 2). When the temperature was increased to 130 °C, the individual nanosheets were observed as a major morphology, and hydroxyapatite NFHNs were minor; in addition, polyhedra were formed as a minor morphology (Fig. 3a). When the temperature was increased to 160 °C, the NFHNs were not observed, and the sample consisted of mixed morphologies of nanosheets and polyhedra (Fig. 3b). When the temperature was further enhanced to 200 °C, the NFHNs disappeared, and they totally transformed to polyhedra (Fig. 3c and d). These results indicate that the microwave heating temperature has a significant influence on the crystal phase and morphology of the product. At a lower temperature (100 °C), the hydroxyapatite NFHNs were obtained. However, at a higher temperature (200 °C), polyhedra were formed.
 | ||
| Fig. 3 SEM micrographs of the samples prepared using Ca(CH3COO)2·H2O and NaH2PO4·2H2O by a microwave-assisted hydrothermal method at different temperatures for 5 min. (a) 130 °C; (b) 160 °C; (c, d) 200 °C. | ||
We also investigated the effect of microwave heating time on the morphology of product (Fig. 4). The hydroxyapatite NFHNs formed by microwave heating for only 1 min, however, in addition to flower-like hierarchical nanostructures, some sheet-like structures also coexisted, as shown in Fig. 4a and b. As discussed above, the product prepared at 100 °C for 5 min was composed of well-defined flower-like hierarchical nanostructures assembled with nanosheets (Fig. 2 and 4c). When the microwave heating time was increased to 30 min, some NFHNs collapsed and polyhedra formed, as shown in Fig. 4d.
 | ||
| Fig. 4 SEM micrographs of the samples prepared using Ca(CH3COO)2·H2O and NaH2PO4·2H2O by a microwave-assisted hydrothermal method at 100 °C for different times. (a, b) 1 min; (c) 5 min; (d) 30 min. | ||
As discussed above, the morphology of the product varied from nanosheets to flower-like hierarchical nanostructures to polyhedra by adjusting the experimental conditions. The formation process of hydroxyapatite NFHNs is shown in Scheme 2. The hydroxyapatite nanosheets form at the early stage by the chemical reaction, nucleation and crystal growth under microwave-hydrothermal conditions. The surface energy of newly formed nanosheets is high, and these nanosheets act as the building blocks and self-assemble to form NFHNs so that their surface energy can be reduced for stability. When the microwave heating temperature is high enough, the NFHNs are not stable in aqueous solution under microwave-hydrothermal conditions, and the polyhedra form through dissolution and recrystallization.
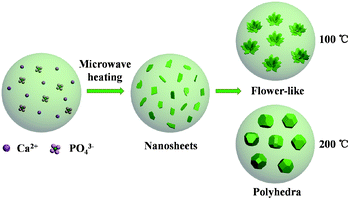 | ||
| Scheme 2 Schematic illustration for the formation of flower-like hierarchical nanostructures and polyhedra. | ||
According to the XRD results, the crystal phase of NFHNs formed at 100 °C for 5 min was hydroxyapatite (Fig. 1b), while the crystal phase of polyhedra prepared at 200 °C was β-tricalcium phosphate (β-TCP) (Fig. 1f). It has been found that there was a phase transformation from hydroxyapatite to β-tricalcium phosphate when the microwave heating temperature increased from 100 °C to 200 °C. The morphology change was mainly attributed to this phase transformation. The polyhedra formed at 200 °C exhibited a relatively high crystallinity. The reason for the phase transformation may be related to the microwave-hydrothermal environment, heating temperature, Ca/P ratio, etc. The detailed mechanism needs to be further investigated.
Hydroxyapatite nanostructured materials are considered to be an ideal nanocarrier for proteins and DNA due to their good biocompatibility. In this study, we used the as-prepared hydroxyapatite NFHNs as the carrier for proteins and DNA. Bovine serum albumin (BSA) and hemoglobin (Hb) were used as model proteins and fish sperm DNA was used as a model DNA to evaluate the protein and DNA loading capacity of the as-prepared hydroxyapatite NFHNs. The adsorption of BSA and Hb molecules in hydroxyapatite NFHNs was detected by FT-IR analysis (Fig. 5). Hydroxyapatite NFHNs were identified by the characteristic peaks of PO43− located at 563, 603, 1031 and 1092 cm−1. The amide peaks located at 1653 and 1538 cm−1 were also observed, indicating the presence of BSA or Hb molecules.
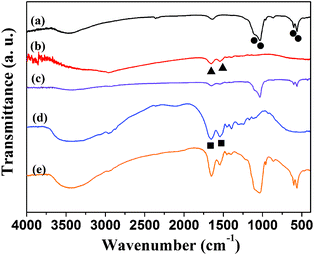 | ||
| Fig. 5 FT-IR spectra. (a) Hydroxyapatite NFHNs without drug loading; (b) hemoglobin (Hb); (c) Hb loaded hydroxyapatite NFHNs; (d) bovine serum albumin (BSA); (e) BSA loaded hydroxyapatite NFHNs. The characteristic peaks for phosphate, Hb and BSA are labelled as circles, triangles and squares, respectively. | ||
Fig. 6a shows the zeta potential values of the as-prepared hydroxyapatite NFHNs before and after loading Hb, BSA and DNA. The as-prepared polyhedra were adopted as a control sample, and the zeta potential values of polyhedra before and after loading Hb, BSA and DNA are shown in Fig. 6b for comparison. The zeta potential value of hydroxyapatite NFHNs was −12.7 mV in deionized water and it changed to be −12.1, −20.2 and −17.8 mV after loading Hb, BSA and DNA, respectively. The change in the zeta potential value was caused by the adsorbed protein or DNA molecules on the surface of hydroxyapatite NFHNs. Hb is a neutral protein and is uncharged in neutral water, so after loading Hb in hydroxyapatite NFHNs, the zeta potential value slightly changed. However, the zeta potential values of hydroxyapatite NFHNs after loading BSA and DNA were −20.2 and −17.8 mV, respectively, because both BSA and DNA molecules are negatively charged in neutral water. The zeta potential value of polyhedra was −15.7 mV and it changed to be −15.7, −24.6 and −18.4 mV after loading Hb, BSA and DNA. The variation trend of zeta potential values of polyhedra after loading protein or DNA is similar to that of hydroxyapatite NFHNs.
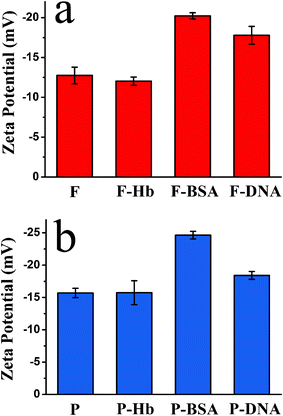 | ||
| Fig. 6 Zeta potential values in aqueous solution. (a) The as-prepared hydroxyapatite NFHNs before and after loading Hb, BSA and DNA; (b) the polyhedra before and after loading Hb, BSA and DNA (F represents flower-like hydroxyapatite and P represents polyhedra). | ||
The protein loading capacity of the as-prepared hydroxyapatite NFHNs was investigated by varying the initial protein concentrations, and the experimental results are shown in Fig. 7. The polyhedra were used as a control sample and protein loading capacities of polyhedra are also shown in Fig. 7 for comparison.
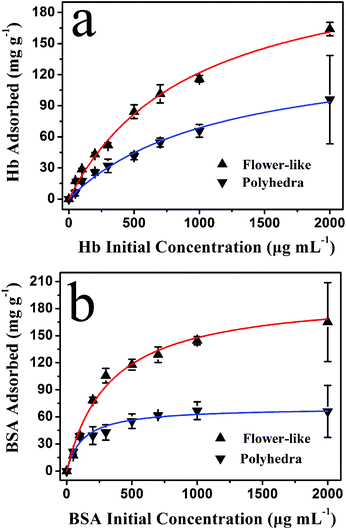 | ||
| Fig. 7 The protein loading capacity of the as-prepared hydroxyapatite NFHNs and polyhedra versus initial protein concentrations (experimental data are labelled as triangles). The isotherm simulations using the Langmuir equation for protein adsorption are also shown as solid curves. (a) Hb; (b) BSA. | ||
The protein loading capacity of hydroxyapatite NFHNs increased with increasing initial protein concentration, and it finally reached a saturated value. The protein loading capacities for BSA and Hb were determined to be as high as 165 mg g−1 and 164 mg g−1, respectively, at an initial protein concentration of 2000 μg mL−1. However, the loading capacities of polyhedra for Hb and BSA were 96 mg g−1 and 66 mg g−1 at an initial protein concentration of 2000 μg mL−1, which are much lower than that of hydroxyapatite NFHNs. The higher protein loading capacity of hydroxyapatite NFHNs can be attributed to three-dimensional hierarchical nanostructures and relatively high specific surface area. The Brunauer–Emmett–Teller (BET) specific surface areas of hydroxyapatite NFHNs and polyhedra were determined to be 54.4 and 27.1 m2 g−1, respectively. The higher BET specific surface area of hydroxyapatite NFHNs may be ascribed to the hierarchical nanostructures assembled by nanosheets. The hydroxyapatite NFHNs can provide a large number of binding sites for protein molecules. Therefore, the protein loading capacity of hydroxyapatite NFHNs is significantly increased compared with that of polyhedra.
The Hb and BSA adsorption data of hydroxyapatite NFHNs and polyhedra were analyzed using Langmuir adsorption isotherms (the solid curves in Fig. 7). The adsorption behaviors of the two proteins are consistent with a typical Langmuir isotherm, illustrating that the protein adsorption on hydroxyapatite NFHNs and polyhedra was a monolayer adsorption mechanism. These results indicate that the as-prepared hydroxyapatite NFHNs have a high loading capacity for proteins and are promising for the application as the protein nanocarrier.
Fig. 8 shows the adsorption kinetics curves of Hb on the as-prepared hydroxyapatite NFHNs at different Hb initial concentrations of 80 and 2000 μg mL−1. The adsorption rates were very high at the early stage (less than 5 min) of the adsorption process, and then slowly reduced and reached equilibrium values at about 20 min. The adsorption kinetics results show that the as-prepared hydroxyapatite NFHNs have a rapid adsorption ability for proteins. And the high initial adsorption rate and short adsorption equilibrium time indicate that the surface of as-prepared hydroxyapatite NFHNs has a high density of binding sites for protein adsorption.
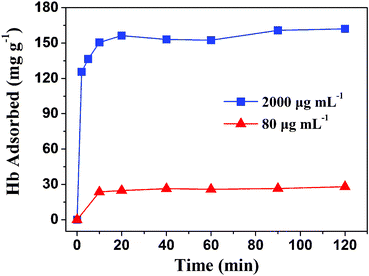 | ||
| Fig. 8 Adsorption kinetics of Hb on the as-prepared hydroxyapatite NFHNs at different Hb initial concentrations of 80 and 2000 μg mL−1. | ||
The DNA loading capacity of as-prepared hydroxyapatite NFHNs was also investigated, as shown in Fig. 9. The fish sperm DNA was chosen as a model DNA. Fig. 9 shows that the DNA loading capacity of as-prepared hydroxyapatite NFHNs increased with increasing initial concentration of DNA in the range from 100 to 1000 μg mL−1. The loading capacity of DNA reached 112 mg g−1 for fish sperm DNA at a DNA initial concentration of 1000 μg mL−1. These results show that the as-prepared hydroxyapatite NFHNs are promising for the application as the DNA nanocarrier.
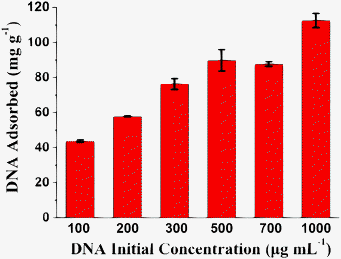 | ||
| Fig. 9 The loading capacity of hydroxyapatite NFHNs for fish sperm DNA at different DNA initial concentrations ranging from 100 to 1000 μg mL−1. | ||
The solubility of hydroxyapatite increases with the decrease of the environmental pH value, and hydroxyapatite can be dissolved as nontoxic ions (Ca2+ and PO43− ions) in aqueous solution with a sufficiently low pH value. It is well known that the pH values in tumors and inflammatory tissues are lower than those in blood and normal tissues. The acidic cellular environments such as endosomes (pH ≈ 5.0) and lysosomes (pH ≈ 4.5) exhibit even lower pH values.21,22 Thus, hydroxyapatite nanostructured materials can be designed and used as pH-sensitive drug nanocarriers that can respond to physiopathological pH signals to control the drug release process.
The Hb release behavior of hydroxyapatite NFHNs was conducted at different pH values (pH = 7.2, 5.5 and 4.8) in PBS solution at 37 °C. As shown in Fig. 10, the Hb release rates were high in the first 2.5 h at different pH values. After the initial rapid release stage, the Hb release rate reduced gradually and maintained at similar release rates between 2.5 and 12 h for different pH values. The cumulative Hb release percentage in PBS with a pH 7.2 reached 20% at a release time of 15 h. In contrast, the cumulative Hb release percentages in PBS with pH 5.5 and 4.8 reached 26% and 32%, respectively. The higher release rate and higher cumulative release percentage of Hb at lower pH values may be attributed to increased dissolution of hydroxyapatite. It was reported that proteins adsorbed on hydroxyapatite were bound strongly and their release from the surface was mainly dependent on hydroxyapatite dissolution.23 Thus, the enhanced hydroxyapatite dissolution at lower pH values led to increased protein release.24 These protein release experiments show that the as-prepared hydroxyapatite NFHNs have a good pH-controlled release function and are promising for the application as pH-sensitive drug nanocarriers.
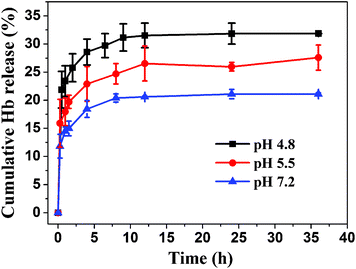 | ||
| Fig. 10 Cumulative release of Hb from Hb loaded hydroxyapatite NFHNs at different pH values of 4.8, 5.5 and 7.2 in PBS at 37 °C. | ||
4 Conclusion
In summary, we have developed a surfactant-free rapid microwave-assisted hydrothermal method for the preparation of hydroxyapatite nanosheet-assembled flower-like hierarchical nanostructures. The morphology of the product is dependent on the experimental conditions such as the microwave heating temperature and heating time. Hydroxyapatite NFHNs can be obtained at a lower temperature (100 °C), and polyhedra can be obtained at a higher temperature (200 °C). It has been found that hydroxyapatite NFHNs are promising nanocarriers for proteins and DNA. The loading capacities of as-prepared hydroxyapatite NFHNs for hemoglobin (Hb) and bovine serum albumin (BSA) are determined to be as high as 164 mg g−1 and 165 mg g−1, respectively. The pH-controlled Hb release behavior of hydroxyapatite NFHNs in phosphate buffer saline has been demonstrated. The experimental results reveal that the as-prepared hydroxyapatite NFHNs have a high protein and DNA loading capacity and pH-controlled protein release function. Thus, the as-prepared hydroxyapatite NFHNs are promising for protein and DNA adsorption and pH-controlled protein release applications.Acknowledgements
Financial support from the National Basic Research Program of China (973 Program, No. 2012CB933600), the National Natural Science Foundation of China (51172260, 51121064, 51102258), the Science and Technology Commission of Shanghai (11nm0506600, 1052nm06200) and CAS/SAFEA International Partnership Program for Creative Research Teams is gratefully acknowledged.References
- S. V. Dorozhkin and M. Epple, Angew. Chem., Int. Ed., 2002, 41, 3130–3146 CrossRef CAS.
- F. Chen, Q. L. Tang, Y. J. Zhu, K. W. Wang, M. L. Zhang, W. Y. Zhai and J. Chang, Acta Biomater., 2010, 6, 3013–3020 CrossRef CAS.
- F. Chen, Y. J. Zhu, K. W. Wang and K. L. Zhao, CrystEngComm, 2011, 13, 1858–1863 RSC.
- M. Jevtić, M. Mitrić, S. Škapin, B. Jančar, N. Ignjatović and D. Uskoković, Cryst. Growth Des., 2008, 8, 2217–2222 Search PubMed.
- K. W. Wang, Y. J. Zhu, X. Y. Chen, W. Y. Zhai, Q. Wang, F. Chen, J. Chang and Y. R. Duan, Chem.–Asian J., 2010, 5, 2477–2482 CrossRef CAS.
- K. W. Wang, Y. J. Zhu, F. Chen, G. F. Cheng and Y. H. Huang, Mater. Lett., 2011, 65, 2361–2363 CrossRef CAS.
- S. I. Stupp and P. V. Braun, Science, 1997, 277, 1242–1248 CrossRef CAS.
- M. Y. Ma, Y. J. Zhu, L. Li and S. W. Cao, J. Mater. Chem., 2008, 18, 2722–2727 RSC.
- Y. Cai and R. Tang, J. Mater. Chem., 2008, 18, 3775–3787 RSC.
- C. M. Zhang, J. Yang, Z. W. Quan, P. P. Yang, C. X. Li, Z. Y. Hou and J. Lin, Cryst. Growth Des., 2009, 9, 2725–2733 CAS.
- C. Qi, Y. J. Zhu, B. Q. Lu, X. Y. Zhao, J. Zhao and F. Chen, J. Mater. Chem., 2012, 22, 22642–22650 RSC.
- K. Kandori, T. Kuroda, S. Togashi and E. Katayama, J. Phys. Chem. B, 2011, 115, 653–659 CrossRef CAS.
- E. Fujii, M. Ohkubo, K. Tsuru, S. Hayakawa, A. Osaka, K. Kawabata, C. Bonhomme and F. Babonneau, Acta Biomater., 2006, 2, 69–74 CrossRef.
- S. Takemoto, Y. Kusudo, K. Tsuru, S. Hayakawa, A. Osaka and S. Takashima, J. Biomed. Mater. Res. A, 2004, 69A, 544–551 CrossRef CAS.
- H. Tolou, Anal. Biochem., 1993, 215, 156–158 CrossRef CAS.
- C. Chen and H. Okayama, Mol. Cell. Biol., 1987, 7, 2745–2752 CAS.
- E. Orrantia and P. L. Chang, Exp. Cell Res., 1990, 190, 170–174 CrossRef CAS.
- J. K. Burkholder, J. Decker and N. S. Yang, J. Immunol. Methods, 1993, 165, 149–156 CrossRef CAS.
- M. Jordan, A. Schallhorn and F. M. Wurm, Nucleic Acids Res., 1996, 24, 596–601 CrossRef CAS.
- P. Girard, L. Porte, T. Berta, M. Jordan and F. M. Wurm, Cytotechnology, 2001, 35, 175–180 CrossRef CAS.
- Q. Yang, S. Wang, P. Fan, L. F. Wang, Y. Di, K. F. Lin and F. S. Xiao, Chem. Mater., 2005, 17, 5999–6003 CrossRef CAS.
- H. J. Lee, S. E. Kim, I. K. Kwon, C. Park, C. Kim, J. Yang and S. C. Lee, Chem. Commun., 2010, 46, 377–379 RSC.
- L. Jongpaiboonkit, T. Franklin-Ford and W. L. Murphy, Adv. Mater., 2009, 21, 1960–1963 CrossRef CAS.
- T. Matsumoto, M. Okazaki, M. Inoue, S. Yamaguchi, T. Kusunose, T. Toyonaga, Y. Hamada and J. Takahashi, Biomaterials, 2004, 25, 3807–3812 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
